Are Aflatoxin Residues in Chicken Products a Real or Perceived Human Dietary Risk?
Abstract
:1. Introduction
2. Results
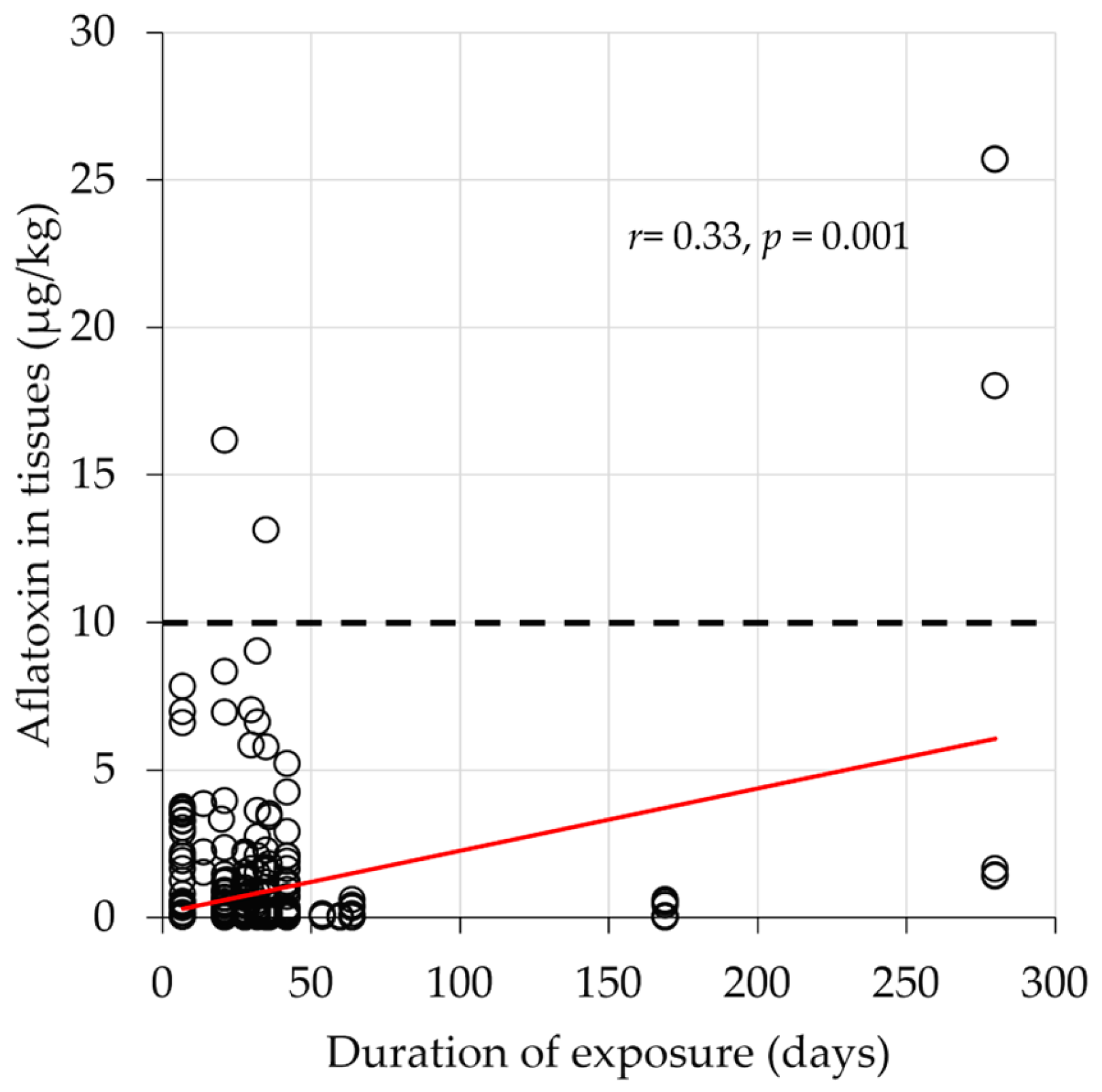
2.1. Characteristics of the Included Studies and the Aflatoxin Exposure Profiles in the Evaluated Chickens
2.2. Aflatoxin Carryover to Various Chicken Tissues
2.2.1. Aflatoxin Accumulation in Chicken Muscle
2.2.2. Aflatoxin Accumulation in Chicken Liver
2.2.3. Aflatoxin Accumulation in Chicken Eggs
2.2.4. Aflatoxin Accumulation in Chicken Kidney
| Tissue (Total Number of Trials) | Aflatoxin in Feeds | Number of Trials (%) with Different Ranges of Aflatoxin Concentration (µg/kg) in Chicken Tissues | |||||
|---|---|---|---|---|---|---|---|
| Concentration (µg/kg) | Number of Trials | <1 | 1–5 | 5–10 | 10–20 | >20 | |
| Total (334) | 0.1−100 | 80 | 73 (91) | 6 (8) | 1 (1) | ||
| 100−300 | 35 | 27 (77) | 6 (17) | 1 (3) | 1 (3) | ||
| >300 | 219 | 161 (74) | 45 (20) | 9 (4) | 2 (1) | 2 (1) | |
| AFB1 only (192) | 0.1−100 | 52 | 48 (92) | 3 (5) | 1 (2) | 1 (2) | |
| 100−300 | 25 | 17 (68) | 6 (24) | 2 (8) | |||
| >300 | 115 | 65 (56) | 38 (33) | 8 (7) | 2 (2) | 2 (2) | |
| Liver (103) | 0.1−100 | 24 | 21 (88) | 2 (8) | 1 (4) | ||
| 100−300 | 12 | 8 (67) | 3 (25) | 1 (8) | |||
| >300 | 67 | 38 (57) | 23 (34) | 6 (9) | |||
| Muscle (91) | 0.1−100 | 21 | 20 (95) | 1 (5) | |||
| 100−300 | 17 | 14 (82) | 2 (12) | 1 (6) | |||
| >300 | 53 | 40 (75) | 10 (19) | 1(2) | 2 (4) | ||
| Eggs (58) | 0.1−100 | 13 | 13 (100) | ||||
| 100−300 | 4 | 4 (100) | |||||
| >300 | 41 | 38 (93) | 3 (7) | ||||
| Kidneys (35) | 0.1−100 | 10 | 7 (70) | 3 (30) | |||
| 100−300 | 1 | 1 (100) | |||||
| >300 | 24 | 20 (84) | 2 (8) | 1 (4) | 1 (4) | ||
| Gizzards (25) | 100−300 | 1 | 1 (100) | ||||
| >300 | 24 | 15 (63) | 7 (29) | 2 (8) | |||
| Skin (8) | 0.1−100 | 8 | 8 (100) | ||||
| Heart (9) | 0.1−100 | 4 | 4 (100) | ||||
| >300 | 5 | 5 (100) | |||||
| Spleen (5) | >300 | 5 | 5 (100) | ||||
2.2.5. Aflatoxin Accumulation in Chicken Gizzards
2.2.6. Aflatoxin Accumulation in Chicken Skin, Heart, and Spleen
2.3. Aflatoxin Buffering Capacity by Tissue
2.4. Effect of Aflatoxin Source Used to Contaminate Feed
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Data Collection and Curation
5.2. Data Quality Assessment
5.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blount, W.P. Turkey X disease. J. Brit. Turk. Fed. 1961, 9, 52–54. [Google Scholar]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front. Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.J.; Schilter, B.; Tritscher, A.M.; Stadler, R.H. Contaminants of milk and dairy products: Contamination resulting from farm and dairy practices. In Encyclopedia of Dairy Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 887–897. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2019, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Amirkhizi, B.; Arefhosseini, S.R.; Ansarin, M.; Nemati, M. Aflatoxin B in eggs and chicken livers by dispersive liquid-liquid microextraction and HPLC. Food Addit. Contam. Part. B Surveill. 2015, 8, 245–249. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Abboodi, A.R.; Awawdeh, M.A.; Jbour, S. Assessment of mycotoxins (deoxynivalenol, zearalenone, aflatoxin B1 and fumonisin B1) in hen’s eggs in Jordan. Heliyon 2022, 8, e11017. [Google Scholar] [CrossRef]
- Sineque, A.R.; Macuamule, C.L.; Dos Anjos, F.R. Aflatoxin B1 contamination in chicken livers and gizzards from industrial and small abattoirs, measured by ELISA technique in Maputo, Mozambique. Int. J. Environ. Res. Public. Health 2017, 14, 951. [Google Scholar] [CrossRef]
- Khan, M.Z.; Hameed, M.R.; Hussain, T.; Khan, A.; Javed, I.; Ahmad, I.; Hussain, A.; Saleemi, M.K.; Islam, N.-U. Aflatoxin residues in tissues of healthy and sick broiler birds at market age in Pakistan: A one year study. Pak. Vet. J. 2013, 33, 423–427. [Google Scholar]
- Alam, S.; Khan, N.A.; Muhammad, A.; Jan, I.; Hashmi, M.S.; Khan, A.; Khan, M.O. Carryover of aflatoxin B1 from feed to broilers’ tissues and its effect on chicken performance. Fresenius Environ. Bull. 2020, 29, 214–221. [Google Scholar]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Elolimy, A.A.; Alqhtani, A.H.; Al-Garadi, M.A.; Abudabos, A.M. Protective effect of date pits on growth performance, carcass traits, blood indices, intestinal morphology, nutrient digestibility, and hepatic aflatoxin residues of aflatoxin B1-exposed broilers. Agriculture 2022, 12, 476. [Google Scholar] [CrossRef]
- Ali, A.; Khatoon, A.; Almohaimeed, H.M.; Al-Sarraj, F.; Albiheyri, R.; Alotibi, I.; Abidin, Z.U. Mitigative potential of novel Lactobacillus plantarum TISTR 2076 against the aflatoxins-associated oxidative stress and histopathological alterations in liver and kidney of broiler chicks during the entire growth period. Toxins 2022, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Al-Ruwaili, M.; Alkhalaileh, N.I.; Herzallah, S.M.; Rawashdeh, A.; Fataftah, A.; Holley, R. Reduction of aflatoxin B1 residues in meat and organs of broiler chickens by lactic acid bacteria. Pak. Vet. J. 2018, 38, 325–328. [Google Scholar] [CrossRef]
- Ashry, A.; Taha, N.M.; Lebda, M.A.; Abdo, W.; El-Diasty, E.M.; Fadl, S.E.; Morsi Elkamshishi, M. Ameliorative effect of nanocurcumin and Saccharomyces cell wall alone and in combination against aflatoxicosis in broilers. BMC Vet. Res. 2022, 18, 178. [Google Scholar] [CrossRef]
- Bintvihok, A.; Thiengnin, S.; Doi, K.; Kumagai, S. Residues of aflatoxins in the liver, muscle and eggs of domestic fowls. J. Vet. Med. Sci. 2002, 64, 1037–1039. [Google Scholar] [CrossRef]
- Bintvihok, A.; Kositcharoenkul, S. Effect of dietary calcium propionate on performance, hepatic enzyme activities and aflatoxin residues in broilers fed a diet containing low levels of aflatoxin B1. Toxicon 2006, 47, 41–46. [Google Scholar] [CrossRef]
- Chen, C.; Pearson, A.M.; Coleman, T.H.; Gray, J.I.; Pestka, J.J.; Aust, S.D. Tissue deposition and clearance of aflatoxins from broiler chickens fed a contaminated diet. Food Chem. Toxicol. 1984, 22, 447–451. [Google Scholar] [CrossRef]
- Denli, M.; Blandon, J.C.; Guynot, M.E.; Salado, S.; Perez, J.F. Effects of dietary afladetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B1. Poult. Sci. 2009, 88, 1444–1451. [Google Scholar] [CrossRef]
- Fernández, A.; Verde, M.T.; Gascón, M.; Ramos, J.J.; Gómez, J. Aflatoxin and its metabolites in tissues from laying hens and broiler chickens fed a contaminated diet. J. Sci. Food Agric. 1994, 65, 407–414. [Google Scholar] [CrossRef]
- Fowler, J.; Li, W.; Bailey, C. Effects of a calcium bentonite clay in diets containing aflatoxin when measuring liver residues of aflatoxin B1 in starter broiler chicks. Toxins 2015, 7, 3455–3464. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Khan, M.Z.; Khan, A.; Javed, I.; Hussain, Z. Effects of individual and combined administration of ochratoxin A and aflatoxin B1 in tissues and eggs of White Leghorn breeder hens. J. Sci. Food Agric. 2012, 92, 1540–1544. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Ma, Q.; Fan, Y.; Ji, C.; Zhang, J.; Liu, T.; Zhao, L. The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality and mycotoxins residues in eggs of layers and the protective effect of Bacillus subtilis biodegradation product. Food Chem. Toxicol. 2016, 90, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Keutchatang, F.D.T.; Tchuenchieu, A.K.; Nguegwouo, E.; Mouafo, H.T.; Ntsama, I.S.B.; Kansci, G.; Medoua, G.N. Occurrence of total aflatoxins, aflatoxin B, and ochratoxin A in chicken and eggs in some Cameroon urban areas and population dietary exposure. J. Environ. Public. Health 2022, 2022, 5541049. [Google Scholar] [CrossRef]
- Micco, C.; Miraglia, M.; Benelli, L.; Onori, R.; Ioppolo, A.; Mantovani, A. Long term administration of low doses of mycotoxins in poultry. 2. Residues of ochratoxin A and aflatoxins in broilers and laying hens after combined administration of ochratoxin A and aflatoxin B1. Food Addit. Contam. 1988, 5, 309–314. [Google Scholar] [CrossRef]
- Neeff, D.V.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Dakovic, A.; Murarolli, R.A.; Oliveira, C.A.F. In vitro and in vivo efficacy of a hydrated sodium calcium aluminosilicate to bind and reduce aflatoxin residues in tissues of broiler chicks fed aflatoxin B1. Poult. Sci. 2013, 92, 131–137. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Kobashigawa, E.; Reis, T.A.; Mestieri, L.; Albuquerque, R.; Corrêa, B. Aflatoxin B1 residues in eggs of laying hens fed a diet containing different levels of the mycotoxin. Food Addit. Contam. 2000, 17, 459–462. [Google Scholar] [CrossRef]
- Pandey, I.; Chauhan, S.S. Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin B1. Br. Poult. Sci. 2007, 48, 713–723. [Google Scholar] [CrossRef]
- Poloni, V.; Magnoli, A.; Fochesato, A.; Cristofolini, A.; Caverzan, M.; Merkis, C.; Montenegro, M.; Cavaglieri, L. A Saccharomyces cerevisiae RC016-based feed additive reduces liver toxicity, residual aflatoxin B1 levels and positively influences intestinal morphology in broiler chickens fed chronic aflatoxin B1-contaminated diets. Anim. Nutr. 2020, 6, 31–38. [Google Scholar] [CrossRef]
- Resanovic, R.; Sinovec, Z. Effects of limited feeding of aflatoxin B1 contaminated feed on the performance of broilers. Mycotoxin Res. 2006, 22, 183–188. [Google Scholar] [CrossRef]
- Rizzi, L.; Simioli, M.; Roncada, P.; Zaghini, A. Aflatoxin B1 and clinoptilolite in feed for laying hens: Effects on egg quality, mycotoxin residues in livers, and hepatic mixed-function oxygenase activities. J. Food Prot. 2003, 66, 860–865. [Google Scholar] [CrossRef]
- Stefanović, D.; Marinković, D.; Trailović, S.; Vasiljević, M.; Farkaš, H.; Raj, J.; Tolimir, N.; Radulović, S.; Nešić, V.; Trailović, J.N.; et al. Evaluation of effectiveness of a novel multicomponent mycotoxins detoxification agent in the presence of AFB1 and T-2 toxin on broiler chicks. Microorganisms 2023, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, W.; Wang, X.H.; Han, M.Y.; Muhammad, I.; Zhang, X.Y.; Sun, X.Q.; Cui, X.X. Water-soluble substances of wheat: A potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019, 98, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Wolzak, A.; Pearson, A.M.; Coleman, T.H.; Pestka, J.J.; Gray, J.I. Aflatoxin deposition and clearance in the eggs of laying hens. Food Chem. Toxicol. 1985, 23, 1057–1061. [Google Scholar] [CrossRef]
- Wolzak, A.; Pearson, A.M.; Coleman, T.H.; Pestka, J.J.; Gray, J.I.; Chen, C. Aflatoxin carryover and clearance from tissues of laying hens. Food Chem. Toxicol. 1986, 24, 37–41. [Google Scholar] [CrossRef]
- Yang, J.; Bai, F.; Zhang, K.; Lv, X.; Bai, S.; Zhao, L.; Peng, X.; Ding, X.; Li, Y.; Zhang, J. Effects of feeding corn naturally contaminated with AFB1 and AFB2 on performance and aflatoxin residues in broilers. Czech J. Anim. Sci. 2012, 57, 506–515. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, L.; Xu, J.; Wang, Y.; Wang, Y. Effects of moldy corn on the performance, antioxidant capacity, immune function, metabolism and residues of mycotoxins in eggs, muscle, and edible viscera of laying hens. Poult. Sci. 2023, 102, 102502. [Google Scholar] [CrossRef]
- Zuo, R.; Chang, J.; Yin, Q.; Wang, P.; Yang, Y.; Wang, X.; Wang, G.; Zheng, Q. Effect of the combined probiotics with aflatoxin B1-degrading enzyme on aflatoxin detoxification, broiler production performance, and hepatic enzyme gene expression. Food Chem. Toxicol. 2013, 59, 470–475. [Google Scholar] [CrossRef]
- Goodwin, L.D.; Leech, N.L. Understanding correlation: Factors that affect the size of r. J. Exp. Educ. 2006, 74, 249–266. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- FAO. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO: Rome, Italy, 2004. [Google Scholar]
- EC Commission. Regulation (EU) No 165/2010 of 26 February 2010 amending regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, 50, 8–12. Available online: https://eur-lex.europa.eu/eli/reg/2010/165/oj/eng (accessed on 12 February 2025).
- FDA Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed; USFDA: Washington, DC, USA, 2000. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-action-levels-poisonous-or-deleterious-substances-human-food-and-animal-feed (accessed on 12 February 2025).
- Dohlman, E. Mycotoxin Hazards and Regulations. In International Trade and Food Safety: Economic Theory and Case Studies; Buzby, J.C., Ed.; USDA Agricultural Report no. 828; United States Department of Agriculture: Davis, California, USA, 2003; pp. 97–108. Available online: https://arefiles.ucdavis.edu/uploads/filer_public/2014/05/19/lcfoodsafettrade03.pdf (accessed on 12 February 2025).
- FDA. Compliance Policy Guides Sec. 683.100 Action Levels for Aflatoxins in Animal Feeds; FDA: Washington, DC, USA, 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-683100-action-levels-aflatoxins-animal-feeds (accessed on 12 February 2025).
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [PubMed]
- Fayokun, C.O.; Adegoke, G.O. Studies on residual aflatoxin B1 in poultry feeds and poultry products. J. Food Sci. Technol. 2000, 37, 311–314. [Google Scholar]
- Hamouda, M.; Fadl, S.E.; Lebda, M.A.; El-Diasty, E.M.; Taha, N.M. Trials for reducing the dangerous effect on poultry fed on aflatoxin contaminated ration using nano-curcumin. BMC Vet. Res. 2025, 21, 4519. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Alharthi, A.S.; Aljumaah, R.S.; Alabdullatif, A.A.; Elolimy, A.A.; Abudabos, A.M. Evaluating the effectiveness of Toxfin and Novasil as dietary aflatoxin-binding agents in broilers for sustaining hepatic antioxidant capacity and intestinal health status during aflatoxin B1 exposure. Mycotox Res. 2025, 41, 145–157. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mahmoud, A.S.; Ahmed, N.A.; El-Desoky, N.I. Investigating the potential of silymarin and/or Spirulina platensis to attenuate the deleterious consequences of aflatoxin contamination in broilers' feeds. J. Anim. Plant Sci. 2024, 34, 899–908. [Google Scholar] [CrossRef]
- Mohammadi, H.; Khosravi, A.; Seidavi, A.; Ghasemi, H.A.; Chamani, M.; Laudadio, V. Beneficial effects of advanced chelate technology-based 7-minerals in aflatoxin-B1 challenged broilers: Toxin residue reduction, serum biochemical improvement and modulation of the mRNA expression of NF-κB and Nrf2, and genes within their pathways. J. Sci. Food Agric. 2024, 104, 1422–1432. [Google Scholar] [CrossRef]
- Amminikutty, A.C.; Veldman, A.; Bederska-Lojewska, D.; Szuba-Trznadel, A. Turmeric powder counteracts oxidative stress and reduces aflatoxin B1 content in the liver of broilers exposed to the EU maximum levels of the mycotoxin. Environ. Sci. Pollut. Res. 2023, 30, 7761–7772. [Google Scholar] [CrossRef]
- Ochieng, P.E.; Croubels, S.; Kemboi, D.; Okoth, S.; De Baere, S.; Cavalier, E.; Kang’ethe, E.; Faas, J.; Doupovec, B.; Gathumbi, J.; et al. Effects of aflatoxins and fumonisins, alone or in combination, on performance, health, and safety of food products of broiler chickens, and mitigation efficacy of bentonite and fumonisin esterase. J. Agric. Food Chem. 2023, 71, 13462–13473. [Google Scholar] [CrossRef]
- Raj, J.; Farkaš, H.; Jakovčević, Z.; Vasiljević, M.; Kumar, R.; Asrani, R.K. Effects of supplemented multicomponent mycotoxin detoxifying agent in laying hens fed aflatoxin B1 and T2-toxin contaminated feeds. Poult. Sci. 2023, 102, 102795. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]




| COA (µg/kg) | DOF (Days) | Country | Type | N | Tissues Evaluated | Diagnostic Test | Ref. |
|---|---|---|---|---|---|---|---|
| 100, 200, 400 | 42 | Pakistan | Broiler | 120 | Liver Muscle | HPLC | [10] |
| 250 | 20 | Saudi Arabia | Broiler | 360 | Liver | HPLC | [11] |
| 300, 600 | 42 | Pakistan | Broiler | 180 | Kidney Liver | ELISA | [12] |
| 110, 117, 129, 345, 361, 385 | 21, 28, 35, 42 | Saudi Arabia | Broiler | 100 | Gizzard Kidney Liver Muscle | HPLC | [13] |
| NR | NR | Iran | NR | 50 | Eggs Liver | HPLC | [6] |
| 250 | 35 | Egypt | Broiler | 150 | Liver Muscle | NR | [14] |
| 3000 | 7 | Japan | Broiler Layer | 64 | Liver Muscle | HPLC | [15] |
| 50, 100 | 42 | Japan | Layer Duck | 270 | Liver Muscle | HPLC | [16] |
| 1300, 2100 | 35 | USA | Broiler | 48 | Gizzard Muscle | NR | [17] |
| 1,000 | 42 | Spain | Broiler | 120 | Liver | HPLC | [18] |
| 2500, 5000 | 32 | Spain | Layer | 80 | Egg Muscle Liver Gizzard Kidney | TLC | [19] |
| 600, 1200, 1800 | 7, 14, 21 | USA | Broiler | 336 | Liver | TLC | [20] |
| 5 | 21 | Spain | Layer | 72 | Kidney Liver Muscle | HPLC | [21] |
| 1600, 3200, 6400 | 7 | Pakistan | Broiler | 2,000 | Liver Muscle | HPLC | [22] |
| 123 | 42 | China | Layer | 336 | Eggs | HPLC | [23] |
| 5.8, 8.2 | NR | Cameroon | Layer | 48 | Eggs Liver Muscle | ELISA | [24] |
| NR | NR | Pakistan | Broiler | 264 | Kidney Liver Muscle | HPLC | [9] |
| 50 | 36, 64, 169 | Italy | Broiler | 163 | Kidney Liver Muscle Skin | HPLC | [25] |
| 2500 | 21 | Columbia | Broiler | 100 | Kidney Liver | HPLC | [26] |
| 100, 300, 500 | 54 | Brazil | Layer | 93 | Eggs Muscle | TLC | [27] |
| NR | NR | United Arab Emirates | NR | NR | Eggs | LS-MS/MS | [7] |
| 2500, 3900 | 280 | India | Layer | 144 | Eggs Muscle | NR | [28] |
| 100 | 22 | Argentina | Broiler | 25 | Liver | HPLC | [29] |
| 45 | 21 | Serbia & Montenegro | Broiler | 1,000 | Liver | TLC | [30] |
| 2500 | 28 | Italy | Layer | 96 | Liver | EIA | [31] |
| NR | NR | Mozambique | Broiler | NR | Gizzard Liver | ELISA | [8] |
| 100 | 42 | Serbia | Broiler | 96 | Liver Muscle | LC-MS/MS | [32] |
| 5000 | 28 | China | Broiler | NR | Muscle | HPLC | [33] |
| 1700, 3300 | 28 | USA | Layer | 16 | Eggs | TLC | [34] |
| 1700, 3300 | 28 | USA | Layer | 80 | Heart Gizzard Kidney Liver Muscle Spleen | TLC | [35] |
| 37, 69, 82, 130 | 21 | China | Broiler | 1,200 | Eggs Liver | HPLC-MS/MS | [36] |
| 0.1, 25, 52, 77 | 60 | China | Broiler | 180 | Eggs Heart Liver Muscle | HPLC-MS/MS | [37] |
| 47, 450 | 30 | China | Broiler | 75 | Liver Muscle | ELISA | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelenga, M.; Matumba, L.; Sitali, M.C.; Kachala, B.; Nambuzi, V.; Mwenifumbo, M.; Gama, A.P.; Mwanza, M.; Monjerezi, M.; Leslie, J.F. Are Aflatoxin Residues in Chicken Products a Real or Perceived Human Dietary Risk? Toxins 2025, 17, 179. https://doi.org/10.3390/toxins17040179
Chelenga M, Matumba L, Sitali MC, Kachala B, Nambuzi V, Mwenifumbo M, Gama AP, Mwanza M, Monjerezi M, Leslie JF. Are Aflatoxin Residues in Chicken Products a Real or Perceived Human Dietary Risk? Toxins. 2025; 17(4):179. https://doi.org/10.3390/toxins17040179
Chicago/Turabian StyleChelenga, Madalitso, Limbikani Matumba, Muloongo C. Sitali, Bertha Kachala, Verson Nambuzi, Merning Mwenifumbo, Aggrey Pemba Gama, Mulunda Mwanza, Maurice Monjerezi, and John F. Leslie. 2025. "Are Aflatoxin Residues in Chicken Products a Real or Perceived Human Dietary Risk?" Toxins 17, no. 4: 179. https://doi.org/10.3390/toxins17040179
APA StyleChelenga, M., Matumba, L., Sitali, M. C., Kachala, B., Nambuzi, V., Mwenifumbo, M., Gama, A. P., Mwanza, M., Monjerezi, M., & Leslie, J. F. (2025). Are Aflatoxin Residues in Chicken Products a Real or Perceived Human Dietary Risk? Toxins, 17(4), 179. https://doi.org/10.3390/toxins17040179






