Development and Preclinical Testing of a Novel Neurodenervant in the Rat: C3 Transferase Mitigates Botulinum Toxin’s Adverse Effects on Muscle Mechanics †
Abstract
1. Introduction
2. Results
2.1. Study 1: Effects of Combined Administration of BTX-A and C3 Transferase on Muscle Mechanics
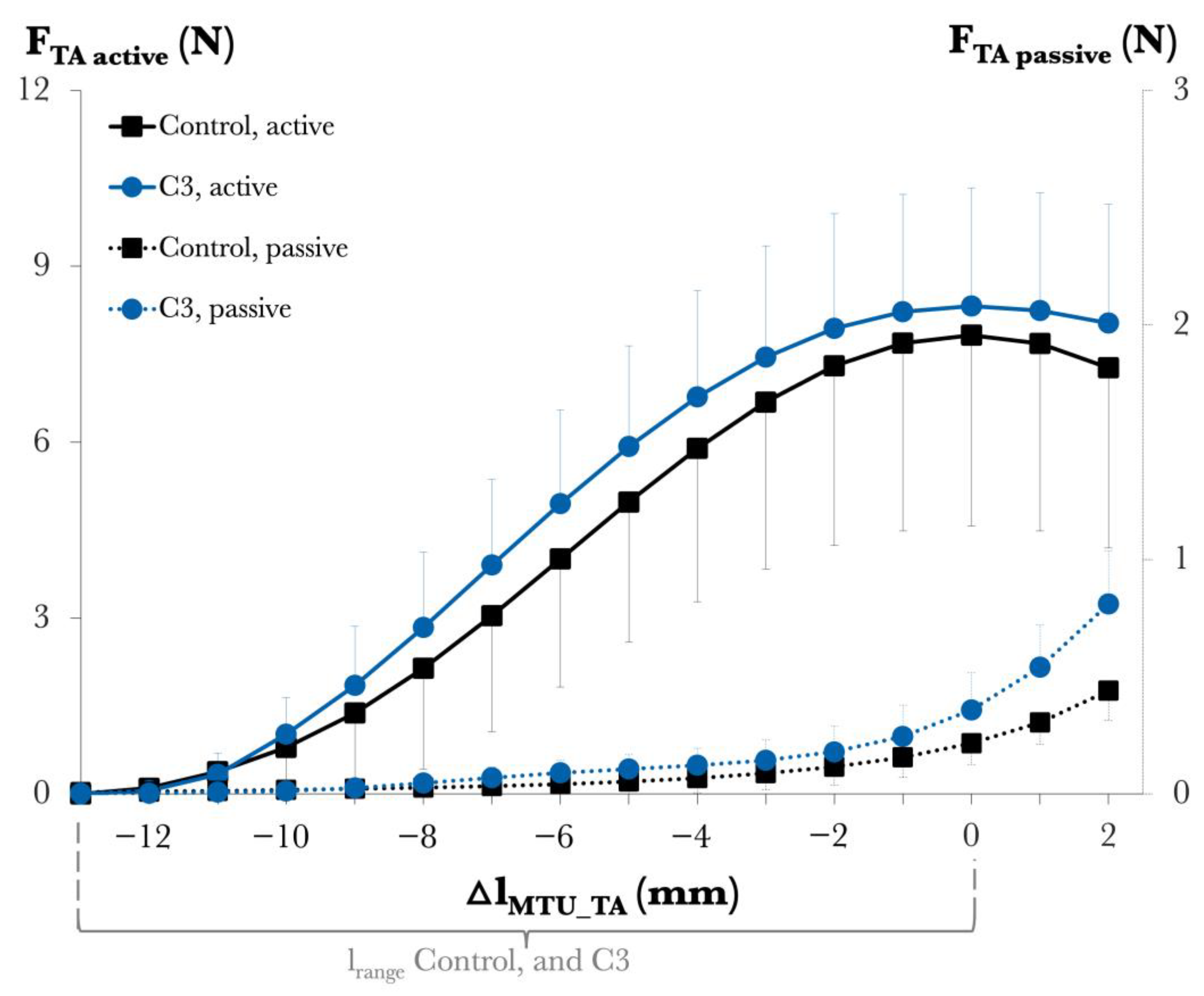
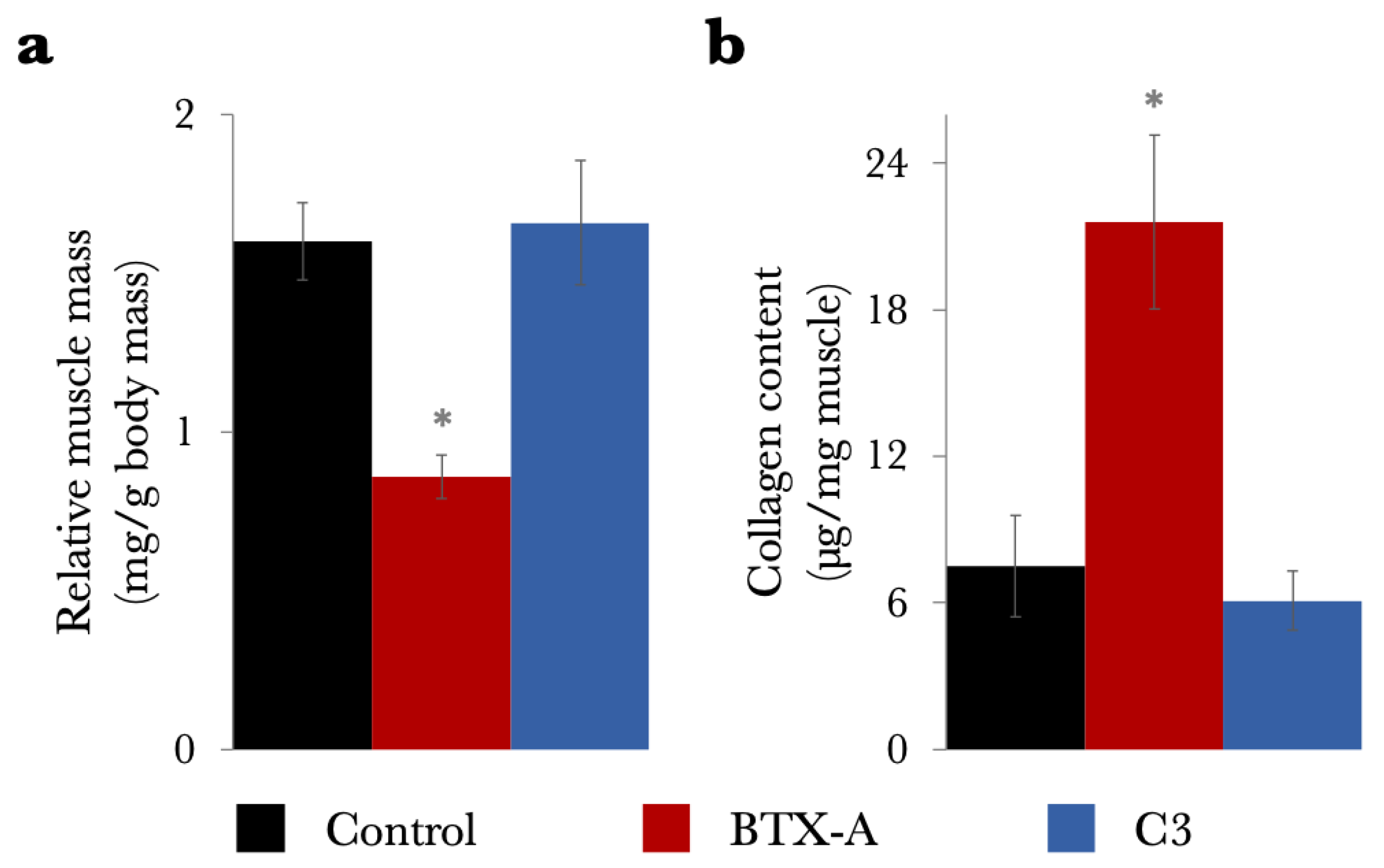
2.2. Study 2: Effects of C3 Transferase Alone on Muscle Mechanics and Structure
3. Discussion
3.1. Injection Protocols in Experimental Models and Clinical Comparisons
3.2. Addressing Muscle Atrophy and Growth Challenges
3.3. Muscle Mechanical Changes Induced by Combined Injection
3.4. Limitations and Future Directions
4. Conclusions
5. Materials and Methods
5.1. Study 1: Assessment of Effects of Combined Administration of BTX-A and C3 Transferase on Muscle Mechanics
5.1.1. Injection Protocol
5.1.2. Surgical Procedure
5.1.3. Experimental Setup and Procedure
5.2. Study 2: Assessment of the Effects of C3 Transferase Alone on Muscle Mechanics and Structure
5.3. Data Processing
5.4. Statistical Analyses
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BTX-A | botulinum toxin type A |
| CP | cerebral palsy |
| ECM | extracellular matrix |
| EDL | extensor digitorum longus |
| EHL | extensor hallucis longus |
| TA | tibialis anterior |
References
- Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of Cerebral Palsy in Europe: A Collaboration of Cerebral Palsy Surveys and Registers. Dev. Med. Child. Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Cosgrove, A.P.; Corry, I.S.; Graham, H.K. Botulinum Toxin in the Management of the Lower Limb in Cerebral Palsy. Dev. Med. Child. Neurol. 1994, 36, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; Alter, K.E.; Chambers, H. New Clinical and Research Trends in Lower Extremity Management for Ambulatory Children with Cerebral Palsy. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Kaya Keles, C.S.; Ates, F. Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles. Toxins 2022, 14, 772. [Google Scholar] [CrossRef]
- Bakheit, A.M.O. Management of Muscle Spasticity. Crit. Rev. Phys. Rehabil. Med. 1996, 8, 235–252. [Google Scholar] [CrossRef]
- Love, S.C.; Valentine, J.P.; Blair, E.M.; Price, C.J.; Cole, J.H.; Chauvel, P.J. The Effect of Botulinum Toxin Type A on the Functional Ability of the Child with Spastic Hemiplegia a Randomized Controlled Trial. Eur. J. Neurol. 2001, 8, 50–58. [Google Scholar] [CrossRef]
- Deschrevel, J.; Andries, A.; Maes, K.; De Beukelaer, N.; Corvelyn, M.; Staut, L.; De Houwer, H.; Costamagna, D.; Desloovere, K.; Van Campenhout, A.; et al. Short-Term Effects of Botulinum Toxin-A Injection on the Medial Gastrocnemius Histological Features in Ambulant Children with Cerebral Palsy: A Longitudinal Pilot Study. Toxins 2024, 16, 69. [Google Scholar] [CrossRef]
- De Beukelaer, N.; Weide, G.; Huyghe, E.; Vandekerckhove, I.; Hanssen, B.; Peeters, N.; Uytterhoeven, J.; Deschrevel, J.; Maes, K.; Corvelyn, M.; et al. Reduced Cross-Sectional Muscle Growth Six Months after Botulinum Toxin Type-A Injection in Children with Spastic Cerebral Palsy. Toxins 2022, 14, 139. [Google Scholar] [CrossRef]
- Fortuna, R.; Horisberger, M.; Vaz, M.A.; Herzog, W. Do Skeletal Muscle Properties Recover Following Repeat Onabotulinum Toxin A Injections? J. Biomech. 2013, 46, 2426–2433. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Arikan, Ö.E.; Ates, F. BTX-A Administration to the Target Muscle Affects Forces of All Muscles within an Intact Compartment and Epimuscular Myofascial Force Transmission. J. Biomech. Eng. 2012, 134, 111002. [Google Scholar] [CrossRef]
- Ates, F.; Yucesoy, C.A. Effects of Botulinum Toxin Type A on Non-Injected Bi-Articular Muscle Include a Narrower Length Range of Force Exertion and Increased Passive Force. Muscle Nerve 2014, 49, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, C.A.; Ates, F. BTX-A Has Notable Effects Contradicting Some Treatment Aims in the Rat Triceps Surae Compartment, Which Are Not Confined to the Muscles Injected. J. Biomech. 2018, 66, 78–85. [Google Scholar] [CrossRef]
- Ates, F.; Yucesoy, C.A. Botulinum Toxin Type-A Affects Mechanics of Non-Injected Antagonistic Rat Muscles. J. Mech. Behav. Biomed. Mater. 2018, 84, 208–216. [Google Scholar] [CrossRef]
- Gough, M.; Fairhurst, C.; Shortland, A.P. Botulinum Toxin and Cerebral Palsy: Time for Reflection? Dev. Med. Child Neurol. 2005, 47, 709–712. [Google Scholar] [CrossRef]
- Turkoglu, A.N.; Huijing, P.A.; Yucesoy, C.A. Mechanical Principles of Effects of Botulinum Toxin on Muscle Length-Force Characteristics: An Assessment by Finite Element Modeling. J. Biomech. 2014, 47, 1565–1571. [Google Scholar] [CrossRef]
- Turkoglu, A.N.; Yucesoy, C.A. Simulation of Effects of Botulinum Toxin on Muscular Mechanics in Time Course of Treatment Based on Adverse Extracellular Matrix Adaptations. J. Biomech. 2016, 49, 1192–1198. [Google Scholar] [CrossRef]
- Kaya, C.S.; Yılmaz, E.O.; Akdeniz-Doğan, Z.D.; Yucesoy, C.A. Long-Term Effects with Potential Clinical Importance of Botulinum Toxin Type-A on Mechanics of Muscles Exposed. Front. Bioeng. Biotechnol. 2020, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.O.; Kaya, C.S.; Akdeniz-Doğan, Z.D.; Yucesoy, C.A. Long-Term BTX-A Effects on Bi-Articular Muscle: Higher Passive Force, Limited Length Range of Active Force Production and Unchanged Intermuscular Interactions. J. Biomech. 2021, 126, 110627. [Google Scholar] [CrossRef] [PubMed]
- Rohrbeck, A.; Just, I. Cell Entry of C3 Exoenzyme from Clostridium Botulinum. Curr. Top. Microbiol. Immunol. 2017, 406, 97–118. [Google Scholar] [CrossRef]
- Parizi, M.; Howard, E.W.; Tomasek, J.J. Regulation of LPA-Promoted Myofibroblast Contraction: Role of Rho, Myosin Light Chain Kinase, and Myosin Light Chain Phosphatase. Exp. Cell Res. 2000, 254, 210–220. [Google Scholar] [CrossRef]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Kaya Keles, C.S.; Yucesoy, C.A. A Novel Botulinum Toxin Formula, Which Diminishes the Adverse Effects of BTX-A on Muscular Mechanics. Gait Posture 2024, 113, 111–112. [Google Scholar] [CrossRef]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The Updated European Consensus 2009 on the Use of Botulinum Toxin for Children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2010, 1, 45–66. [Google Scholar] [CrossRef]
- Frasson, E.; Dall’Ora, E.; Bordignon, M.; Brigo, F.; Tocco, P.; Primon, D.; Didonè, G.; Vicentini, S.; Fiaschi, A.; Bertolasi, L. Spread of Botulinum Neurotoxin Type A at Standard Doses Is Inherent to the Successful Treatment of Spastic Equinus Foot in Cerebral Palsy: Short-Term Neurophysiological and Clinical Study. J. Child Neurol. 2012, 27, 587–593. [Google Scholar] [CrossRef]
- Koman, L.A.; Mooney, J.F.; Smith, B.; Goodman, A.; Mulvaney, T. Management of Cerebral Palsy with Botulinum-a Toxin: Preliminary Investigation. J. Pediatr. Orthop. 1993, 13, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Molenaers, G.; van Campenhout, A.; Fagard, K.; de Cat, J.; Desloovere, K. The Use of Botulinum Toxin A in Children with Cerebral Palsy, with a Focus on the Lower Limb. J. Child. Orthop. 2010, 4, 183–195. [Google Scholar] [CrossRef]
- Shaari, C.M.; Sanders, I. Quantifying How Location and Dose of Botulinum Toxin Injections Affect Muscle Paralysis. Muscle Nerve 1993, 16, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy. Toxins 2015, 7, 1629–1648. [Google Scholar] [CrossRef]
- Friedman, B.C.; Goldman, R.D. Use of Botulinum Toxin A in Management of Children with Cerebral Palsy. Can. Fam. Physician 2011, 57, 1006–1073. [Google Scholar]
- Boyd, R.; Graham, H.K. Botulinum Toxin A in the Management of Children with Cerebral Palsy: Indications and Outcome. Eur. J. Neurol. 1997, 4, 15–21. [Google Scholar]
- Ma, J.; Elsaidi, G.A.; Smith, T.L.; Walker, F.O.; Tan, K.H.; Martin, E.; Koman, L.A.; Smith, B.P. Time Course of Recovery of Juvenile Skeletal Muscle after Botulinum Toxin A Injection: An Animal Model Study. Am. J. Phys. Med. Rehabil. 2004, 83, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, R.; Aurélio Vaz, M.; Rehan Youssef, A.; Longino, D.; Herzog, W. Changes in Contractile Properties of Muscles Receiving Repeat Injections of Botulinum Toxin (Botox). J. Biomech. 2011, 44, 39–44. [Google Scholar] [CrossRef]
- Morbiato, L.; Carli, L.; Johnson, E.A.; Montecucco, C.; Molgó, J.; Rossetto, O. Neuromuscular Paralysis and Recovery in Mice Injected with Botulinum Neurotoxins A and C. Eur. J. Neurosci. 2007, 25, 2697–2704. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Tang, M.J.; Herzog, W.; Howard, J.J.; Graham, H.K. Sarcopenia, Cerebral Palsy, and Botulinum Toxin Type A. JBJS Rev. 2019, 7, e4. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Ertl-Wagner, B.; Britsch, S.; Schröder, J.M.; Nikolin, S.; Weis, J.; Müller-Felber, W.; Koerte, I.; Stehr, M.; Berweck, S.; et al. Muscle Biopsy Substantiates Long-Term MRI Alterations One Year after a Single Dose of Botulinum Toxin Injected into the Lateral Gastrocnemius Muscle of Healthy Volunteers. Mov. Disord. 2009, 24, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Verhaegen, A.; Pans, S.; Molenaers, G. Botulinum Toxin Type A Injections in the Psoas Muscle of Children with Cerebral Palsy: Muscle Atrophy after Motor End Plate-Targeted Injections. Res. Dev. Disabil. 2013, 34, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Elliott, C.; Valentine, J.; Stannage, K.; Bear, N.; Donnelly, C.J.; Shipman, P.; Reid, S. Muscle Volume Alterations after First Botulinum Neurotoxin A Treatment in Children with Cerebral Palsy: A 6-Month Prospective Cohort Study. Dev. Med. Child. Neurol. 2018, 60, 1165–1171. [Google Scholar] [CrossRef]
- Mathevon, L.; Michel, F.; Decavel, P.; Fernandez, B.; Parratte, B.; Calmels, P. Muscle Structure and Stiffness Assessment after Botulinum Toxin Type A Injection. A Systematic Review. Ann. Phys. Rehabil. Med. 2015, 58, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Kerr Graham, H.; Barrett, R.; Lichtwark, G. The Effects of Botulinum Toxin Injection Frequency on Calf Muscle Growth in Young Children with Spastic Cerebral Palsy: A 12-Month Prospective Study. J. Child. Orthop. 2013, 7, 425–433. [Google Scholar] [CrossRef]
- Hanssen, B.; Peeters, N.; Vandekerckhove, I.; de Beukelaer, N.; Bar-On, L.; Molenaers, G.; van Campenhout, A.; Degelaen, M.; van den Broeck, C.; Calders, P.; et al. The Contribution of Decreased Muscle Size to Muscle Weakness in Children with Spastic Cerebral Palsy. Front. Neurol. 2021, 12, 692582. [Google Scholar] [CrossRef]
- Goudriaan, M.; Nieuwenhuys, A.; Schless, S.H.; Goemans, N.; Molenaers, G.; Desloovere, K. A New Strength Assessment to Evaluate the Association between Muscle Weakness and Gait Pathology in Children with Cerebral Palsy. PLoS ONE 2018, 13, e0191097. [Google Scholar] [CrossRef] [PubMed]
- Speth, L.; Janssen-Potten, Y.; Rameckers, E.; Defesche, A.; Winkens, B.; Becher, J.; Smeets, R.; Vles, H. Effects of Botulinum Toxin A and/or Bimanual Task-Oriented Therapy on Upper Extremity Activities in Unilateral Cerebral Palsy: A Clinical Trial. BMC Neurol. 2015, 15, 143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elvrum, A.K.G.; Brændvik, S.M.; Sæther, R.; Lamvik, T.; Vereijken, B.; Roeleveld, K. Effectiveness of Resistance Training in Combination with Botulinum Toxin-A on Hand and Arm Use in Children with Cerebral Palsy: A Pre-Post Intervention Study. BMC Pediatr. 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Yaraskavitch, M.; Leonard, T.; Herzog, W. Botox Produces Functional Weakness in Non-Injected Muscles Adjacent to the Target Muscle. J. Biomech. 2008, 41, 897–902. [Google Scholar] [CrossRef]
- Longino, D.; Butterfield, T.A.; Herzog, W. Frequency and Length-Dependent Effects of Botulinum Toxin-Induced Muscle Weakness. J. Biomech. 2005, 38, 609–613. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Koopman, B.H.F.J.M.; Baan, G.C.; Grootenboer, H.J.; Huijing, P.A. Effects of Inter- and Extramuscular Myofascial Force Transmission on Adjacent Synergistic Muscles: Assessment by Experiments and Finite-Element Modeling. J. Biomech. 2003, 36, 1797–1811. [Google Scholar] [CrossRef]
- Yucesoy, C.A. Epimuscular Myofascial Force Transmission Implies Novel Principles for Muscular Mechanics. Exerc. Sport Sci. Rev. 2010, 38, 128–134. [Google Scholar] [CrossRef]
- Wilke, J.; Schleip, R.; Yucesoy, C.A.; Banzer, W. Not Merely a Protective Packing Organ? A Review of Fascia and Its Force Transmission Capacity. J. Appl. Physiol. 2017, 124, 234–244. [Google Scholar] [CrossRef]
- Ates, F.; Temelli, Y.; Yucesoy, C.A. Effects of Antagonistic and Synergistic Muscles’ Co-Activation on Mechanics of Activated Spastic Semitendinosus in Children with Cerebral Palsy. Hum. Mov. Sci. 2018, 57, 103–110. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Turkoğlu, A.N.; Umur, S.; Ates, F. Intact Muscle Compartment Exposed to Botulinum Toxin Type a Shows Compromised Intermuscular Mechanical Interaction. Muscle Nerve 2015, 51, 106–116. [Google Scholar] [CrossRef]
- Kaya, C.S.; Bilgili, F.; Akalan, N.E.; Temelli, Y.; Ateş, F.; Yucesoy, C.A. Intraoperative Experiments Combined with Gait Analyses Indicate That Active State Rather than Passive Dominates the Spastic Gracilis Muscle’s Joint Movement Limiting Effect in Cerebral Palsy. Clin. Biomech. 2019, 68, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.S.; Bilgili, F.; Akalan, N.E.; Yucesoy, C.A. Intraoperative Testing of Passive and Active State Mechanics of Spastic Semitendinosus in Conditions Involving Intermuscular Mechanical Interactions and Gait Relevant Joint Positions. J. Biomech. 2020, 103, 109755. [Google Scholar] [CrossRef] [PubMed]
- Gruner, J.A.; Altman, J.; Spivack, N. Effects of Arrested Cerebellar Development on Locomotion in the Rat. Cinematogr. Electromyogr. Analysis. Exp. Brain Res. 1980, 40, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.G. Determination of Hydroxyproline Content as a Measure of Fibrosis in Nondystrophic and Dystrophic Skeletal Muscle. Available online: https://www.treat-nmd.org/wp-content/uploads/2023/07/MDX-DMD_M.1.2.006.pdf (accessed on 13 December 2024).
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models; McGraw-Hill/Irwin: Homewood, IL, USA, 1996. [Google Scholar]
- Neuman, R.E.; Logan, M.A. The Determination of Collagen and Elastin in Tissues. J. Biol. Chem. 1950, 186, 549–556. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya Keles, C.S.; Akdeniz Dogan, Z.D.; Yucesoy, C.A. Development and Preclinical Testing of a Novel Neurodenervant in the Rat: C3 Transferase Mitigates Botulinum Toxin’s Adverse Effects on Muscle Mechanics. Toxins 2025, 17, 234. https://doi.org/10.3390/toxins17050234
Kaya Keles CS, Akdeniz Dogan ZD, Yucesoy CA. Development and Preclinical Testing of a Novel Neurodenervant in the Rat: C3 Transferase Mitigates Botulinum Toxin’s Adverse Effects on Muscle Mechanics. Toxins. 2025; 17(5):234. https://doi.org/10.3390/toxins17050234
Chicago/Turabian StyleKaya Keles, Cemre Su, Zeynep D. Akdeniz Dogan, and Can A. Yucesoy. 2025. "Development and Preclinical Testing of a Novel Neurodenervant in the Rat: C3 Transferase Mitigates Botulinum Toxin’s Adverse Effects on Muscle Mechanics" Toxins 17, no. 5: 234. https://doi.org/10.3390/toxins17050234
APA StyleKaya Keles, C. S., Akdeniz Dogan, Z. D., & Yucesoy, C. A. (2025). Development and Preclinical Testing of a Novel Neurodenervant in the Rat: C3 Transferase Mitigates Botulinum Toxin’s Adverse Effects on Muscle Mechanics. Toxins, 17(5), 234. https://doi.org/10.3390/toxins17050234





