Protocol and Demographics of the RELY-CD Study: Assessing Long-Term Clinical Response to Botulinum Neurotoxin in Cervical Dystonia
Abstract
1. Introduction
2. Results
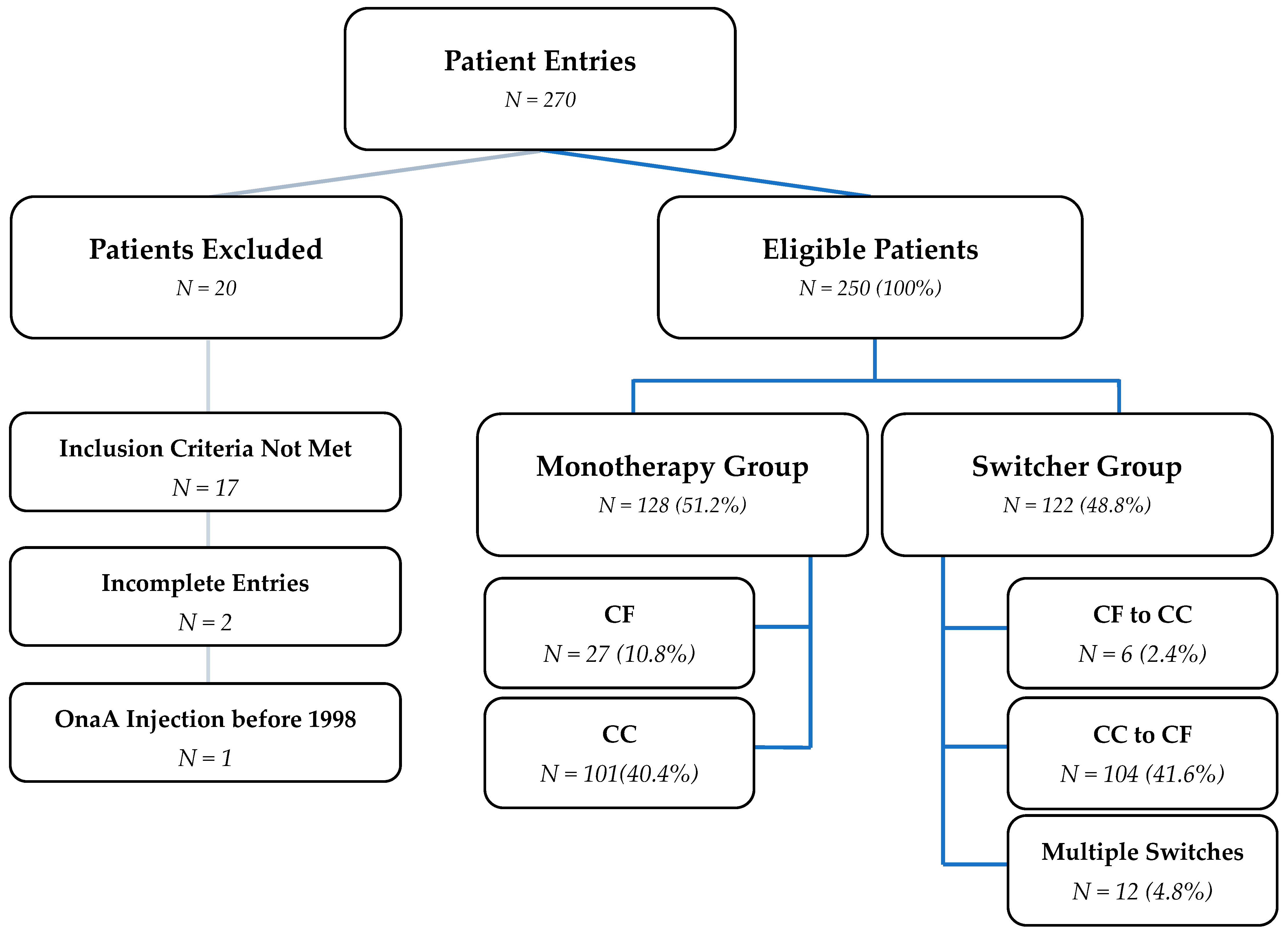
2.1. Data Collection and Treatment Groups
2.2. Formulation-Switching Subgroup: Switchers
2.3. Baseline Demographics
2.4. Symptom Onset and CD Diagnosis
3. Discussion
3.1. DEff and Its Implications
3.2. Demographics and Clinical Characteristics
3.3. Time to Diagnosis and Treatment
3.4. Dose Conversion
3.5. Treatment Response
3.6. Limitations of Statistical Analysis
4. Conclusions
5. Methods
5.1. Study Design
5.2. Patient Population
- Clinical diagnosis of cervical dystonia (according to the definition of dystonia and focal isolated dystonia described in Albanese et al.) [1].
- Adults (m/f/d) 18-64 years of age at start of the BoNT/A treatment.
- Treatment with BoNT/A for at least 7 consecutive years.
- Complete history of BoNT/A formulations.
- Patients treated with only either complex-containing or complex-free BoNT/A formulations.
- Complete documentation of BoNT/A dose per specified muscle. BoNT/A formulation and same efficacy outcome for ≥2 visits in 2nd and 7th treatment years.
- Patient had no known drug addiction or mental illness that was judged to interfere with BoNT/A treatment according to the treating physician.
- Patient was never treated with a botulinum toxin type B formulation.
- Patient did not suffer from additional chronic diseases which may interfere with BoNT/A treatment (e.g., multiple sclerosis or amyotrophic lateral sclerosis).
- Patient did not receive a different BoNT/A formulation for a different indication (in the therapeutic or aesthetic field).
- Patient’s written informed consent if required by local and/or national law.
5.3. Data Collection
5.4. Study Outcomes
5.4.1. Primary Outcome
- (a)
- Disease progression;
- (b)
- Developing drug resistance;
- (c)
- Complete drug resistance;
- (d)
- Physical changes (e.g., weight gain/loss);
- (e)
- Psychological trigger (e.g., stress and depression).
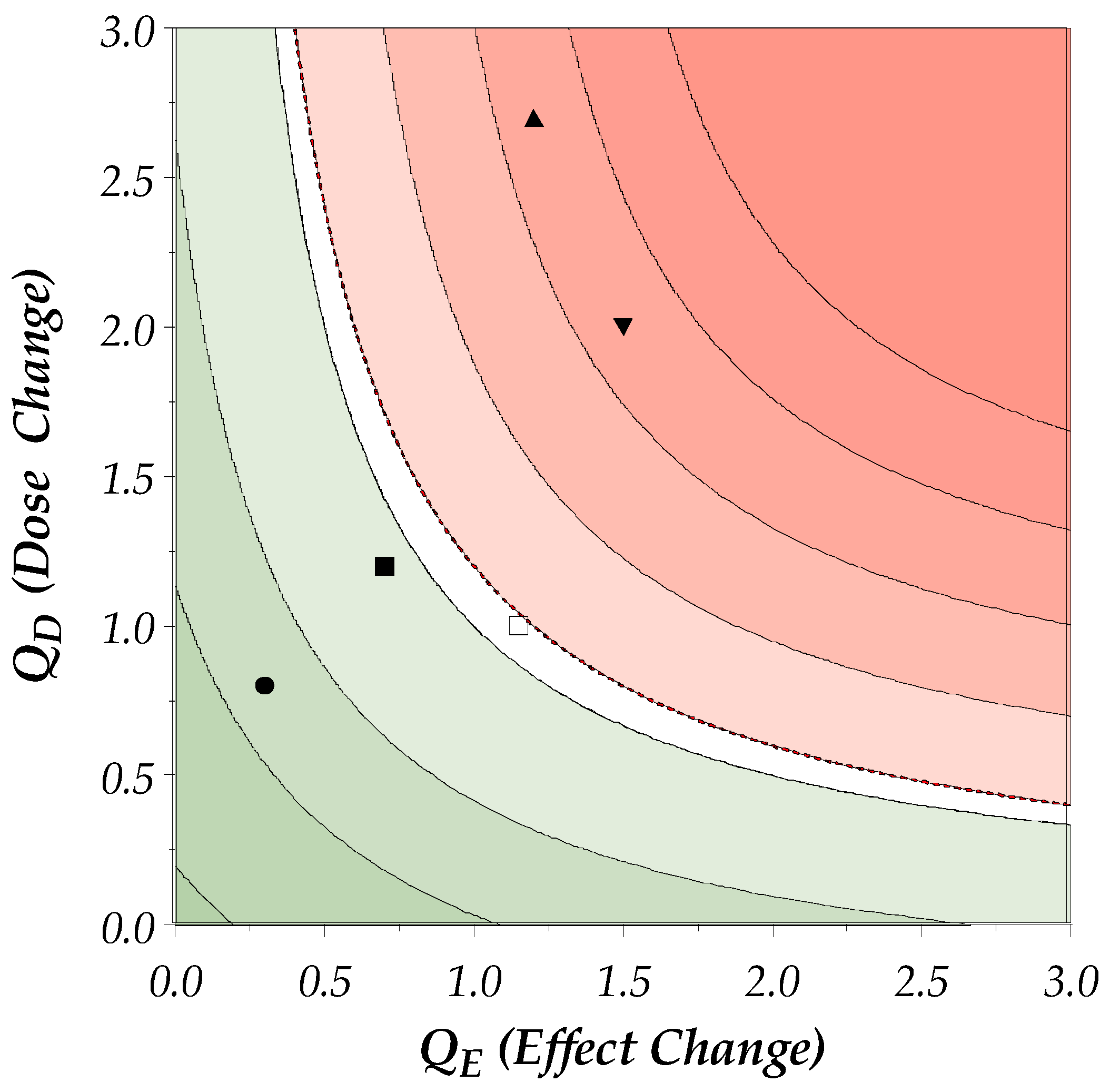
5.4.2. Calculation of the DEff
- 1.
- Multiplicative Relationship:
- Interaction Between Dose and Efficacy: The DEff aims to capture the combined effect of changes in both the dose and efficacy. By multiplying QD and QE, the DEff reflects how changes in the dose and efficacy interact with each other. If either the dose or efficacy changes significantly, the product will highlight this interaction more effectively than a mean would.
- Sensitivity to Changes: Multiplication is more sensitive to changes in either parameter. For example, if the dose increases significantly but the efficacy decreases, the product will show a more pronounced effect, indicating a potential issue with treatment resistance.
- 2.
- Geometric Mean Concept:
- Proportional Changes: The use of the product aligns with the concept of the geometric mean, which is suitable for proportional changes. The geometric mean is often used in situations where values are multiplicative rather than additive.
- Normalization: Multiplying QD and QE normalizes the DEff, making it easier to compare across different patients and treatment regimens. It provides a single, unified measure that captures the overall treatment effect.
- 3.
- Clinical Relevance:
- Thresholds for Clinical Significance: The product of QD and QE allows for the establishment of clinically meaningful thresholds.
- Highlighting Extremes: By using the product, the DEff can highlight extreme cases where either the dose or efficacy changes drastically. This is important for identifying patients who may be developing resistance or experiencing significant changes in treatment response.
5.4.3. Visualization of the DEff
5.4.4. Secondary Endpoints
5.4.5. Other Endpoints and Safety
- Clinician’s Rationale for Observed Changes Potentially Related to Immunogenicity
5.4.6. Quality of Life
5.5. Statistics
- Sample Size
- Analysis Sets
- Full Analysis Set (FAS)
- ○
- All patients enrolled who met the selection criteria.
- Monotherapy Analysis Set = Monotherapy Group
- ○
- CC monotherapy group (including switches between CC products);
- ○
- CF monotherapy group.
- Switcher Analysis Set = Switcher Group
- ○
- CF to CC group;
- ○
- CC to CF group;
- ○
- All switchers, including multiple switches between CC and CF.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MHDA | mouse hemidiaphragm assay |
| MPN | mouse phrenic nerve |
| NRS | Numeric Rating Scale |
| onaA | onabotulinumtoxinA |
| PEGR | Patient Evaluation of Global Response |
| PGIC | Patient’s Global Impression of Change |
| QoL | quality of life |
| RELY-CD | Real-World Evidence of Longevity of BoNT/A in Cervical Dystonia |
| SF-36 | Short-Form 36 |
| TWSTRS | Toronto Western Spasmodic Torticollis Rating Scale |
| US | ultrasound |
| VAS | Visual Analog Scale |
| aboA | abobotulinumtoxinA |
| AEs | adverse events |
| BoNT/A | botulinum neurotoxin type A |
| CD | cervical dystonia |
| CDQ-24 | Craniocervical Dystonia Questionnaire 24 |
| CGIC | Clinician’s Global Impression of Change |
| DEff | dose–effect parameter |
| eCRF | electronic case report form |
| EMA | European Medical Agency |
| EMG | electromyography |
| EQ-5D | EuroQol 5 Dimensions |
| FAS | full analysis set |
| incoA | incobotulinumtoxinA |
| kDa | kilodalton |
| m/f/d | male/female/diverse |
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and Classification of Dystonia: A Consensus Update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, N.; Jankovic, J. Botulinum Toxin in the Treatment of Cervical Dystonia: Evidence-Based Review. Dystonia 2022, 1, 10655. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High Prevalence of Neutralizing Antibodies after Long-Term Botulinum Neurotoxin Therapy. Neurology 2019, 92, E48–E54. [Google Scholar] [CrossRef]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and Risk Factors of Antibody-Induced Secondary Failure of Botulinum Neurotoxin Therapy. Neurology 2020, 94, E2109–E2120. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C.; Janeway, C. Janeway’s Immunobiology, 9th ed.; Garland Science: New York, NY, USA, 2017; ISBN 9780815345053. [Google Scholar]
- Hefter, H.; Rosenthal, D.; Bigalke, H.; Moll, M. Clinical Relevance of Neutralizing Antibodies in Botulinum Toxin Long-Term Treated Still-Responding Patients with Cervical Dystonia. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419892078. [Google Scholar] [CrossRef]
- Benecke, R. Clinical Relevance of Botulinum Toxin Immunogenicity. BioDrugs 2012, 26, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J.; Dressler, D. Clinical Relevance of Immunoresistance to Botulinum Therapy. In Botulinum Toxin Therapy Manual for Dystonia and Spasticity; InTech: London, UK, 2016. [Google Scholar]
- Boehncke, W.H.; Brembilla, N.C. Immunogenicity of Biologic Therapies: Causes and Consequences. Expert. Rev. Clin. Immunol. 2018, 14, 513–523. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research Administration. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products. Available online: https://www.fda.gov/media/85017/download (accessed on 9 February 2025).
- Bellows, S.; Jankovic, J. Immunogenicity Associated with Botulinum Toxin Treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef]
- Rahman, E.; Carruthers, J.D.A. Immunogenicity of Botulinum Toxin A: Insights. Dermatol. Surg. 2024, 50, S117–S126. [Google Scholar] [CrossRef]
- Merz Pharmaceuticals GmbH. Xeomin 100 Units Powder for Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/6202/smpc (accessed on 9 February 2025).
- Ipsen Ltd. Dysport 300 Units Powder for Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/964/smpc (accessed on 9 February 2025).
- AbbVie Ltd. BOTOX 100 Allergan Units Powder for Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/859/smpc (accessed on 9 February 2025).
- Matsumura, T.; Fujinaga, Y. Functional Analysis of Botulinum Hemagglutinin (HA). In Lectin Purification and Analysis. Methods in Molecular Biology; Hirabayashi, J., Ed.; Humana: New York, NY, USA, 2020; Volume 2132, pp. 191–200. ISBN 978-1-0716-0430-4. [Google Scholar]
- Eisele, K.H.; Fink, K.; Vey, M.; Taylor, H.V. Studies on the Dissociation of Botulinum Neurotoxin Type A Complexes. Toxicon 2011, 57, 555–565. [Google Scholar] [CrossRef]
- Kerscher, M.; Wanitphakdeedecha, R.; Ma, M.D.; Trindade De Almeida, A.; Maas, C.; Frevert, J. IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J. Drugs Dermatol. 2019, 18, 52–57. [Google Scholar] [PubMed]
- Frevert, J. Pharmaceutical, Biological, and Clinical Properties of Botulinum Neurotoxin Type A Products. Drugs R&D 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Yang, W.; Lindo, P.; Singh, B.R. Type A Botulinum Neurotoxin Complex Proteins Differentially Modulate Host Response of Neuronal Cells. Toxicon 2014, 82, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.; Chang, T.W.; Cai, S.; Lindo, P.; Riding, S.; Zhou, Y.; Ravichandran, E.; Singh, B.R. Immunological Characterization of the Subunits of Type A Botulinum Neurotoxin and Different Components of Its Associated Proteins. Toxicon 2009, 53, 616–624. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Ürer, B.; Brauns, R.; Rosenthal, D.; Lee, J.I.; Albrecht, P.; Hefter, H. Clinical Implications of Difference in Antigenicity of Different Botulinum Neurotoxin Type A Preparations: Clinical Take-Home Messages from Our Research Pool and Literature. Toxins 2020, 12, 499. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Jansen, A.; Brauns, R.; Ürer, B.; Bigalke, H.; Hartung, H.P.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; et al. Significantly Lower Antigenicity of Incobotulinumtoxin than Abo- or Onabotulinumtoxin. J. Neurol. 2023, 270, 788–796. [Google Scholar] [CrossRef]
- Bigalke, H.; Rummel, A. Botulinum Neurotoxins: Qualitative and Quantitative Analysis Using the Mouse Phrenic Nerve Hemidiaphragm Assay (MPN). Toxins 2015, 7, 4895–4905. [Google Scholar] [CrossRef]
- Waeschle, B.; Vézina, D.; Masso, J.M.; Comes, G.; Stark, H.; Albrecht, P. Samuel Belzberg 6th International Symposium Abstract Book. In Proceedings of the Samuel Belzberg 6th International Dystonia Symposium Abstract Book, Dublin, Ireland, 1–3 June 2023; Dystonia Medical Research Foundation: Chicago, IL, USA, 2023; pp. 36–37. [Google Scholar]
- Waeschle, B.; Stark, H.; Albrecht, P. Characterization of Routine Botulinum Toxin (BoNT) Therapy and Patient Population in Cervical Dystonia. Mov. Disord. 2022, 37, Abstract S264. [Google Scholar]
- Hefter, H.; Spiess, C.; Rosenthal, D. Very Early Reduction in Efficacy of Botulinum Toxin Therapy for Cervical Dystonia in Patients with Subsequent Secondary Treatment Failure: A Retrospective Analysis. J. Neural Transm. 2014, 121, 513–519. [Google Scholar] [CrossRef]
- Castelão, M.; Marques, R.E.; Duarte, G.S.; Rodrigues, F.B.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum Toxin Type A Therapy for Cervical Dystonia. Cochrane Database Syst. Rev. 2017, 2017, CD003633. [Google Scholar] [CrossRef]
- Norris, S.A.; Jinnah, H.A.; Espay, A.J.; Klein, C.; Brüggemann, N.; Barbano, R.L.; Malaty, I.A.C.; Rodriguez, R.L.; Vidailhet, M.; Roze, E.; et al. Clinical and Demographic Characteristics Related to Onset Site and Spread of Cervical Dystonia. Mov. Disord. 2016, 31, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.P.; Cardoso, F.; Comella, C.; Defazio, G.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Kaji, R.; et al. Isolated Cervical Dystonia: Diagnosis and Classification. Mov. Disord. 2023, 38, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Rafee, S.; Al-Hinai, M.; Hutchinson, M. Adult-Onset Idiopathic Cervical Dystonia. Eur. Med. J. 2022, 7, 69–76. [Google Scholar] [CrossRef]
- Bailey, G.A.; Martin, E.; Peall, K.J. Cognitive and Neuropsychiatric Impairment in Dystonia. Curr. Neurol. Neurosci. Rep. 2022, 22, 699–708. [Google Scholar] [CrossRef]
- Benson, M.; Albanese, A.; Bhatia, K.P.; Cavillon, P.; Cuffe, L.; König, K.; Reinhard, C.; Graessner, H. Development of a Patient Journey Map for People Living with Cervical Dystonia. Orphanet J. Rare Dis. 2022, 17, 130. [Google Scholar] [CrossRef]
- Tiderington, E.; Goodman, E.M.; Rosen, A.R.; Hapner, E.R.; Johns, M.M.; Evatt, M.L.; Freeman, A.; Factor, S.; Jinnah, H.A. How Long Does It Take to Diagnose Cervical Dystonia? J. Neurol. Sci. 2013, 335, 72–74. [Google Scholar] [CrossRef]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef]
- Ravenni, R.; De Grandis, D.; Mazza, A. Conversion Ratio between Dysport and Botox in Clinical Practice: An Overview of Available Evidence. Neurol. Sci. 2013, 34, 1043–1048. [Google Scholar] [CrossRef]
- Dashtipour, K.; Mari, Z.; Jankovic, J.; Adler, C.H.; Schwartz, M.; Brin, M.F. Minimal Clinically Important Change in Patients with Cervical Dystonia: Results from the CD PROBE Study. J. Neurol. Sci. 2019, 405, 116413. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Rosenthal, D.; Moll, M. High Botulinum Toxin-Neutralizing Antibody Prevalence Under Long-Term Cervical Dystonia Treatment. Mov. Disord. Clin. Pract. 2016, 3, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Deuschl, G.; Nebe, A.; Feifel, E.; Wissel, J.; Benecke, R.; Kessler, K.R.; Ceballos-Baumann, A.O.; Ohly, A.; Oertel, W.; et al. What Is the Optimal Dose of Botulinum Toxin A in the Treatment of Cervical Dystonia? Results of a Double Blind, Placebo Controlled, Dose Ranging Study Using Dysport®. J. Neurol. Neurosurg. Psychiatry 1998, 64, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Evidente, V.G.H.; Fernandez, H.H.; Ledoux, M.S.; Brashear, A.; Grafe, S.; Hanschmann, A.; Comella, C.L. A Randomized, Double-Blind Study of Repeated IncobotulinumtoxinA (Xeomin®) in Cervical Dystonia. J. Neural Transm. 2013, 120, 1699–1707. [Google Scholar] [CrossRef]
| Total | CF | CC | ||||
|---|---|---|---|---|---|---|
| n | % of Non-missing | n | % of Non-missing | n | % of Non-missing | |
| Total Number of Patients | 128 | 27 | 101 | |||
| Demographic Characteristics | ||||||
| Sex | 128 | 100% | 27 | 100% | 101 | 100% |
| Missing | 0 | 0 | 0 | |||
| Male | 44 | 34.4% | 11 | 40.7% | 33 | 32.8% |
| Female | 84 | 65.6% | 16 | 59.3% | 68 | 67.3% |
| Intersex | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Age at First Injection Visit (Years) | 128 | 100% | 27 | 100% | 101 | 100% |
| Missing | 0 | 0 | 0 | |||
| 18–29 | 5 | 3.9% | 1 | 3.7% | 4 | 4.0% |
| 30–39 | 26 | 20.3% | 5 | 18.5% | 21 | 20.8% |
| 40–49 | 41 | 32.0% | 8 | 29.7% | 33 | 32.7% |
| 50–59 | 32 | 25.0% | 8 | 29.6% | 24 | 23.8% |
| 60–64 | 24 | 18.8% | 5 | 18.5% | 19 | 18.8% |
| Clinical Characteristics | ||||||
| Etiology of CD | 114 | 100% | 27 | 100% | 87 | 100% |
| Missing | 14 | 0 | 143 | |||
| Idiopathic | 110 | 96.5% | 26 | 96.3% | 84 | 96.6% |
| Inheritance | 2 | 1.8% | 1 | 3.7% | 1 | 1.1% |
| Acquired (e.g., Brain Injury) | 2 | 1.8% | 0 | 0.0% | 2 | 2.3% |
| Concomitant Diseases | 111 | 100% | 26 | 100% | 85 | 100% |
| Missing | 17 | 1 | 16 | |||
| No | 83 | 74.8% | 20 | 76.9% | 63 | 74.1% |
| Yes | 28 | 25.2% | 6 | 23.1% | 22 | 25.9% |
| Endocrine Disorders | 4 | 3.6% | 2 | 7.7% | 2 | 2.4% |
| Investigations | 1 | 0.9% | 1 | 3.8% | 0 | 0.0% |
| Metabolism and Nutritional Disorders | 3 | 2.7% | 0 | 0.0% | 3 | 3.5% |
| Musculoskeletal and Connective Tissue Disorders | 6 | 5.4% | 1 | 3.8% | 5 | 5.9% |
| Nervous System Disorders | 1 | 0.9% | 0 | 0.0% | 1 | 1.2% |
| Psychiatric Disorders | 13 | 11.7% | 1 | 3.8% | 12 | 14.1% |
| Vascular Disorders | 8 | 7.2% | 1 | 3.8% | 7 | 8.2% |
| Other Relevant Diseases | 2 | 1.8% | 2 | 7.7% | 0 | 0.0% |
| Concomitant Medication | 111 | 100% | 26 | 100% | 85 | 100% |
| Missing | 17 | 1 | 16 | |||
| No | 80 | 72.1% | 22 | 84.6% | 58 | 68.2% |
| Yes | 31 | 27.9% | 4 | 15.4% | 27 | 31.8% |
| Non-Steroidal Anti-Inflammatory Drugs | 5 | 4.5% | 0 | 0.0% | 5 | 5.9% |
| Antidepressant Medication of any Route | 11 | 9.9% | 2 | 7.7% | 9 | 10.6% |
| Opioid Analgesics | 1 | 0.9% | 0 | 0.0% | 1 | 1.2% |
| Medication used for Treatment of Focal Dystonia | 14 | 12.6% | 1 | 3.8% | 13 | 15.3% |
| Other Oral Medication Potentially Interfering with BoNT/A Treatment | 8 | 7.2% | 1 | 3.8% | 7 | 8.2% |
| Medication and Treatment Known to Interfere with BoNT/A Treatment | 6 | 5.4% | 0 | 0.0% | 6 | 7.1% |
| Total | CF to CC | CC to CF | ||||
|---|---|---|---|---|---|---|
| n | % of Non-Missing | n | % of Non-Missing | n | % of Non-Missing | |
| Total Number of Patients | 122 | 6 | 104 | |||
| Demographic Characteristics | ||||||
| Sex | 122 | 100% | 6 | 100% | 104 | 100% |
| Missing | 0 | 0 | 0 | |||
| Male | 37 | 30.3% | 2 | 33.3% | 31 | 29.8% |
| Female | 85 | 69.7% | 4 | 66.7% | 73 | 70.2% |
| Intersex | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Age at First Injection Visit (Years) | 122 | 100% | 6 | 100% | 104 | 100% |
| Missing | 0 | 0 | 0 | |||
| 18–29 | 13 | 10.7% | 0 | 0.0% | 13 | 12.5% |
| 30–39 | 17 | 13.9% | 1 | 16.7% | 14 | 13.5% |
| 40–49 | 29 | 23.8% | 2 | 33.3% | 21 | 20.2% |
| 50–59 | 40 | 32.8% | 3 | 50.0% | 36 | 34.6% |
| 60–64 | 23 | 18.9% | 0 | 0.0% | 20 | 19.2% |
| Clinical Characteristics | ||||||
| Etiology of CD | 116 | 100% | 6 | 100% | 98 | 100% |
| Missing | 6 | 0 | 6 | |||
| Idiopathic | 115 | 99.1% | 6 | 100% | 97 | 99.0% |
| Inheritance | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Acquired (e.g., Brain Injury) | 1 | 0.9% | 0 | 0.0% | 1 | 1.0% |
| Concomitant Diseases | 118 | 100% | 6 | 100% | 100 | 100% |
| Missing | 4 | 0 | 4 | |||
| No | 103 | 87.3% | 5 | 83.3% | 88 | 88.0% |
| Yes | 15 | 12.7% | 1 | 16.7% | 12 | 12.0% |
| Cardiac Disorders | 1 | 0.8% | 0 | 0.0% | 1 | 1.0% |
| Gastrointestinal disorders | 1 | 0.8% | 0 | 0.0% | 1 | 1.0% |
| Metabolism and Nutritional Disorders | 3 | 2.5% | 0 | 0.0% | 2 | 2.0% |
| Musculoskeletal and Connective Tissue Disorders | 1 | 0.8% | 0 | 0.0% | 1 | 1.0% |
| Psychiatric Disorders | 7 | 5.9% | 1 | 16.7% | 5 | 5.0% |
| Vascular Disorders | 7 | 5.9% | 0 | 0.0% | 5 | 5.0% |
| Concomitant Medication | 117 | 100% | 6 | 100% | 99 | 100% |
| Missing | 5 | 0 | 5 | |||
| No | 101 | 86.3% | 5 | 83.3% | 86 | 86.9% |
| Yes | 16 | 13.7% | 1 | 16.7% | 13 | 13.1% |
| Non-Steroidal Anti-Inflammatory Drugs | 2 | 1.7% | 0 | 0.0% | 2 | 2.0% |
| Antidepressant Medication of any Route | 8 | 6.8% | 1 | 16.7% | 6 | 6.1% |
| Opioid Analgesics | 1 | 0.9% | 0 | 0.0% | 1 | 1.0% |
| Medication used for Treatment of Focal Dystonia | 5 | 4.3% | 0 | 0.0% | 4 | 4.0% |
| Other Oral Medication Potentially Interfering with BoNT/A Treatment | 5 | 4.3% | 0 | 0.0% | 5 | 5.1% |
| Medication and Treatment Known to Interfere with BoNT/A Treatment | 1 | 0.9% | 0 | 0.0% | 1 | 1.0% |
| Time Since CD Symptoms Onset (Years) | Total (n = 127) | CF (n = 27) | CC (n = 100) |
| Mean (SD) | 4.2 (5.1) | 2.8 (3.7) | 4.5 (5.3) |
| Median (IQR) | 2.0 (1; 6) | 1.0 (1; 4) | 3.0 (1; 7) |
| Min, Max | 0, 33 | 0, 17 | 0, 33 |
| Time Since CD Diagnosis (Years) | Total (n = 128) | CF (n = 27) | CC (n = 101) |
| Mean (SD) | 1.3 (2.4) | 0.5 (1.1) | 1.4 (2.7) |
| Median (IQR) | 0.3 (0; 1.3) | 0.2 (0; 0.4) | 0.3 (0; 1.5) |
| Min, Max | 0, 14.2 | 0, 4.2 | 0, 14.2 |
| Time Since CD Symptoms Onset (Years) | Total (n = 122) | CF to CC (n = 6) | CC to CF (n = 104) |
| Mean (SD) | 4.5 (5.0) | 2.7 (2.3) | 4.6 (5.3) |
| Median (IQR) | 2.0 (1; 7) | 2.0 (2; 3) | 2.0 (1; 7) |
| Min, Max | 0, 23 | 0, 7 | 0, 23 |
| Time Since CD Diagnosis (Years) | Total (n = 122) | CF to CC (n = 6) | CC to CF (n = 104) |
| Mean (SD) | 1.4 (2.9) | 0.3 (0.4) | 1.4 (3.0) |
| Median (IQR) | 0.3 (0.1; 1.2) | 0.1 (0; 0.3) | 0.3 (0.1; 1.2) |
| Min, Max | −0.1, 17.3 | 0, 1.0 | −0.1, 17.3 |
| Parameter | Description |
|---|---|
| Demographics |
|
| Efficacy Outcomes (in order of priority for DEff calculation) |
|
| Quality of Life |
|
| Clinical Parameters |
|
| Scale | Threshold for Clinical Meaningfulness |
|---|---|
| 7-point GICS (CGIC, PGIC) 7-point VAS 7-point Likert 7-point NRS | ≥5 on GICS (+1, at least “minimally improved”) or 1-point improvement on VAS/Likert/NRS [39]. |
| 8-point PEGR 10-point PEGR 8–10-point VAS 8–10-point Likert 8–10-point NRS | ≥+2 compared to baseline [42]. |
| Clinical Change | Cause/Rationale |
|---|---|
| Dose Increase Effect Duration Decrease Decrease in Efficacy Lack of Efficacy Patient-Reported Dissatisfaction Other (Free text) | Disease Progression Developing Drug Resistance Complete Drug Resistance Physical Changes (e.g., weight gain/loss) Psychological Trigger (e.g., stress. depression) Other (Free text) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waeschle, B.; Lee, J.-I.; Kölsche, T.; Jansen, R.; Banach, M.; Ochudlo, S.; Tyślerowicz, M.; Sobolewski, P.; Sánchez Valiente, S.; López-Valdés, E.; et al. Protocol and Demographics of the RELY-CD Study: Assessing Long-Term Clinical Response to Botulinum Neurotoxin in Cervical Dystonia. Toxins 2025, 17, 180. https://doi.org/10.3390/toxins17040180
Waeschle B, Lee J-I, Kölsche T, Jansen R, Banach M, Ochudlo S, Tyślerowicz M, Sobolewski P, Sánchez Valiente S, López-Valdés E, et al. Protocol and Demographics of the RELY-CD Study: Assessing Long-Term Clinical Response to Botulinum Neurotoxin in Cervical Dystonia. Toxins. 2025; 17(4):180. https://doi.org/10.3390/toxins17040180
Chicago/Turabian StyleWaeschle, Benjamin, John-Ih Lee, Tristan Kölsche, Robin Jansen, Marta Banach, Stanislaw Ochudlo, Małgorzata Tyślerowicz, Piotr Sobolewski, Sara Sánchez Valiente, Eva López-Valdés, and et al. 2025. "Protocol and Demographics of the RELY-CD Study: Assessing Long-Term Clinical Response to Botulinum Neurotoxin in Cervical Dystonia" Toxins 17, no. 4: 180. https://doi.org/10.3390/toxins17040180
APA StyleWaeschle, B., Lee, J.-I., Kölsche, T., Jansen, R., Banach, M., Ochudlo, S., Tyślerowicz, M., Sobolewski, P., Sánchez Valiente, S., López-Valdés, E., Mir, P., Jesús, S., Ojeda-Lepe, E., Papuć, E., Sánchez Alonso, P., Salazar, G., Comes, G., Stark, H., & Albrecht, P. (2025). Protocol and Demographics of the RELY-CD Study: Assessing Long-Term Clinical Response to Botulinum Neurotoxin in Cervical Dystonia. Toxins, 17(4), 180. https://doi.org/10.3390/toxins17040180





