Protective Effect of Ethoxyquin and N-acetylcysteine on Biochemical and Pathological Changes Induced by Chronic Exposure to Aflatoxins in Laying Hens
Abstract
1. Introduction
2. Results
2.1. Animal Performance
2.2. Biochemical Analysis
2.2.1. Reduced Glutathione
2.2.2. Glutathione S-Transferases
2.2.3. Gamma Glutamyltransferase
2.2.4. Alanine Aminotransferase and Aspartate Aminotransferase
2.2.5. Total Proteins
2.3. Morphological Changes
2.3.1. Relative Weight of Liver and Kidneys
2.3.2. Histopathology
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Birds and Housing
5.2. Experimental Design
5.3. Production and Quantification of Total Aflatoxins
5.4. Collection of Biological Samples
5.5. Biochemical and Histopathological Analysis
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fawaz, M.A.; Hassan, H.A.; Abdel Wareth, A.A.A. Aflatoxins in poultry feed: Present status and future concerns. SVU-IJAS 2022, 4, 113–124. [Google Scholar] [CrossRef]
- Ruan, H.; Lu, Q.; Wu, J.; Qin, J.; Sui, M.; Sun, X.; Shi, Y.; Luo, J.; Yang, M. Hepatotoxicity of food-borne mycotoxins: Molecular mechanism, anti-hepatotoxic medicines and target prediction. Crit. Rev. Food. Sci. Nutr. 2022, 62, 2281–2308. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Ferronato, G.; Alqhtani, A.H.; Al-Garadi, M.A.; Al-sornokh, H.; Abudabos, A.M. The efficacy of bentonite and zeolite in reducing aflatoxin B1 toxicity on production performance and intestinal and hepatic health of broiler chickens. Ital. J. Anim. Sci. 2022, 21, 1181–1189. [Google Scholar] [CrossRef]
- Lin, L.; Fu, P.; Chen, N.; Gao, N.; Cao, Q.; Yue, K.; Xu, T.; Zhang, C.; Zhang, C.; Liu, F.; et al. Total flavonoids of Rhizoma Drynariae protect hepatocytes against aflatoxin B1-induced oxidative stress and apoptosis in broiler chickens. Ecotoxicol. Environ. Saf. 2022, 230, 113148. [Google Scholar] [CrossRef]
- Ochieng, P.E.; Kemboi, D.C.; Okoth, S.; De Baere, S.; Cavalier, E.; Kang’ethe, E.; Doupovec, B.; Gathumbi, J.; Scippo, M.L.; Antonissen, G.; et al. Aflatoxins and fumonisins co-contamination effects on laying hens and use of mycotoxin detoxifiers as a mitigation strategy. Mycotoxin Res. 2025, 41, 63–75. [Google Scholar] [CrossRef]
- Kövesi, B.; Cserháti, M.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Lack of dose- and time-dependent effects of aflatoxin B1 on gene expression and enzymes associated with lipid peroxidation and the glutathione redox system in chicken. Toxins 2020, 12, 84. [Google Scholar] [CrossRef]
- Bozzo, G.; Pugliese, N.; Samarelli, R.; Schiavone, A.; Dimuccio, M.M.; Circella, E.; Bonerba, E.; Ceci, E.; Camarda, A. Ochratoxin A and aflatoxin B1 detection in laying hens for omega 3-enriched eggs production. Agriculture 2023, 13, 138. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAO. Available online: https://www.fao.org/poultry-production-products/socio-economic-aspects/economic-aspects/es/ (accessed on 20 June 2025).
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Falowo, A.B.; Oloruntola, O.D.; Atiba, O.I.; Ayodele, O.A.; Olarotimi, O.J.; Gbore, F.A. Growth performance, carcass quality, immune response, and production economics of broiler chickens fed avocado seed meal under feed restriction. Transl. Anim. Sci. 2025, 9, txaf047. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef]
- Valdivia, A.G.; Martinez, A.; Damian, F.J.; Quezada, T.; Ortiz, R.; Martinez, C.; Llamas, J.; Rodriguez, M.L.; Yamamoto, L.; Jaramillo, F.; et al. Efficacy of N-acetylcysteine to reduce the effects of aflatoxin B1 intoxication in broiler chickens. Poult. Sci. 2001, 80, 727–734. [Google Scholar] [CrossRef]
- Çam, Y.; Eraslan, G.; Atasever, A.; Eren, M.; Liman, B.C.; Şeybek, N. Efficacy of N-acetylcysteine on aflatoxicosis in rabbits. Pol. J. Environ. Stud. 2008, 17, 189–197. [Google Scholar]
- Błaszczyk, A.; Augustyniak, A.; Skolimowski, J. Ethoxyquin: An antioxidant used in animal feed. Int. J. Food Sci. 2013, 2013, 585931. [Google Scholar] [CrossRef] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives, Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Scientific Opinion on the safety and efficacy of a feed additive consisting of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species (FEFANA asbl). EFSA J. 2022, 20, 7166. [Google Scholar] [CrossRef]
- Olariu, R.M.; Fiţ, N.I.; Bouari, C.M.; Nadăş, G.C. Mycotoxins in broiler production: Impacts on growth, immunity, vaccine efficacy, and food safety. Toxins 2025, 17, 261. [Google Scholar] [CrossRef]
- Martínez-de-Anda, A.; Valdivia, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz, R.; Quezada, T.; de Luna, M.C.; Rodríguez, M.L. Effects of aflatoxin chronic intoxication in renal function of laying hens. Poult. Sci. 2010, 89, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Song, B.; Song, Z. Hidden hazards revealed: Mycotoxins and their masked forms in poultry. Toxins 2024, 16, 137. [Google Scholar] [CrossRef]
- Pinton, P.; Terciolo, C.; Payros, D.; Oswald, I.P. Mycotoxins hazard: The European view. Curr. Opin. Food Sci. 2025, 63, 101306. [Google Scholar] [CrossRef]
- Ediage, E.N.; Van Poucke, C.; De Saeger, S. A multi-analyte LC–MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 177, 397–404. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliott, C.T.; Campbell, K. Current trends in rapid tests for mycotoxins. Food Addit. Contam. Part A 2019, 36, 800–814. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The existing methods and novel approaches in mycotoxins’ detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef] [PubMed]
- Zabiulla, I.; Malathi, V.; Swamy, H.V.L.N.; Naik, J.; Pineda, L.; Han, Y. The efficacy of a smectite-based mycotoxin binder in reducing aflatoxin B1 toxicity on performance, health and histopathology of broiler chickens. Toxins 2021, 13, 856. [Google Scholar] [CrossRef]
- Alternative Medicine Review (AMR). AMR Monographs, Volume 1. N-Acetylcysteine; Thorne Research, Inc.: Dover, ID, USA, 2003. [Google Scholar]
- Arfsten, D.P.; Johnson, E.W.; Thitoff, A.R.; Jung, A.E.; Wilfong, E.R.; Lohrke, S.M.; Bausman, T.A.; Eggers, J.S.; Bobb, A.J. Impact of 30-day oral dosing with N-acetyl-l-cysteine on sprague-dawley rat physiology. Int. J. Toxicol. 2004, 23, 239–247. [Google Scholar] [CrossRef]
- Ozaras, R.; Tahan, V.; Aydin, S.; Uzun, H.; Kaya, S.; Senturk, H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J. Gastroenterol. 2003, 9, 125–128. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of aflatoxins induces severe hepatotoxicity through multiple mechanisms. Front. Pharmacol. 2021, 11, 605823. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.H.; Abushelaibi, A.; Lim, S.E.; Loh, J.Y.; et al. Aflatoxin contamination: An overview on health issues, detection and management strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in glutathione content in liver diseases: An update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Ikeda, Y.; Fujii, J. The emerging roles of γ-glutamyl peptides produced by γ-glutamyltransferase and the glutathione synthesis system. Cells 2023, 12, 2831. [Google Scholar] [CrossRef]
- Sun, L.H.; Zhang, N.Y.; Zhu, M.K.; Zhao, L.; Zhou, J.C.; Qi, D.S. Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome p450 isozymes and up-regulation of 6 selenoprotein genes in chick liver. J. Nutr. 2016, 146, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Petkova, T.; Milanova, A. Absorption of N-acetylcysteine in healthy and Mycoplasma gallisepticum-infected chickens. Vet. Sci. 2021, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.Y.; Kryl’skii, E.D.; Brazhnikova, D.A.; Popova, T.N.; Shikhaliev, K.S.; Shulgin, K.K.; Matasova, L.V.; Popov, S.S.; Zhaglin, D.A.; Zakharova, A.A.; et al. Novel antioxidant, deethylated ethoxyquin, protects against carbon tetrachloride induced hepatotoxicity in rats by inhibiting NLRP3 inflammasome activation and apoptosis. Antioxidants 2021, 10, 122. [Google Scholar] [CrossRef]
- Wang, S.; Bottje, W.; Maynard, P.; Dibner, J.; Shermer, W. Effect of Santoquin and oxidized fat on liver and intestinal glutathione in broilers. Poult. Sci. 1997, 76, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gao, X.; Yuan, J. Substituting ethoxyquin with tea polyphenols and propyl gallate enhanced feed oxidative stability, broiler hepatic antioxidant capacity and gut health. Poult. Sci. 2024, 103, 104368. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res.–Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Judah, D.J.; McLellan, L.I.; Kerr, L.A.; Peacock, S.D.; Neal, G.E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J. 1991, 279, 385–398. [Google Scholar] [CrossRef]
- Bammler, T.K.; Slone, D.H.; Eaton, D.L. Effects of dietary oltipraz and ethoxyquin on aflatoxin B1 biotransformation in non-human primates. Toxicol. Sci. 2000, 54, 30–41. [Google Scholar] [CrossRef]
- Henson, K.L.; Stauffer, G.; Gallagher, E.P. Induction of glutathione S-transferase activity and protein expression in brown bullhead (Ameiurus nebulosus) liver by ethoxyquin. Toxicol. Sci. 2001, 62, 54–60. [Google Scholar] [CrossRef][Green Version]
- Elbasuni, S.S.; Ibrahim, S.S.; Elsabagh, R.; Nada, M.O.; Elshemy, M.A.; Ismail, A.K.; Mansour, H.M.; Ghamry, H.I.; Ibrahim, S.F.; Alsaati, I.; et al. The preferential therapeutic potential of Chlorella vulgaris against aflatoxin-induced hepatic injury in quail. Toxins 2022, 14, 843. [Google Scholar] [CrossRef]
- Moloi, T.P.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mabaso, N.H.; Ndlovu, Z. Aflatoxin B1-induced hepatotoxicity through mitochondrial dysfunction, oxidative stress, and inflammation as central pathological mechanisms: A review of experimental evidence. Toxicology 2024, 509, 153983. [Google Scholar] [CrossRef] [PubMed]
- de Luna-López, M.C.; Valdivia-Flores, A.G.; Rangel-Muñoz, E.J.; Hernández-Valdivia, E.; Quezada-Tristán, T.; Jaramillo-Juárez, F.; Ortiz-Martínez, R. Time-dependent changes in performance, biochemistry, and histology in dairy calves with acute aflatoxicosis. Vet. Sci. 2025, 12, 273. [Google Scholar] [CrossRef]
- Bajaj, P.; Chowdhury, S.K.; Yucha, R.; Kelly, E.J.; Xiao, G. Emerging Kidney models to investigate metabolism, transport, and toxicity of drugs and xenobiotics. Drug Metab. Dispos. 2018, 46, 1692–1702. [Google Scholar] [CrossRef]
- Klatt, O.C.; de Brouwer, L.; Hendriks, F.; Dehne, E.M.; Ataç Wagegg, B.; Jennings, P.; Wilmes, A. Human and rat renal proximal tubule in vitro models for ADME applications. Arch. Toxicol. 2025, 99, 1613–1641. [Google Scholar] [CrossRef]
- Quezada, T.T.; Jaramillo, J.F.; Valdivia, F.A.; Reyes, S.J.L.; Ortiz, M.R.; Rodríguez, V.M.L. Comparative study of the concentration of reduced glutathione and the activity of gamma-glutamyl-transferase and glutathione transferase in liver and kidney of broilers and rats. Vet. Mex. 2002, 33, 125–135. [Google Scholar]
- Wang, X.H.; Li, W.; Wang, X.H.; Han, M.Y.; Muhammad, I.; Zhang, X.Y.; Sun, X.Q.; Cui, X.X. Water-soluble substances of wheat: A potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019, 98, 136–149. [Google Scholar] [CrossRef]
- Fernandez, A.; Verde, M.T.; Gascon, M.; Ramos, J.; Gomez, J.; Luco, D.F.; Chavez, G. Variations of clinical biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed. Avian Pathol. 1994, 23, 37–47. [Google Scholar] [CrossRef]
- Raju, M.V.; Rama Rao, S.V.; Radhika, K.; Panda, A.K. Effect of amount and source of supplemental dietary vegetable oil on broiler chickens exposed to aflatoxicosis. Br. Poult. Sci. 2005, 46, 587–594. [Google Scholar] [CrossRef]
- Jansen van Rensburg, C.; Van Rensburg, C.E.J.; Van Ryssen, J.B.J.; Casey, N.H.; Rottinghaus, G.E. In vitro and in vivo assessment of humic acid as an aflatoxin binder in broiler chickens. Poult. Sci. 2006, 85, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M.; Ball, H.W.; Barrett, M.C.; Clark, H.L.; Judah, D.J.; Williamson, G.; Neal, G.E. Mechanism of action of dietary chemoprotective agents in rat liver: Induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis 1997, 18, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- De Feo, P.; Lucidi, P. Liver protein synthesis in physiology and in disease states. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Milki, S.; Abdeta, D. Public health impact of aflatoxin. J. Bacteriol. Mycol. Open Access 2023, 11, 34–39. [Google Scholar] [CrossRef]
- Riahi, I.; Pérez-Vendrell, A.M.; Ramos, A.J.; Brufau, J.; Esteve-Garcia, E.; Schulthess, J.; Marquis, V. Biomarkers of deoxyni-valenol toxicity in chickens with special emphasis on metabolic and welfare parameters. Toxins 2021, 13, 217. [Google Scholar] [CrossRef]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-acetylcysteine and its immunomodulatory properties in humans and domesticated animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Daubry, T.M.E.; Adienbo, O.M.; Ovili-Odili, B.Z.; Chinko, B.C. N-acetyl cysteine and zinc sulfate attenuates acute crude oil-induced oxidative stress and testicular structural damage in male wistar rats. Asian J. Biol. 2024, 20, 98–108. [Google Scholar] [CrossRef]
- Elokil, A.; Li, S.; Chen, W.; Farid, O.; Abouelezz, K.; Zohair, K.; Nassar, F.; El-Komy, E.; Farag, S.; Elattrouny, M. Ethoxyquin attenuates enteric oxidative stress and inflammation by promoting cytokine expressions and symbiotic microbiota in heat-stressed broilers. Poult. Sci. 2024, 103, 103761. [Google Scholar] [CrossRef] [PubMed]
- Makinia, M. Study of performance broiler chickens fed of contaminated wheat by aflatoxin and ammoniac. Int. J. Ad. Biol. Biomed. Res. 2014, 2, 2001–2007. [Google Scholar]
- Subramaniam, S.; Sabran, M.R.; Stanslas, J.; Kirby, B.P. Effect of aflatoxin B1 exposure on the progression of depressive-like behavior in rats. Front. Nutr. 2022, 9, 1032810. [Google Scholar] [CrossRef] [PubMed]
- Ortatatli, M.; Oğuz, H.; Hatίpoğlu, F.; Karaman, M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005, 78, 61–68. [Google Scholar] [CrossRef]
- Triana-García, P.A.; Gutierrez-Espinosa, M.C.; Eslava-Mocha, P.R. Rendimiento productivo e hígado graso en tilapia híbrida (Oreochromis spp): Influencia de dos fuentes de lípidos. Orinoquia 2013, 17, 183–196. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic steatohepatitis clinical research network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Ashraf, K.; Gul, S.T.; Naseem, M.N.; Sajid, M.S.; Mohsin, M.; He, C.; Zubair, M.; Khan, A. Toxicopathological effects of feeding aflatoxins B1 in broilers and its amelioration with indigenous mycotoxin binder. Ecotoxicol. Environ. Saf. 2020, 187, 109712. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Food Additives Permitted in Feed and Drinking Water of Animals. In The Code of Federal Regulations; 9/04/2025, Title 21, Chapter I, Subchapter E, Part 573; pp. 587–588. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-E/part-573 (accessed on 14 January 2022).
- Hy-Line. Available online: https://www.hyline.com/spanish/literatura/w-36 (accessed on 14 January 2022).
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994; Available online: https://scholar.google.com/scholar_lookup?title=Nutrient%20Requirements%20of%20Poultry&publication_year=1994& (accessed on 14 January 2022).
- de Luna-López, M.C.; Valdivia-Flores, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz-Martínez, R.; Quezada-Tristán, T. Association between Aspergillus flavus colonization and aflatoxins production in immature grains of maize genotypes. J. Food Sci. Eng. 2013, 12, 688–698. [Google Scholar] [CrossRef]
- Trucksess, M.W. Natural toxins. In Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2, pp. 1–64. [Google Scholar]
- Gella, F.J.; Olivella, T.; Cruz, M.; Arenas, J.; Moreno, R.; Durban, R.; Gómez, J.A. A simple procedure for routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin. Chim. Acta 1985, 153, 241–247. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Hørder, M.; Rej, R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2). J. Clin. Chem. Clin. Biochem. 1986, 24, 481–495. [Google Scholar] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology (Armed Forces Institute of Pathology); The American Registry of Pathology: Washington, DC, USA, 1994. [Google Scholar]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.J.A.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef]

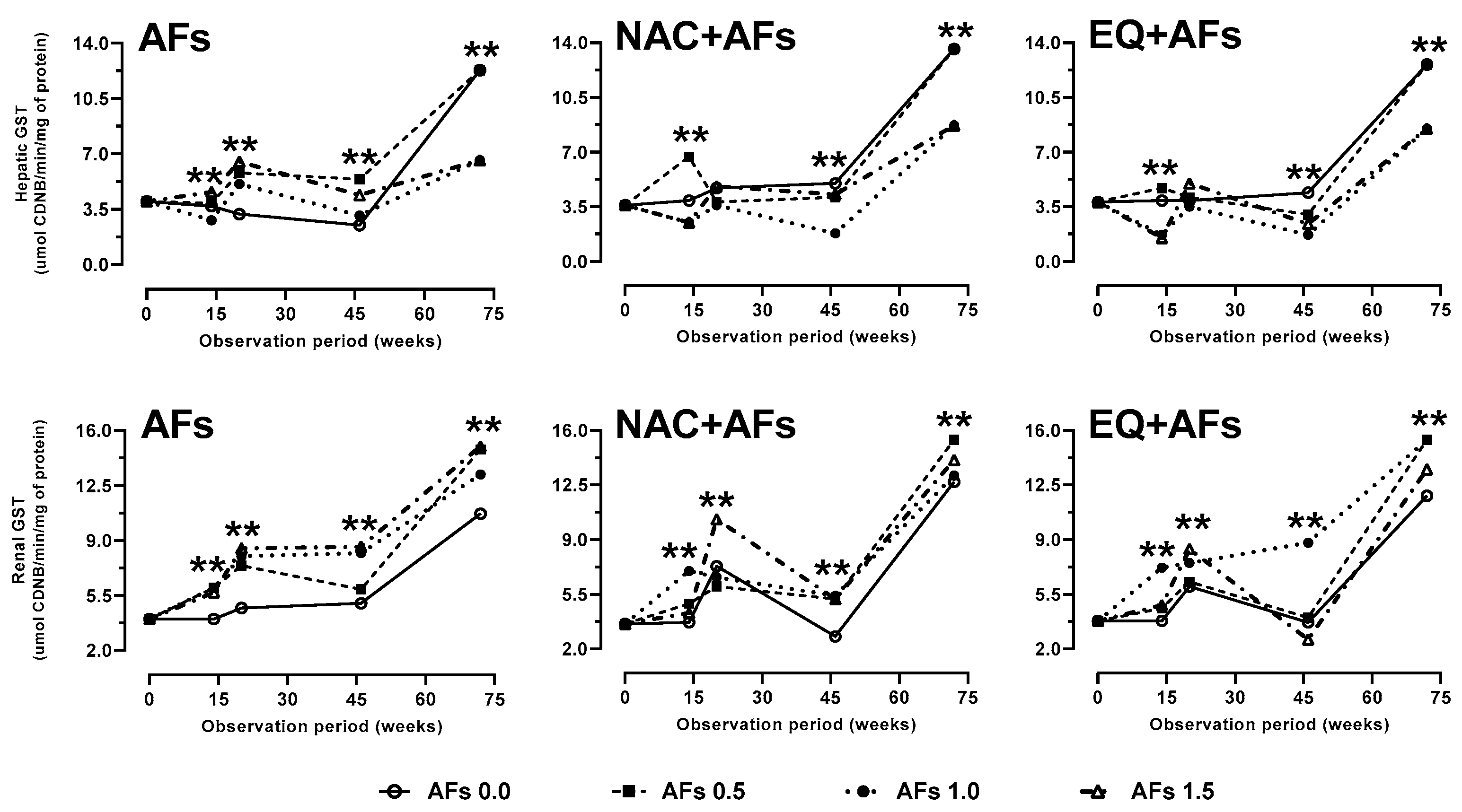



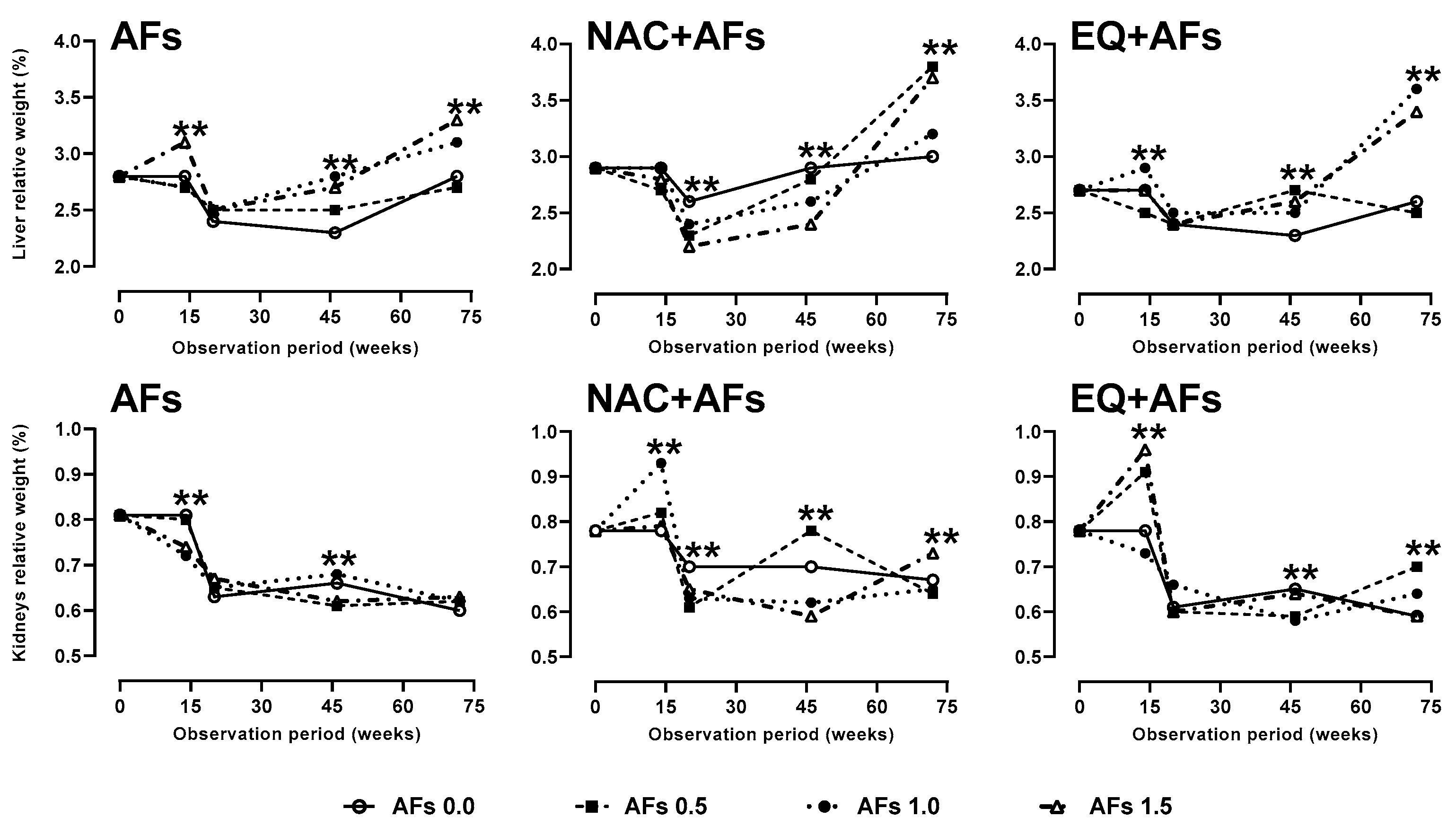


| Performance | Time (wk) | Control * | Aflatoxins * | N-acetylcysteine * | Ethoxyquin * |
|---|---|---|---|---|---|
| Feed Intake (g/d) | 14–20 | 81.2 ± 2.1 a | 80.0 ± 1.30 b | 80.0 ± 0.85 b | 80.8 ± 1.87 ab |
| 21–46 | 112 ± 1.61 a | 98.4 ± 1.35 b | 112 ± 2.1 a | 111 ± 2.2 a | |
| 47–72 | 104 ± 3.7 a | 96.2 ± 1.95 b | 104 ± 4.3 a | 103 ± 1.72 a | |
| Hen-day egg production (%) ** | 14–20 | 78.6 ± 3.7 a | 65.3 ± 2.8 b | 78.6 ± 1.56 a | 65.7 ± 1.70 b |
| 21–46 | 83.6 ± 1.62 c | 88.6 ± 1.28 b | 88.6 ± 0.59 b | 89.8 ± 0.45 a | |
| 47–72 | 81.3 ± 1.08 b | 79.4 ± 1.07 c | 79.9 ± 1.21 c | 84.8 ± 0.53 a | |
| Egg weight (g/egg) | 14–20 | 58.1 ± 0.75 c | 59.6 ± 1.08 a | 59.6 ± 0.59 a | 58.9 ± 0.83 b |
| 21–46 | 64.5 ± 1.96 c | 65.9 ± 1.08 b | 65.9 ± 1.08 b | 68.1 ± 0.28 a | |
| 47–72 | 67.0 ± 0.26 b | 67.3 ± 0.82 b | 67.0 ± 0.82 b | 69.3 ± 1.62 a | |
| Feed efficiency (Laying/feed; kg/ton) | 14–20 | 543 ± 26.9 b | 473 ± 28.1 c | 587 ± 26.9 a | 480 ± 26.9 c |
| 21–46 | 485 ± 24.3 d | 591 ± 17.4 a | 522 ± 13.2 c | 551 ± 12.3 b | |
| 47–72 | 527 ± 25.2 b | 559 ± 12.8 a | 518 ± 24.8 b | 570 ± 15.9 a |
| Week | Effect | Liver | Kidneys | Plasma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prot | GSH | GST | GGT | Prot | GSH | GST | GGT | Prot | ALT | AST | ||
| N-acetylcysteine (NAC) | ||||||||||||
| 14 | Model | 0.067 | 0.163 | 0.001 | 0.036 | 0.017 | 0.000 | 0.001 | 0.016 | 0.000 | 0.000 | 0.000 |
| NAC | 0.373 | 0.990 | 0.472 | 0.314 | 0.908 | 0.124 | 0.392 | 0.102 | 0.109 | 0.085 | 0.385 | |
| AFs | 0.266 | 0.701 | 0.007 | 0.013 | 0.002 | 0.001 | 0.000 | 0.006 | 0.000 | 0.013 | 0.000 | |
| NACxAFs | 0.038 | 0.178 | 0.010 | 0.114 | 0.829 | 0.000 | 0.595 | 0.069 | 0.006 | 0.037 | 0.314 | |
| 20 | Model | 0.609 | 0.001 | 0.409 | 0.285 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.966 | 0.032 |
| NAC | 0.482 | 0.014 | 0.188 | 0.207 | 0.540 | 0.505 | 0.016 | 0.544 | 0.000 | 0.990 | 0.006 | |
| AFB | 0.264 | 0.119 | 0.817 | 0.325 | 0.053 | 0.006 | 0.000 | 0.000 | 0.080 | 0.741 | 0.609 | |
| NACxAFs | 0.505 | 0.898 | 0.100 | 0.729 | 0.031 | 0.004 | 0.068 | 0.002 | 0.003 | 0.813 | 0.006 | |
| 46 | Model | 0.011 | 0.005 | 0.000 | 0.045 | 0.206 | 0.019 | 0.138 | 0.000 | 0.000 | 0.966 | 0.032 |
| NAC | 0.345 | 0.274 | 0.219 | 0.496 | 0.080 | 0.010 | 0.365 | 0.000 | 0.030 | 0.990 | 0.006 | |
| AFs | 0.002 | 0.002 | 0.000 | 0.007 | 0.519 | 0.489 | 0.040 | 0.546 | 0.007 | 0.741 | 0.609 | |
| NACxAFs | 0.708 | 0.840 | 0.490 | 0.455 | 0.046 | 0.286 | 0.256 | 0.000 | 0.000 | 0.813 | 0.006 | |
| 72 | Model | 0.875 | 0.374 | 0.000 | 0.014 | 0.004 | 0.002 | 0.196 | 0.001 | 0.050 | 0.202 | 0.000 |
| NAC | 0.538 | 0.696 | 0.198 | 0.389 | 0.154 | 0.147 | 0.622 | 0.014 | 0.706 | 0.234 | 0.002 | |
| AFs | 0.832 | 0.371 | 0.000 | 0.003 | 0.021 | 0.030 | 0.068 | 0.092 | 0.424 | 0.840 | 0.001 | |
| NACxAFs | 0.805 | 0.631 | 0.923 | 0.211 | 0.009 | 0.556 | 0.326 | 0.000 | 0.073 | 0.893 | 0.000 | |
| Ethoxyquin (EQ) | ||||||||||||
| 14 | Model | 0.719 | 0.020 | 0.000 | 0.000 | 0.006 | 0.006 | 0.050 | 0.498 | 0.000 | 0.000 | 0.000 |
| EQ | 0.537 | 0.559 | 0.120 | 0.204 | 0.815 | 0.059 | 0.573 | 0.453 | 0.188 | 0.495 | 0.113 | |
| AFs | 0.343 | 0.643 | 0.010 | 0.001 | 0.008 | 0.011 | 0.008 | 0.364 | 0.000 | 0.143 | 0.000 | |
| EQxAFs | 0.674 | 0.020 | 0.001 | 0.002 | 0.079 | 0.753 | 0.871 | 0.239 | 0.135 | 0.001 | 0.190 | |
| 20 | Model | 0.057 | 0.789 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.557 | 0.000 | 0.763 | 0.000 |
| EQ | 0.853 | 0.538 | 0.887 | 0.021 | 0.571 | 0.000 | 0.392 | 0.997 | 0.048 | 0.738 | 0.008 | |
| AFs | 0.048 | 0.451 | 0.000 | 0.202 | 0.000 | 0.005 | 0.000 | 0.196 | 0.000 | 0.336 | 0.000 | |
| EQxAFs | 0.176 | 0.518 | 0.029 | 0.000 | 0.294 | 0.000 | 0.203 | 0.727 | 0.000 | 0.666 | 0.710 | |
| 46 | Model | 0.000 | 0.055 | 0.008 | 0.358 | 0.051 | 0.003 | 0.011 | 0.000 | 0.012 | 0.966 | 0.045 |
| EQ | 0.363 | 0.094 | 0.327 | 0.749 | 0.183 | 0.006 | 0.753 | 0.204 | 0.003 | 0.990 | 0.060 | |
| AFs | 0.001 | 0.035 | 0.274 | 0.232 | 0.188 | 0.294 | 0.052 | 0.001 | 0.150 | 0.741 | 0.977 | |
| EQxAFs | 0.005 | 0.388 | 0.007 | 0.595 | 0.021 | 0.000 | 0.117 | 0.001 | 0.022 | 0.813 | 0.007 | |
| 72 | Model | 0.003 | 0.000 | 0.000 | 0.032 | 0.325 | 0.000 | 0.155 | 0.000 | 0.026 | 0.060 | 0.775 |
| EQ | 0.327 | 0.618 | 0.876 | 0.225 | 0.159 | 0.065 | 0.637 | 0.339 | 0.327 | 0.074 | 0.699 | |
| AFs | 0.000 | 0.004 | 0.000 | 0.007 | 0.342 | 0.185 | 0.028 | 0.000 | 0.423 | 0.581 | 0.345 | |
| EQxAFs | 0.195 | 0.007 | 0.185 | 0.456 | 0.117 | 0.000 | 0.716 | 0.203 | 0.015 | 0.848 | 0.823 | |
| Treatment (*) | Microscopic Alteration (**) | Proportion of Lesions (***) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AFs | EQ | NAC | Lymp | HydrD | Steat | Hem | Other | Total | ||
| 0.0 | 0.0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 0.0 | 33.3 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 0.0 | 0.0 | 80.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 0.5 | 0.0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 0.5 | 33.3 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 0.5 | 0.0 | 80.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.3 d) | |
| 1.0 | 0.0 | 0.0 | 15 | 0 | 0 | 0 | 0 | 15 | 20.0 (12.7–27.3 bc) | |
| 1.0 | 33.3 | 0.0 | 4 | 0 | 0 | 0 | 0 | 4 | 5.3 (0.0–12.7 cd) | |
| 1.0 | 0.0 | 80.8 | 9 | 0 | 0 | 0 | 0 | 9 | 12.0 (4.7–19.3 cd) | |
| 1.5 | 0.0 | 0.0 | 15 | 15 | 15 | 7 | 7 | 59 | 78.7 (71.3–86.0 a) | |
| 1.5 | 33.3 | 0.0 | 15 | 4 | 2 | 0 | 0 | 21 | 28.0 (20.7–35.3 b) | |
| 1.5 | 0.0 | 80.8 | 15 | 0 | 0 | 0 | 0 | 15 | 20.0 (12.7–27.3 bc) | |
| Total | 73 | 19 | 17 | 7 | 7 | 123 | ||||
| Treatment (*) | Microscopic Alteration (**) | Proportion of Lesions (***) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFs | EQ | NAC | Con | Linf | Ure | Thro | Hem | Other | Total | ||
| 0.0 | 0.0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.4 e) | |
| 0.0 | 33.3 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.4 e) | |
| 0.0 | 0.0 | 80.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.4 e) | |
| 0.5 | 0.0 | 0.0 | 0 | 4 | 0 | 0 | 0 | 0 | 4 | 4.4 (0.0–11.8 de) | |
| 0.5 | 33.3 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.4 e) | |
| 0.5 | 0.0 | 80.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–7.4 e) | |
| 1.0 | 0.0 | 0.0 | 0 | 15 | 0 | 0 | 0 | 6 | 21 | 16.7 (9.3–24.1 d) | |
| 1.0 | 33.3 | 0.0 | 0 | 15 | 0 | 0 | 0 | 0 | 15 | 16.7 (9.3–24.1 d) | |
| 1.0 | 0.0 | 80.8 | 0 | 15 | 0 | 0 | 0 | 0 | 15 | 16.7 (9.3–24.1 d) | |
| 1.5 | 0.0 | 0.0 | 15 | 15 | 15 | 15 | 12 | 8 | 80 | 88.9 (81.5–96.3 a) | |
| 1.5 | 33.3 | 0.0 | 9 | 15 | 15 | 7 | 0 | 0 | 46 | 51.1 (43.7–58.5 b) | |
| 1.5 | 0.0 | 80.8 | 4 | 15 | 11 | 0 | 0 | 0 | 30 | 33.3 (25.9–40.7 c) | |
| Total | 28 | 94 | 41 | 22 | 12 | 14 | 211 | ||||
| Dietary Regimen ** | Treatment Group | AFs | NAC | EQ |
|---|---|---|---|---|
| mg/kg of Feed | mg/kg BW/d | mg/kg BW/d | ||
| AFs | T1: AFs 0.0 | 0.00 | 0.00 | 0.00 |
| T2: AFs 0.5 | 0.50 | 0.00 | 0.00 | |
| T3: AFs 1.0 | 1.00 | 0.00 | 0.00 | |
| T4: AFs 1.5 | 1.50 | 0.00 | 0.00 | |
| NAC | T5: NAC + 0.5 AFs | 0.00 | 80.8 | 0.00 |
| T6: NAC + 0.5 AFs | 0.50 | 80.8 | 0.00 | |
| T7: NAC + 1.0 AFs | 1.00 | 80.8 | 0.00 | |
| T8: NAC + 1.5 AFs | 1.50 | 80.8 | 0.00 | |
| EQ | T9: EQ + 0.5 AFs | 0.00 | 0.00 | 33.3 |
| T10: EQ + 0.5 AFs | 0.50 | 0.00 | 33.3 | |
| T11: EQ + 1.0 AFs | 1.00 | 0.00 | 33.3 | |
| T12: EQ + 1.5 AFs | 1.50 | 0.00 | 33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de-Luna-López, M.C.; Valdivia-Flores, A.G.; Quezada-Tristán, T.; Ortiz-Martínez, R.; Rangel-Muñoz, E.J.; Hernández-Valdivia, E.; Albarrán-Rodríguez, E.; de Santiago-Díaz, E. Protective Effect of Ethoxyquin and N-acetylcysteine on Biochemical and Pathological Changes Induced by Chronic Exposure to Aflatoxins in Laying Hens. Toxins 2025, 17, 514. https://doi.org/10.3390/toxins17100514
de-Luna-López MC, Valdivia-Flores AG, Quezada-Tristán T, Ortiz-Martínez R, Rangel-Muñoz EJ, Hernández-Valdivia E, Albarrán-Rodríguez E, de Santiago-Díaz E. Protective Effect of Ethoxyquin and N-acetylcysteine on Biochemical and Pathological Changes Induced by Chronic Exposure to Aflatoxins in Laying Hens. Toxins. 2025; 17(10):514. https://doi.org/10.3390/toxins17100514
Chicago/Turabian Stylede-Luna-López, María Carolina, Arturo Gerardo Valdivia-Flores, Teódulo Quezada-Tristán, Raúl Ortiz-Martínez, Erika Janet Rangel-Muñoz, Emmanuel Hernández-Valdivia, Esther Albarrán-Rodríguez, and Elizabeth de Santiago-Díaz. 2025. "Protective Effect of Ethoxyquin and N-acetylcysteine on Biochemical and Pathological Changes Induced by Chronic Exposure to Aflatoxins in Laying Hens" Toxins 17, no. 10: 514. https://doi.org/10.3390/toxins17100514
APA Stylede-Luna-López, M. C., Valdivia-Flores, A. G., Quezada-Tristán, T., Ortiz-Martínez, R., Rangel-Muñoz, E. J., Hernández-Valdivia, E., Albarrán-Rodríguez, E., & de Santiago-Díaz, E. (2025). Protective Effect of Ethoxyquin and N-acetylcysteine on Biochemical and Pathological Changes Induced by Chronic Exposure to Aflatoxins in Laying Hens. Toxins, 17(10), 514. https://doi.org/10.3390/toxins17100514









