Toxigenic Aspergillus Diversity and Mycotoxins in Organic Spanish Grape Berries
Abstract
1. Introduction
2. Results
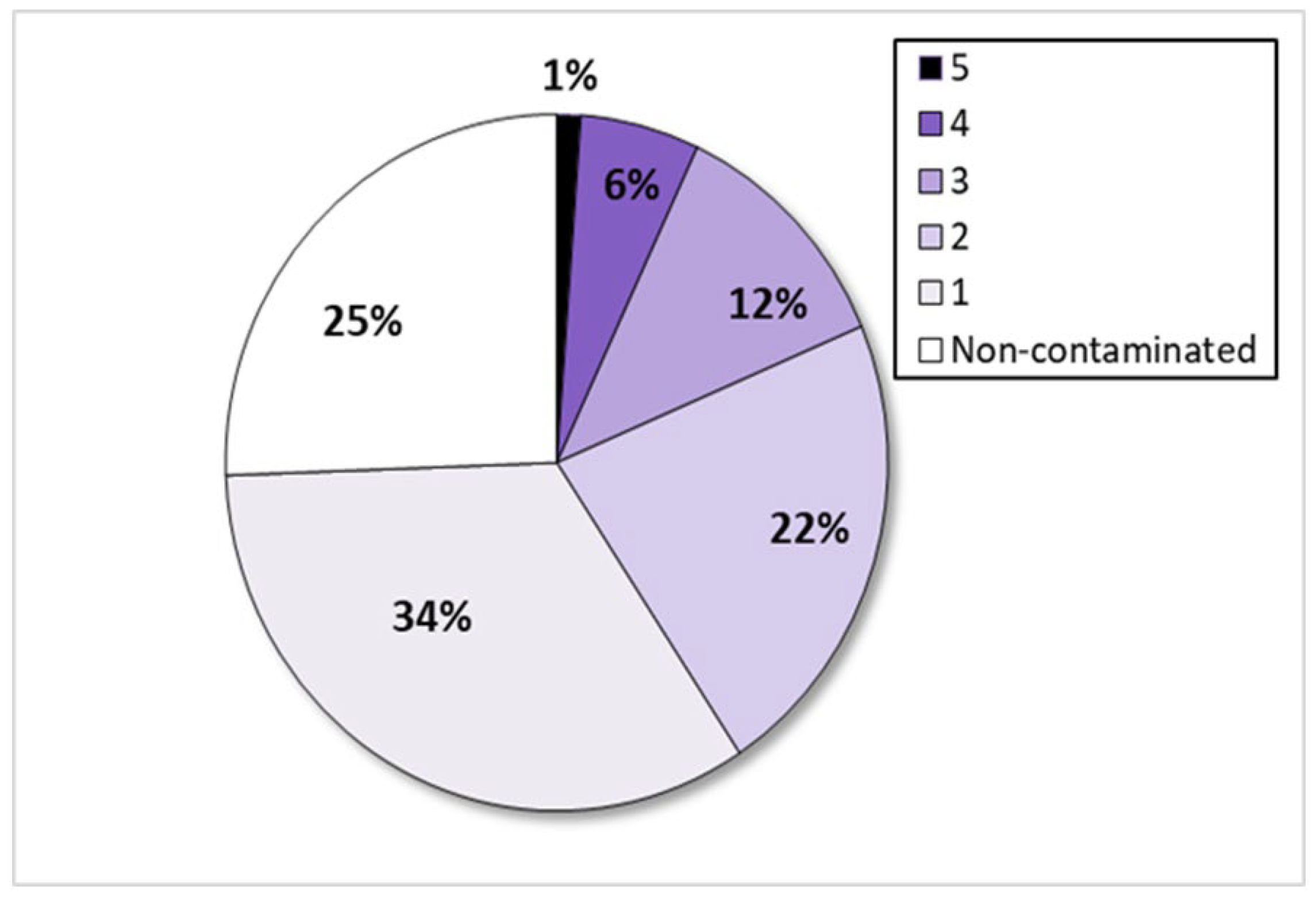
2.1. Occurrence of Toxigenic Aspergillus in Organic Grape Samples
2.2. Mycotoxin Analysis
2.2.1. Determining the Best Extraction Method for UPLC Analysis of OTA
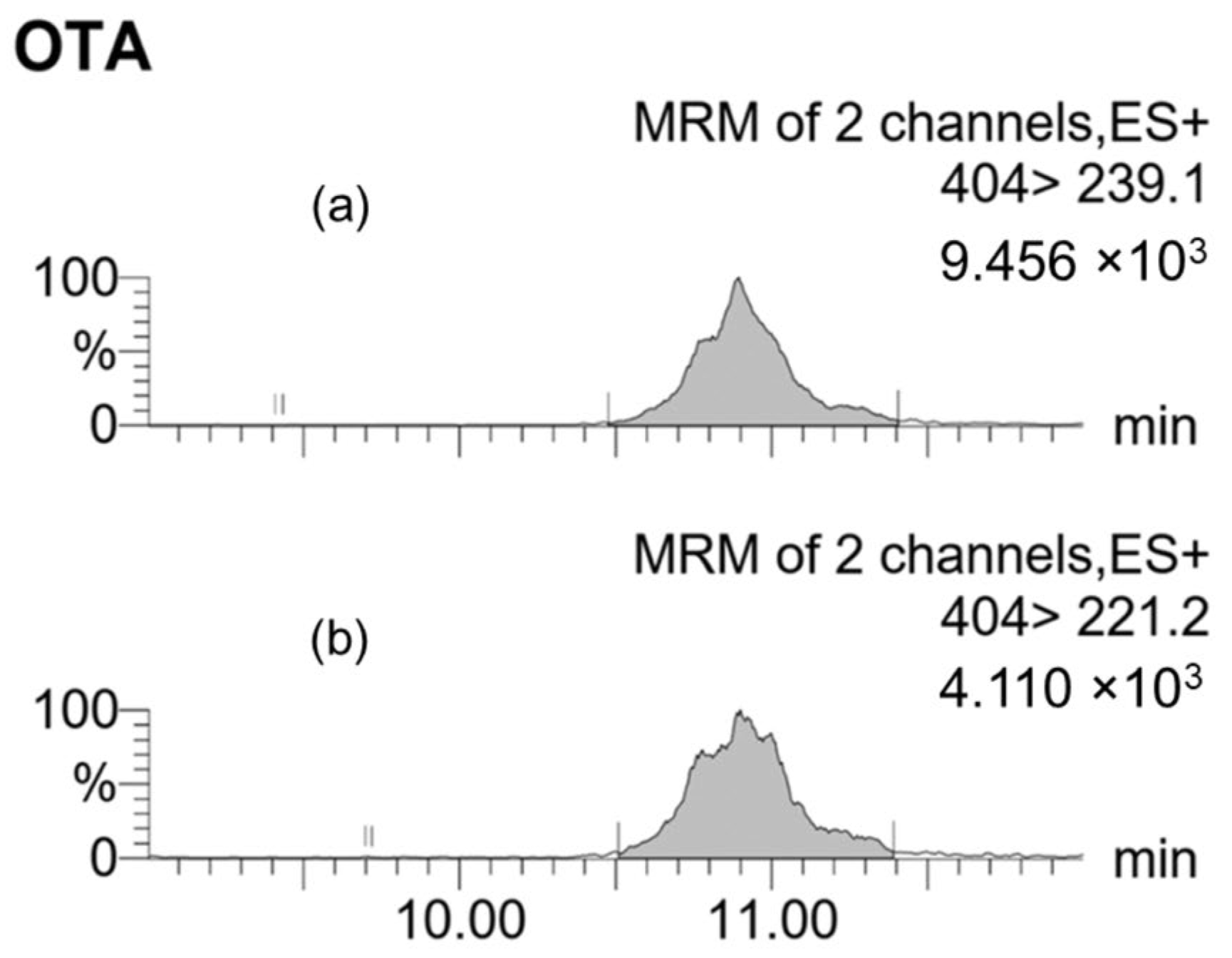
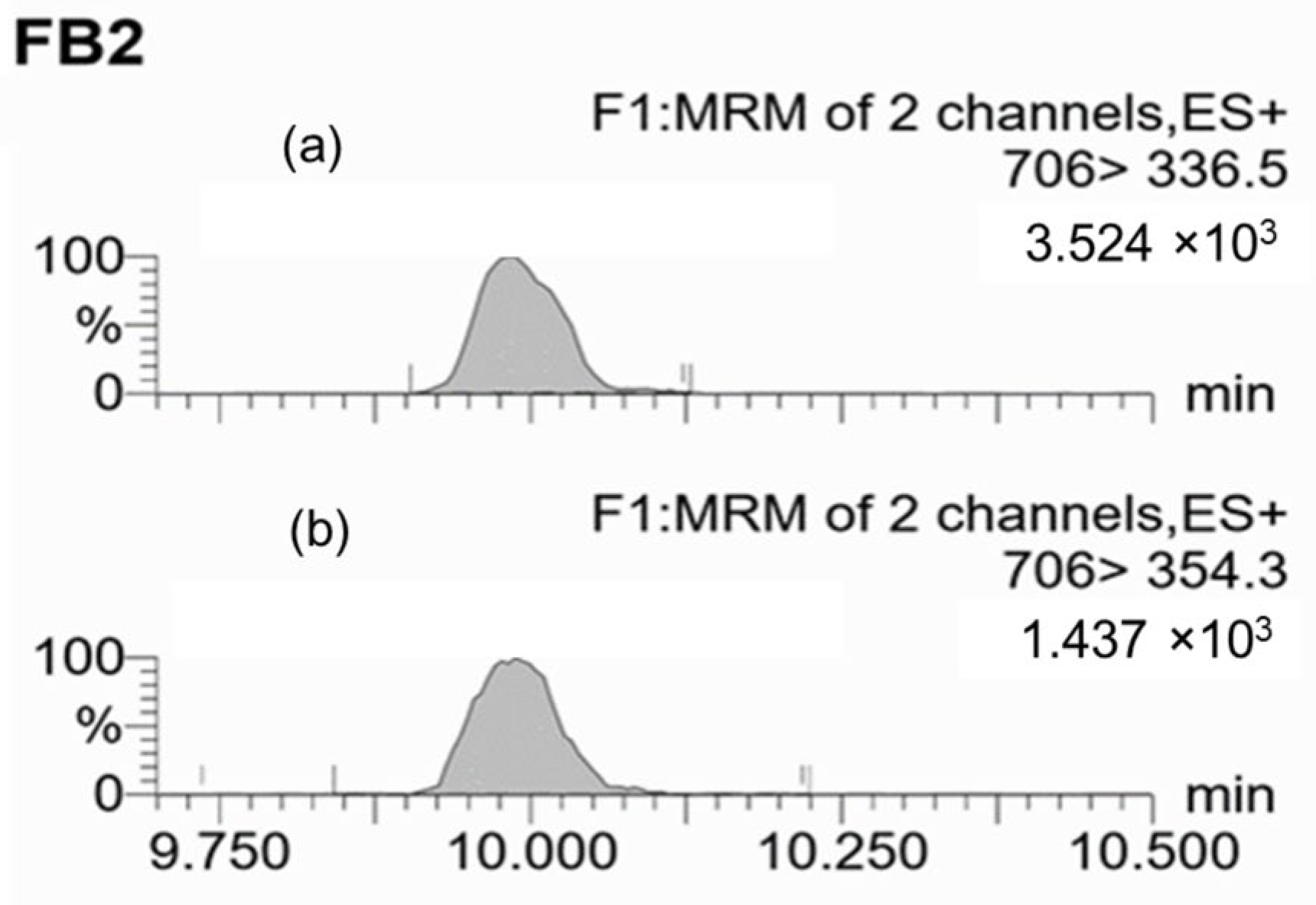
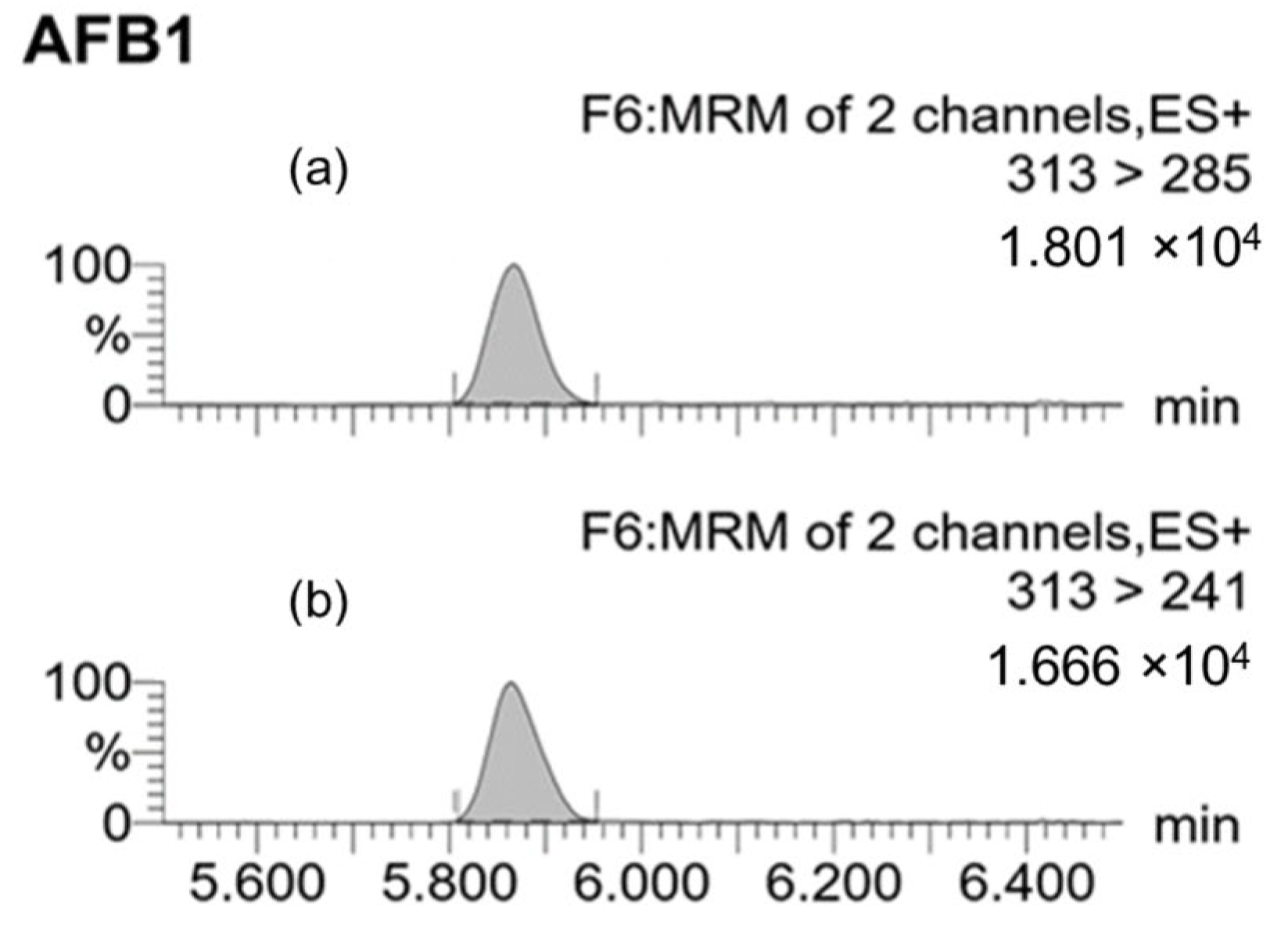
2.2.2. Mycotoxin Detection in Grape Samples
3. Discussion
4. Materials and Methods
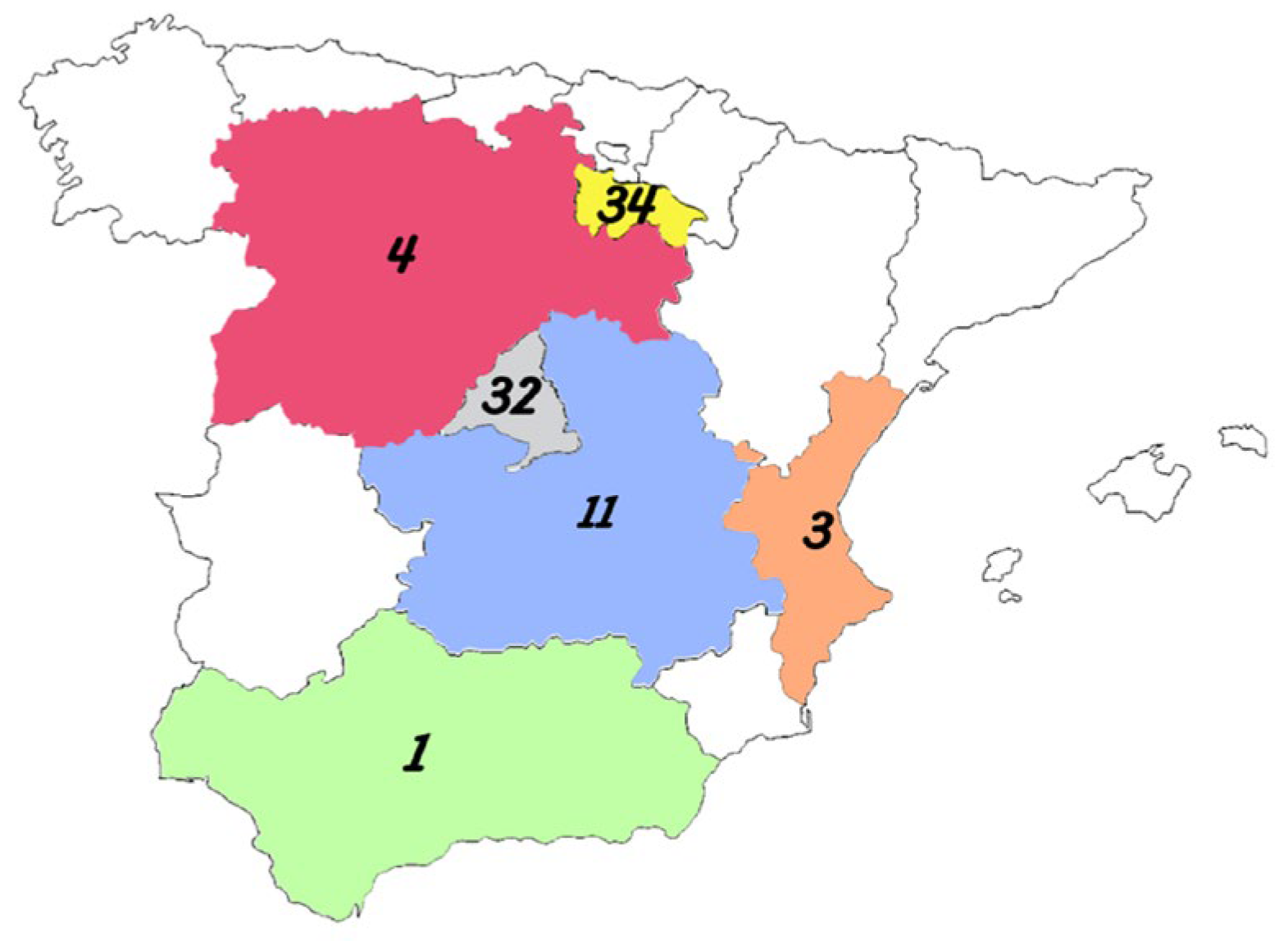
4.1. Collection of Grape Samples
4.2. Occurrence of Toxigenic Aspergillus in Grape Samples
4.3. Mycotoxin Analysis
4.3.1. Reagents and Standards
4.3.2. Assessment of Analytical Methods for OTA Detection
DLLME Method for OTA Analysis
UPLC–ESI–MS/MS Analyses of Extracts from Organic Grapes
Calibration and Validation
4.3.3. Analytical Method for FB2
4.3.4. Analytical Method for AFB1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. State of the World Vine and Wine Sector in 2023. International Organization of Vine and Wine. 2024. Available online: https://www.oiv.int/sites/default/files/documents/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023_0.pdf (accessed on 7 November 2024).
- OIV. Annual Assessment of the World Vine and Wine Sector in 2022, International Organization of Vine and Wine. 2023. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf (accessed on 7 November 2024).
- MAPAMA. Encuesta Sobre Superficies y Rendimientos de Cultivos (ESYRCE). Resultados Provisionales Nacionales y Autonómicos 2024. In Subsecretaría de Agricultura, Pesca y Alimentación. Catálogo de Publicaciones de la Administración General del Estado. Gobierno de España. 2023. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/comentariosespana2024_tcm30-697375.pdf (accessed on 7 November 2024).
- European Commission. Commission regulation (EU) No 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- García-Cela, E.; Crespo-Sempere, A.; Gil-Serna, J.; Porqueres, A.; Marin, S. Fungal diversity, incidence and mycotoxin contamination in grapes from two agro-climatic Spanish regions with emphasis on Aspergillus species. J. Sci. Food Agric. 2015, 95, 1716–1729. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Venâncio, A.; Lima, N.; Guilloux-Bénatier, M.; Rousseaux, S. Predominant mycotoxins, mycotoxigenic fungi and climate change related to wine. Food Res. Int. 2018, 103, 478–491. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Critical reviews in food science and nutrition. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef]
- Bau, M.; Bragulat, M.R.; Abarca, M.L.; Mínguez, S.; Cabañes, F.L. Ochratoxigenic species from Spanish wine grapes. Int. J. Food Microbiol. 2005, 98, 125–130. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Wine contamination with ochratoxins: A review. Beverages 2018, 4, 6. [Google Scholar] [CrossRef]
- Marqués, J.; Castellá, G.; Bragulat, M.R.; Cabañes, F.J. Diversity of Aspergillus section Nigri species from vineyards with different agro-climatic conditions in Catalonia, Spain. Int. J. Food Microbiol. 2025, 430, 111049. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jiang, H.; Xu, J.; Zhang, J.; Li, F. Dynamic fumonisin B2 production by Aspergillus niger intended used in food industry in China. Toxins 2017, 9, 217. [Google Scholar] [CrossRef]
- Mansson, M.; Klejnstrup, M.L.; Phipps, R.K.; Nielsen, K.F.; Frisvad, J.C.; Gotfredsen, C.H.; Larsen, T.O. Isolation and NMR characterization of fumonisin B2 and a new fumonisin B6 from Aspergillus niger. J. Agric. Food Chem. 2009, 58, 949–953. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Frisvad, J.C.; Thrane, U.; Nielsen, K.F. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 2010, 58, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Wu, A. Evaluation of the individual and combined toxicity of fumonisin mycotoxins in human gastric epithelial cells. International Journal of Molecular Sciences. Int. J. Mol. Sci. 2020, 21, 5917. [Google Scholar] [CrossRef]
- Gelderblom, W.; Cawood, M.; Snyman, S.; Vleggaar, R.; Marasas, W. Structure-activity relationships of fumonisins in short-term carcinogenesis and cytotoxicity assays. Food Chem. Toxicol. 1993, 31, 407–414. [Google Scholar] [CrossRef]
- Logrieco, A.; Ferracane, R.; Haidukowsky, M.; Cozzi, G.; Visconti, A.; Ritieni, A. Fumonisin B2 production by Aspergillus niger from grapes and natural occurrence in must. Food Addit. Contam. Part A 2009, 26, 1495–1500. [Google Scholar] [CrossRef]
- Logrieco, A.; Ferracane, R.; Visconti, A.; Ritieni, A. Natural occurrence of fumonisin B2 in red wine from Italy. Food Addit. Contam. Part A 2010, 27, 1136–1141. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Larsen, T.O.; Nielsen, K.F. Widespread occurrence of the mycotoxin fumonisin B2 in wine. J. Agric. Food Chem. 2010, 58, 4853–4857. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.; Hong, S.-B.; Novßkovß, A.; Chen, A.; Arzanlou, M.; Larsen, T.; Sklenßř, F.; Mahakarnchanakul, W. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Klingelhoefer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.; Brüggmann, D.; Groneberg, D.A. Aflatoxin–Publication analysis of a global health threat. Food Control 2018, 89, 280–290. [Google Scholar] [CrossRef]
- Cosme, F.; Ribeiro, M.; Ribeiro, L.F.; Nunes, F.M. Review of mycotoxins in grapes and grape products. In Global Warming and the Wine Industry−Challenges, Innovations and Future Prospects; Cosme, F., Nunes, F.M., Ribeiro, L.F., Eds.; IntechOpen: London, UK, 2024. [Google Scholar]
- EL Khoury, A.E.; Rizk, T.; Lteif, R.; Azouri, H.; Delia, M.-L.; Lebrihi, A. Fungal contamination and Aflatoxin B1 and Ochratoxin A in Lebanese wine–grapes and musts. Food Chem. Toxicol. 2008, 46, 2244–2250. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Further mycotoxin effects from climate change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef]
- Eurostat. Developments in Organic Farming. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?oldid=419176#Total_organic_area (accessed on 11 November 2024).
- European Commission. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://food.ec.europa.eu/document/download/472acca8-7f7b-4171-98b0-ed76720d68d3_en?filename=f2f_action-plan_2020_strategy-info_en.pdf (accessed on 20 March 2024).
- Willer, H.; Trávníček, J.; Schlatter, B. (Eds.) The World of Organic Agriculture. Statistics and Emerging Trends 2024; Research Institute of Organic Agriculture FiBL, Frick, and IFOAM—Organics International: Bonn, Germany, 2024; Available online: http://www.organic-world.net/yearbook/yearbook-2024.html (accessed on 6 November 2024).
- European Commission. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, 150, 1–92. [Google Scholar]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Testempasis, S.I.; Kamou, N.N.; Papadakis, E.-N.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Conventional vs. organic vineyards: Black Aspergilli population structure, mycotoxigenic capacity and mycotoxin contamination assessment in wines, using a new Q-TOF MS-MS detection method. Food Control 2022, 136, 108860. [Google Scholar] [CrossRef]
- Testempasis, S.I.; Papazlatani, C.V.; Karas, P.; Karaoglanidis, G. Vineyard practices reduce the incidence of Aspergillus spp. and alter the composition of carposphere microbiome in grapes (Vitis vinifera L.). Front. Microbiol. 2023, 14, 1257644. [Google Scholar] [CrossRef]
- OIV. Organic Viticulture Is Gaining Terrain. 2021. Available online: https://www.oiv.int/organic-viticulture-is-gaining-terrain (accessed on 7 November 2024).
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Gómez-Albarrán, C.; Melguizo, C.; Patiño, B.; Vázquez, C.; Gil-Serna, J. Diversity of mycobiota in Spanish grape berries and selection of Hanseniaspora uvarum U1 to prevent mycotoxin contamination. Toxins 2021, 13, 649. [Google Scholar] [CrossRef]
- De Souza Ferranti, L.; Pelegrinelli Fungaro, M.H.; Massi, F.P.; Da Silva, J.J.; Penha, R.E.S.; Frisvad, J.C.; Taniwaki, M.H.; Lamanaka, B.T. Diversity of Aspergillus section Nigri on the surface of Vitis labrusca and its hybrid grapes. Int. J. Food Microbiol. 2018, 268, 53–60. [Google Scholar] [CrossRef]
- Lasram, S.; Oueslati, S.; Mliki, A.; Ghorbel, A.; Silar, P.; Chebil, S. Ochratoxin A and ochratoxigenic black Aspergillus species in Tunisian grapes cultivated in different geographic areas. Food Control 2012, 25, 75–80. [Google Scholar] [CrossRef]
- Covarelli, L.; Tini, F.; Perrone, G.; Magistà, D.; Onofri, A.; Beccari, G. Survey on the occurrence of Aspergillus section Nigri species in grapes cultivated in Umbria (central Italy) and influence of several factors on their distribution. J. Plant Pathol. 2025, 107, 229–242. [Google Scholar] [CrossRef]
- Temperini, C.; Kemppainen, M.; Moya, M.; Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic fungi, OTA and fumonisin B2 production by Aspergillus section Nigri isolated from wine grapes and natural occurrence of OTA in wines of Northern Argentinean Patagonia. Int. J. Food Microbiol. 2025, 433, 111135. [Google Scholar] [CrossRef]
- Dutra-Silva, L.; Pereira, G.E.; Batista, L.R.; Matteoli, F.P. Fungal diversity and occurrence of mycotoxin producing fungi in tropical vineyards. World J. Microbiol. Biotechnol. 2021, 37, 112. [Google Scholar] [CrossRef]
- Jensen, B.; Knudsen, I.M.; Andersen, B.; Nielsen, K.F.; Thrane, U.; Jensen, D.F.; Larsen, J. Characterization of microbial communities and fungal metabolites on field grown strawberries from organic and conventional production. Int. J. Food Microbiol. 2013, 160, 313–322. [Google Scholar] [CrossRef]
- Lazzaro, I.; Moretti, A.; Giorni, P.; Brera, C.; Battilani, P. Organic vs conventional farming: Differences in infection by mycotoxin-producing fungi on maize and wheat in northern and central Italy. Crop Prot. 2015, 72, 22–30. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Quejarro, B.J.; Yu, A.; Zhao, A. Comparison of Aspergillus section Nigri species populations in conventional and organic raisin vineyards. Curr. Microbiol. 2019, 76, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13323. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- MITECO. Resumen Anual Climatológico 2019. Spanish National Agency of Meteorology. 2020. Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/vigilancia_clima/resumenes_climat/anuales/res_anual_clim_2019.pdf (accessed on 14 September 2025).
- MITECO. Resumen Anual Climatológico 2020. Spanish National Agency of Meteorology. 2021. Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/vigilancia_clima/resumenes_climat/anuales/res_anual_clim_2020.pdf (accessed on 14 September 2025).
- MITECO. Resumen Anual Climatológico 2021. Spanish National Agency of Meteorology. 2022. Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/vigilancia_clima/resumenes_climat/anuales/res_anual_clim_2021.pdf (accessed on 14 September 2025).
- Battilani, P.; Toscano, P.; der Fels-Klerx, V.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Bock, C.; Mackey, B.; Cotty, P. Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of south-west Arizona. Plant Pathol. 2004, 53, 422–443. [Google Scholar] [CrossRef]
- Sanders, T.H.; Blankenship, P.D.; Cole, R.J.; Hill, R.A. Effect of soil temperature and drought on peanut pod and stem temperatures relative to Aspergillus flavus invasion and aflatoxin contamination. Mycopathologia 1984, 86, 51–54. [Google Scholar] [CrossRef]
- Chuaysrinule, C.; Mahakarnchanakul, W.; Maneeboon, T. Comparative study on the effect of temperature and water activity on Aspergillus flavus and Aspergillus carbonarius isolates growth and mycotoxin production on a chili powder medium. Cogent Food Agric. 2020, 6, 1782097. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Leong, S.; Hocking, A.; Scott, E. Effects of water activity and temperature on the survival of Aspergillus carbonarius spores in vitro. Lett. Appl. Microbiol. 2006, 42, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. pH-controlled dispersive liquid–liquid microextraction for the analysis of ionisable compounds in complex matrices: Case study of ochratoxin A in cereals. Anal. Chim. Acta 2012, 54, 61–66. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Miri, R.; Javidnia, K.; Kobarfard, F.; AliAbadi, M.H.S.; Maham, M.A. Validated dispersive liquid-liquid microextraction method for extraction of ochratoxin A from raisin samples. J. Food Sci. Technol. 2015, 52, 2440–2445. [Google Scholar] [CrossRef][Green Version]
- Ruan, C.; Diao, X.; Li, N.; Zhang, H.; Panga, Y.; Liu, C. Determination of ochratoxin A and citrinin in fruits using ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction with HPLC with fluorescence detection. Anal. Methods 2016, 8, 1586–1594. [Google Scholar] [CrossRef]
- Pallarés, N.; Carballo, D.; Ferrer, E.; Fernández-Franzón, M.; Berrada, H. Mycotoxin dietary exposure assessment through fruit juices consumption in children and adult population. Toxins 2019, 11, 684. [Google Scholar] [CrossRef]
- Cozzi, G.; Paciolla, C.; Haidukowski, M.; De Leonardis, S.; Mulè, G.; Logrieco, A. Increase of fumonisin B2 and ochratoxin A production by black Aspergillus species and oxidative stress in grape berries damaged by powdery mildew. J. Food Prot. 2013, 76, 2031–2036. [Google Scholar] [CrossRef]
- Knudsen, P.B.; Mogensen, J.M.; Larsen, T.O.; Nielsen, K.F. Occurrence of fumonisins B2 and B4 in retail raisins. J. Agric. Food Chem. 2010, 59, 772–776. [Google Scholar] [CrossRef]
- Freire, L.; Braga, A.C.P.; Furtado, M.M.; Delafiori, J.; Dias-Audibert, F.L.; Pereira, G.E.; Reyes, F.G.; Catharino, R.R.; Sant’Ana, A.S. From grape to wine: Fate of ochratoxin A during red, rose, and white winemaking process and the presence of ochratoxin derivatives in the final products. Food Control 2020, 113, 107167. [Google Scholar] [CrossRef]
- Lasram, S.; Mani, A.; Zaied, C.; Chebil, S.; Abid, S.; Bacha, H.; Mliki, A.; Ghorbel, A. Evolution of ochratoxin A content during red and rose vinification. J. Sci. Food Agric. 2008, 88, 1696–1703. [Google Scholar] [CrossRef]
- Lucchetta, G.; Bazzo, I.; Dal Cortivo, G.; Stringher, L.; Bellotto, D.; Borgo, M.; Angelini, E. Occurrence of black Aspergilli and ochratoxin A on grapes in Italy. Toxins 2010, 2, 840–855. [Google Scholar] [CrossRef]
- Serra, R.; Mendonça, C.; Venâncio, A. Fungi and ochratoxin A detected in healthy grapes for wine production. Lett. Appl. Microbiol. 2006, 42, 42–47. [Google Scholar] [CrossRef]
- Leong, S.; Hocking, A.; Pitt, J.I.; Kazi, B.; Emmett, R.; Scott, E. Australian research on ochratoxigenic fungi and ochratoxin A. Int. J. Food Microbiol. 2006, 111, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- European Commission. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union 2009, 309, 71–86. [Google Scholar]
- Leong, S.; Hocking, A.; Scott, E. Aspergillus species producing ochratoxin A: Isolation from vineyard soils and infection of Semillon bunches in Australia. J. Appl. Microbiol. 2007, 102, 124–133. [Google Scholar] [CrossRef]
- Nikolić, M.; Savić, I.; Nikolić, A.; Jauković, M.; Kandić, V.; Stevanović, M.; Stanković, S. Toxigenic species Aspergillus parasiticus originating from maize kernels grown in Serbia. Toxins 2021, 13, 847. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, Á.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Avellone, G.; Di Fiore, A.; Monte, L.; Ogorka, A.Z.T. Determination of aflatoxins and ochratoxins in Sicilian sweet wines by high-performance liquid chromatography with fluorometric detection and immunoaffinity cleanup. Food Anal. Methods 2015, 8, 569–577. [Google Scholar] [CrossRef]
- Pérez-Ortega, P.; Gilbert-López, B.; García-Reyes, J.F.; Ramos-Martos, N.; Molina-Díaz, A. Generic sample treatment method for simultaneous determination of multiclass pesticides and mycotoxins in wines by liquid chromatography–mass spectrometry. J. Chromatogr. A 2012, 1249, 32–40. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Han, S.-Y.; Li, M.; Ma, T.-Z.; Sheng, W.-J.; Zhu, X. Simultaneous analysis of 20 mycotoxins in grapes and wines from Hexi Corridor Region (China): Based on a QuEChERS–UHPLC–MS/MS method. Molecules 2018, 23, 1926. [Google Scholar] [CrossRef]
- Melguizo, C.; Patiño, B.; Ramos, A.J.; Vázquez, C.; Gil-Serna, J. Reconsidering the co-occurrence of Aspergillus flavus in Spanish vineyards and aflatoxins in grapes. Agriculture 2023, 13, 1998. [Google Scholar] [CrossRef]
- Horn, B.; Dorner, J. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl. Environ. Microbiol. 1999, 65, 1444–1449. [Google Scholar] [CrossRef]
- Danne, A.; Thomson, L.; Sharley, D.; Penfold, C.; Hoffmann, A. Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environ. Entomol. 2010, 39, 970–978. [Google Scholar] [CrossRef]
- Guzmán, G.; Cabezas, J.M.; Sánchez-Cuesta, R.; Lora, Á.; Bauer, T.; Strauss, P.; Winter, S.; Zaller, J.; Gómez, J. A field evaluation of the impact of temporary cover crops on soil properties and vegetation communities in southern Spain vineyards. Agric. Ecosyst. Environ. 2019, 272, 135–145. [Google Scholar] [CrossRef]
- Novara, A.; Catania, V.; Tolone, M.; Gristina, L.; Laudicina, V.A.; Quatrini, P. Cover crop impact on soil organic carbon, nitrogen dynamics and microbial diversity in a Mediterranean semiarid vineyard. Sustainability 2020, 12, 3256. [Google Scholar] [CrossRef]
- Chang, P.; Ehrlich, K. Cyclopiazonic acid biosynthesis by Aspergillus flavus. Toxin Rev. 2011, 30, 79–89. [Google Scholar] [CrossRef]
- Zhang, S.; Monahan, B.J.; Tkacz, J.S.; Scott, B. Indole-diterpene gene cluster from Aspergillus flavus. Appl. Environ. Microbiol. 2004, 70, 6875–6883. [Google Scholar] [CrossRef]
- Hymery, N.; Masson, F.; Barbier, G.; Coton, E. Cytotoxicity and immunotoxicity of cyclopiazonic acid on human cells. Toxicol. In Vitro 2014, 28, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Qi, J.; Wang, P.; Liu, C.; Gao, J.M. The chemical structures and biological activities of indole diterpenoids. Nat. Prod. Bioprospect. 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Patiño, B.; González-Salgado, A.; González-Jaén, M.T.; Vázquez, C. PCR detection assays for the ochratoxin-producing Aspergillus carbonarius and Aspergillus ochraceus species. Int. J. Food Microbiol. 2005, 104, 207–214. [Google Scholar] [CrossRef]
- González-Salgado, A.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Highly sensitive PCR-based detection method specific for Aspergillus flavus in wheat flour. Food Addit. Contam. 2008, 25, 758–764. [Google Scholar] [CrossRef]
- Sardiñas, N.; Vázquez, C.; Gil-Serna, J.; González-Jaén, M.T.; Patiño, B. Specific detection of Aspergillus parasiticus in wheat flour using a highly sensitive PCR assay. Food Addit. Contam. 2010, 27, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; Sardiñas, N.; González-Jaén, M.T.; Patiño, B. Discrimination of the main Ochratoxin A-producing species in Aspergillus section Circumdati by specific PCR assays. Int. J. Food Microbiol. 2009, 136, 83–87. [Google Scholar] [CrossRef]
- Bian, C.; Kusuya, Y.; Sklenář, F.; D’hooge, E.; Yaguchi, T.; Ban, S.; Visagie, C.M.; Houbraken, J.; Takahashi, H.; Hubka, V. Reducing the number of accepted species in Aspergillus series Nigri. Stud. Mycol. 2022, 102, 95–132. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L. Detection and discrimination of four Aspergillus section Nigri species by PCR. Lett. Appl. Microbiol. 2015, 60, 188–195. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- De Jesus, C.L.; Bartley, A.; Welch, A.Z.; Berry, J.P. High incidence and levels of ochratoxin A in wines sourced from the United States. Toxins 2018, 10, 1. [Google Scholar] [CrossRef]
- Wei, X.; Wu, D.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y.; Ji, M. Determination of ochratoxin A contamination in grapes, processed grape products and animal-derived products using ultra-performance liquid chromatography-tandem mass spectroscopy system. Sci. Rep. 2018, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M. Determination of ochratoxin A in wines by capillary liquid chromatography with laser induced fluorescence detection using dispersive liquid–liquid microextraction. Food Chem. 2012, 135, 368–372. [Google Scholar] [CrossRef]
- Cagnasso, I.; Mandrile, L.; Tonachini, G.; Berto, S.; Maranzana, A.; Giacomino, A.; Durbiano, F. Comprehensive study on the degradation of ochratoxin A in water by spectroscopic techniques and DFT calculations. RSC Adv. 2019, 9, 19844. [Google Scholar] [CrossRef] [PubMed]
- Romera, D.; Mateo, E.M.; Mateo-Castro, R.; Gómez, J.V.; Gimeno-Adelantado, J.V.; Jiménez, M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chem. 2018, 267, 140–148. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS method for the multi-mycotoxin determination in composite cereal-based samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweigenbaum, J.; Ryu, D.; Cappozzo, J. Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Rastrelli, L. Dispersive liquid–liquid microextraction combined with high-performance liquid chromatography–tandem mass spectrometry for the identification and the accurate quantification by isotope dilution assay of ochratoxin A in wine samples. Anal. Bioanal. Chem. 2011, 399, 1279–1286. [Google Scholar] [CrossRef]
- ICH (International Council for Harmonisation). ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]

| Extraction/Cleanup Method | |||
|---|---|---|---|
| Spiked OTA (ng/g) in Grape Berries | DLLME | LLE | QuEChERS |
| 2 | 70 ± 11 | 29 ± 10 | 46 ± 13 |
| 4 | 79 ± 8 | 35 ± 11 | 56 ± 9 |
| 10 | 90 ± 7 | 44 ± 12 | 65 ± 10 |
| 20 | 93 ± 6 | 55 ± 10 | 76 ± 6 |
| Mycotoxin | Spiking Level (ng/g) | Recovery (%) | RSD of Recovery (%) | LOD (ng/g) | LOQ (ng/g) |
|---|---|---|---|---|---|
| Fumonisin B2 | 10 | 70.3 | 16.2 | 2.2 | 6.6 |
| 20 | 75.2 | 11.6 | |||
| 50 | 80.1 | 8.4 | |||
| 150 | 90.7 | 6.2 | |||
| Aflatoxin B1 | 1 | 73.2 | 13.4 | 0.3 | 0.9 |
| 5 | 77.9 | 9.8 | |||
| 10 | 84.8 | 6.8 | |||
| 20 | 92.3 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melguizo, C.; Tarazona, A.; Gil-Serna, J.; Mateo, F.; Patiño, B.; Mateo, E.M. Toxigenic Aspergillus Diversity and Mycotoxins in Organic Spanish Grape Berries. Toxins 2025, 17, 487. https://doi.org/10.3390/toxins17100487
Melguizo C, Tarazona A, Gil-Serna J, Mateo F, Patiño B, Mateo EM. Toxigenic Aspergillus Diversity and Mycotoxins in Organic Spanish Grape Berries. Toxins. 2025; 17(10):487. https://doi.org/10.3390/toxins17100487
Chicago/Turabian StyleMelguizo, Clara, Andrea Tarazona, Jéssica Gil-Serna, Fernando Mateo, Belén Patiño, and Eva María Mateo. 2025. "Toxigenic Aspergillus Diversity and Mycotoxins in Organic Spanish Grape Berries" Toxins 17, no. 10: 487. https://doi.org/10.3390/toxins17100487
APA StyleMelguizo, C., Tarazona, A., Gil-Serna, J., Mateo, F., Patiño, B., & Mateo, E. M. (2025). Toxigenic Aspergillus Diversity and Mycotoxins in Organic Spanish Grape Berries. Toxins, 17(10), 487. https://doi.org/10.3390/toxins17100487









