T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways
Abstract
1. Introduction
2. Results
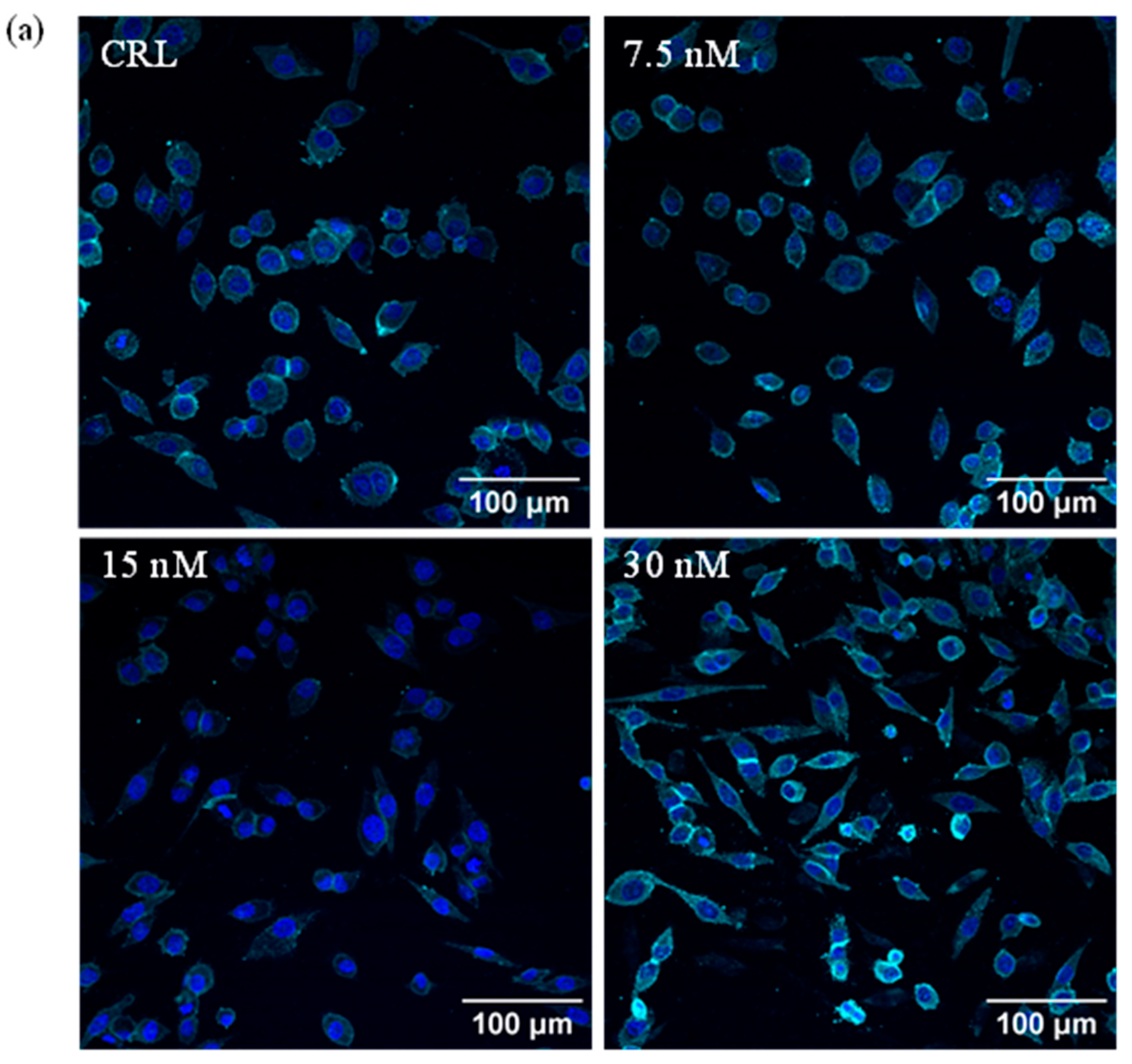
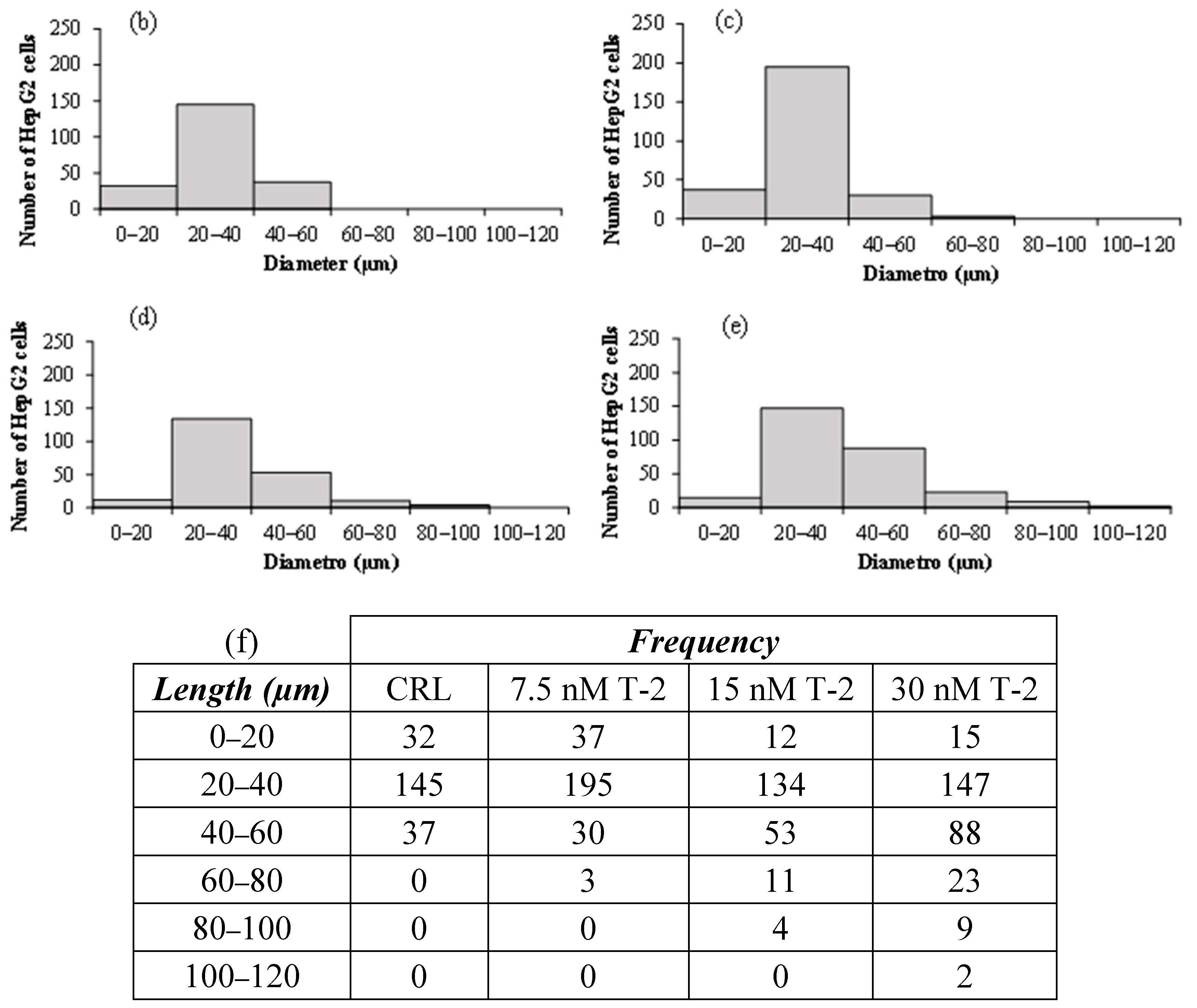
2.1. Analysis of HepG2 Morphology
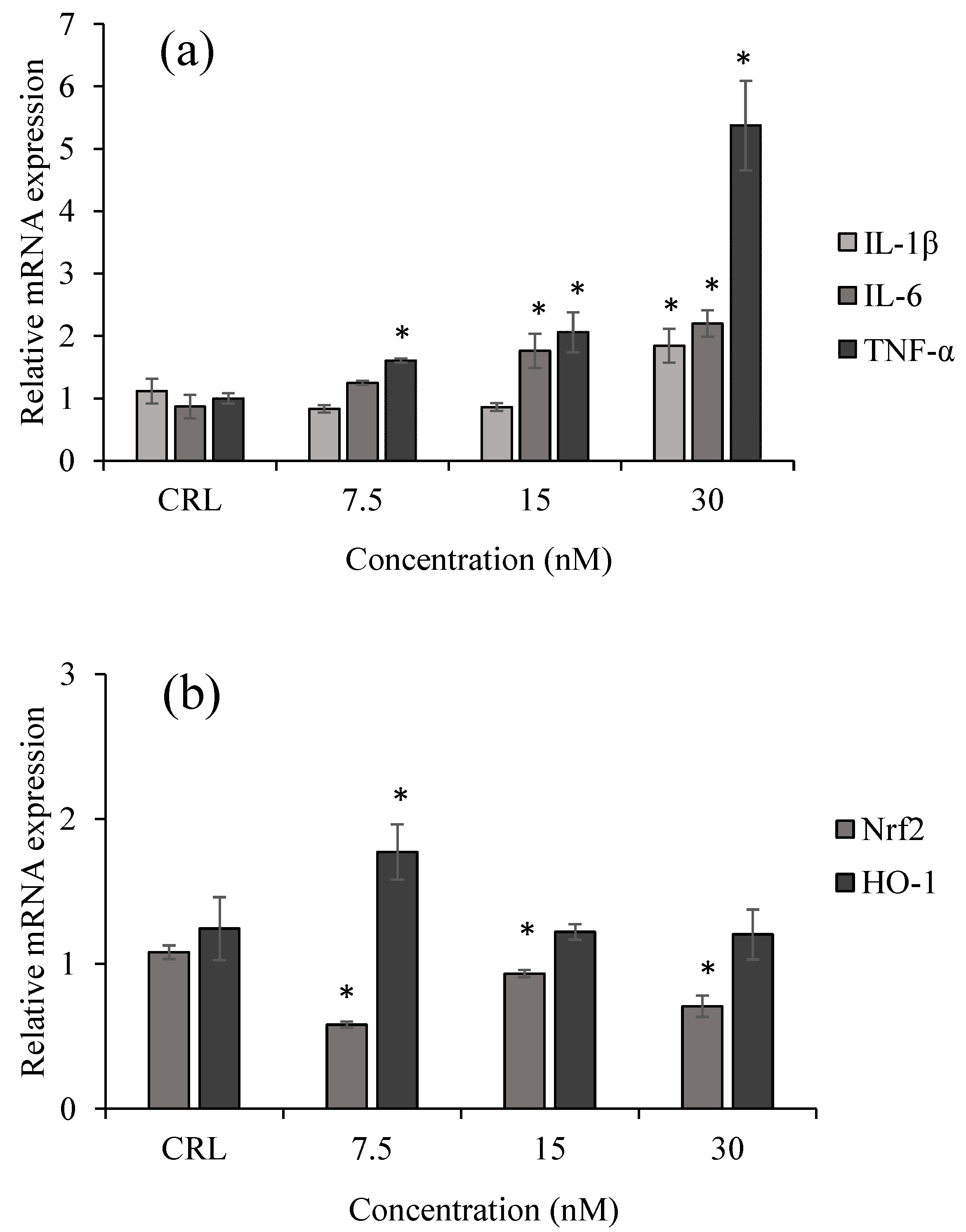
2.2. Inflammatory and Oxidative Response of HepG2 Exposed to T-2 by qPCR
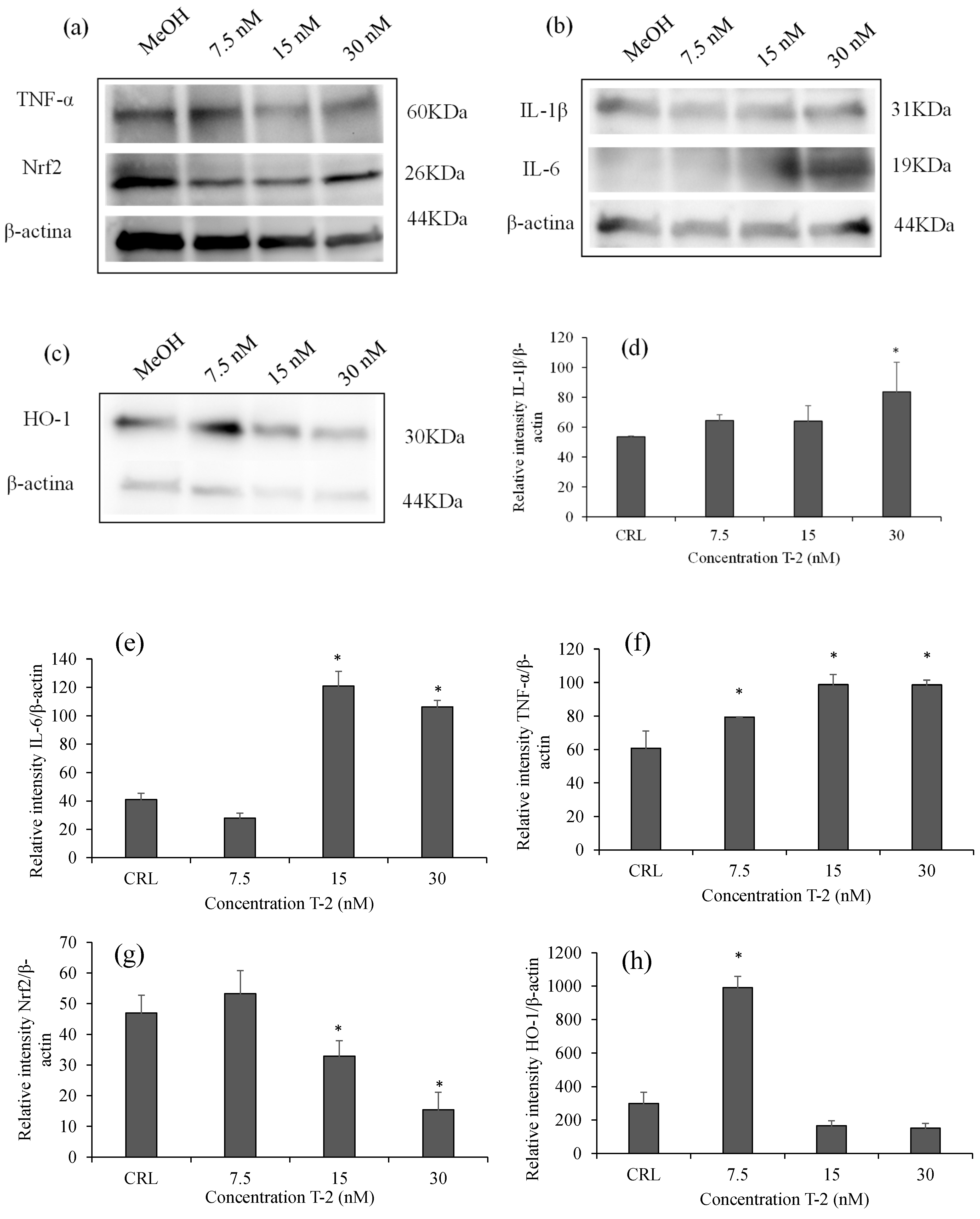
2.3. Effects of T-2 on Inflammatory and Oxidative Proteins by Western Blot
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Cell Culture and Treatment
5.3. Immunofluorescence Detection
5.4. RNA Extraction and Quantification
5.5. Reverse Transcription and qPCR Reaction
5.6. Western Blot Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | catalase |
| cDNA | complementary DNA |
| DAPI | 4′,6-diamidine-2′-phenylindole dihydrochloride |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | diMethyl SulfOxide |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GPx | Glutathione peroxidase |
| GST | Glutathione-S-transferase |
| HO-1 | Heme oxygenase-1 |
| IL-1β | Interleukin-1 β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| kDa | kilodaltons |
| mRNA | Messenger RNA |
| MTT | Methylthiazolte-trazolium salt |
| NBCS | Newborn Calf Serum |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | Nuclear factor erythroid type 2 |
| PBS | Phosphate-Buffered Saline |
| PVDF | Polyvinylidene difluoride |
| qPCR | quantitative polymerase chain reaction |
| ROS | Reactive oxygen species |
| SOD | Superoxido dismutase |
| T-2 | T-2 toxin |
| Tm | melting temperature |
| TNF-α | Tumor Necrosis Factor-α |
References
- Tolosa, J.; Barba, F.J.; Pallarés, N.; Ferrer, E. Mycotoxin Identification and In Silico Toxicity Assessment Prediction in Atlantic Salmon. Mar. Drugs 2020, 18, 629. [Google Scholar] [CrossRef]
- Toutounchi, N.S.; Hogenkamp, A.; Varasteh, S.; Land, B.V.; Garssen, J.; Kraneveld, A.D.; Folkerts, G.; Braber, S. Fusarium Mycotoxins Disrupt the Barrier and Induce IL-6 Release in a Human Placental Epithelium Cell Line. Toxins 2019, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 Toxin Neurotoxicity: Role of Oxidative Stress and Mitochondrial Dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; De Boevre, M.; Zhang, H.; De Ruyck, K.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Wang, Z.; Zhang, S.; et al. Metabolism of T-2 Toxin in Farm Animals and Human in Vitro and in Chickens in Vivo Using Ultra High-Performance Liquid Chromatography- Quadrupole/Time-of-Flight Hybrid Mass Spectrometry Along with Online Hydrogen/Deuterium Exchange Technique. J. Agric. Food Chem. 2017, 65, 7217–7227. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An Update on T-2 Toxin and Its Modified Forms: Metabolism, Immunotoxicity Mechanism, and Human Exposure Assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef] [PubMed]
- Kruber, P.; Trump, S.; Behrens, J.; Lehmann, I. T-2 Toxin Is a Cytochrome P450 1A1 Inducer and Leads to MAPK/P38-but Not Aryl Hydrocarbon Receptor-Dependent Interleukin-8 Secretion in the Human Intestinal Epithelial Cell Line Caco-2. Toxicology 2011, 284, 34–41. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, J.; Zhou, Z.; Zhou, H.; Xu, Y.; Zhang, Q.; Chen, C.; Pi, J. The Spatiotemporal and Paradoxical Roles of NRF2 in Renal Toxicity and Kidney Diseases. Redox Biol. 2025, 79, 103473. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Zeng, L.; Hu, Y.; Gao, R.; Xian, S.; Liao, S.; Huang, J.; Yang, Y.; Liu, J.; et al. Activation of Nrf2/HO-1 Signaling Pathway Exacerbates Cholestatic Liver Injury. Commun. Biol. 2024, 7, 621. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene Attenuates Colistin-Induced Nephrotoxicity in Mice via Activation of the Nrf2/HGo-1 Pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhong, Q.; Cui, H.; Xu, J.; Wei, W. Impact of T-2 Toxin on Intestinal Inflammation and Transcriptional Regulation of Inflammatory Response in Mouse Macrophages. Biochem. Biophys. Rep. 2024, 40, 101840. [Google Scholar] [CrossRef]
- Pei, X.; Ma, S.; Hong, L.; Zuo, Z.; Xu, G.; Chen, C.; Shen, Y.; Liu, D.; Li, C.; Li, D. Molecular Insights of T-2 Toxin Exposure-Induced Neurotoxicity and the Neuroprotective Effect of Dimethyl Fumarate. Food Chem. Toxicol. 2025, 196, 115166. [Google Scholar] [CrossRef]
- Wang, J.; Tao, X.; Liu, Z.; Yan, Y.; Cheng, P.; Liu, B.; Du, H.; Niu, B. Noncoding RNAs in Sepsis-Associated Acute Liver Injury: Roles, Mechanisms, and Therapeutic Applications. Pharmacol. Res. 2025, 212, 107596. [Google Scholar] [CrossRef]
- He, W.; Zhu, J.; Feng, Y.; Liang, F.; You, K.; Chai, H.; Sui, Z.; Hao, H.; Li, G.; Zhao, J.; et al. Neuromorphic-Enabled Video-Activated Cell Sorting. Nat. Commun. 2024, 15, 10792. [Google Scholar] [CrossRef]
- de Hoyos-Vega, J.M.; Hong, H.J.; Stybayeva, G.; Revzin, A. Hepatocyte Cultures: From Collagen Gel Sandwiches to Microfluidic Devices with Integrated Biosensors. APL Bioeng. 2021, 5, 041504. [Google Scholar] [CrossRef] [PubMed]
- Pomothy, J.M.; Szabó, O.; Czimmermann, Á.E.; Babiczky, Á.; Jerzsele, Á.; Pászti-Gere, E. Investigation of the Inflammatory and Oxidative Stress-Inducing Effects of Deoxynivalenol and T-2 Toxin Exposure in Non-Tumorigenic Human Intestinal Cell Model. Toxicon 2021, 200, 78–86. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Pászti, E.A.; Babiczky, Á.; Szóládi, Á.; Jerzsele, Á.; Gere, E.P. Beneficial Effects of Rosmarinic Acid on IPEC-J2 Cells Exposed to the Combination of Deoxynivalenol and T-2 Toxin. Mediat. Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Niu, R.; Guo, D.; Sun, Z. Mechanistic Insights into T-2 Toxin-Induced Thymic Epithelial Cell Injury and Immunotoxicity via the ROS-NF-ΚB-NLRP3 Signaling Axis. J. Agric. Food Chem. 2025, 73, 12961–12977. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dai, J.; Xu, J.; Yang, J.; Zhang, D. Comparative Cytotoxic Effects and Possible Mechanisms of Deoxynivalenol, Zearalenone and T-2 Toxin Exposure to Porcine Leydig Cells In Vitro. Toxins 2022, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Ruiz, M.J. T-2 Toxin and Its Metabolites: Characterization, Cytotoxic Mechanisms and Adaptive Cellular Response in Human Hepatocarcinoma (HepG2) Cells. Food Chem. Toxicol. 2020, 145, 111654. [Google Scholar] [CrossRef]
- Taroncher, M.; Halbig, F.; Rodríguez-Carrasco, Y.; Ruiz, M.J. Stressful Effects of T-2 Metabolites and Defense Capability of HepG2 Cells. Toxins 2022, 14, 841. [Google Scholar] [CrossRef]
- Pei, X.; Jiang, H.; Liu, X.; Li, L.; Li, C.; Xiao, X.; Li, D.; Tang, S. Targeting HMGB1 Inhibits T-2 Toxin-Induced Neurotoxicity via Regulation of Oxidative Stress, Neuroinflammation and Neuronal Apoptosis. Food Chem. Toxicol. 2021, 151, 112134. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Huang, T.; Liu, Y.; Chen, F.; Chen, Y.; Jiang, Y.; Zhang, C.; Yang, X. T-2 Toxin Metabolism and Its Hepatotoxicity: New Insights on the Molecular Mechanism and Detoxification. Environ. Poll. 2023, 330, 121784. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Yuan, F.; Lin, H.; Pavase, T.R. Effects of Brown Seaweed Polyphenols, α-Tocopherol, and Ascorbic Acid on Protein Oxidation and Textural Properties of Fish Mince (Pagrosomus Major) during Frozen Storage. J. Sci. Food Agric. 2017, 97, 1102–1107. [Google Scholar] [CrossRef]
- Janik-Karpinska, E.; Ceremuga, M.; Niemcewicz, M.; Synowiec, E.; Sliwinski, T.; Stela, M.; Bijak, M. DNA Damage Induced by T-2 Mycotoxin in Human Skin Fibroblast Cell Line—Hs68. Int. J. Mol. Sci. 2023, 24, 14458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Velkov, T.; Tang, S.; Dai, C. T-2 Toxin-Induced Toxicity in Neuroblastoma-2a Cells Involves the Generation of Reactive Oxygen, Mitochondrial Dysfunction and Inhibition of Nrf2/HO-1 Pathway. Food Chem. Toxicol. 2018, 114, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Deng, F.; Chen, Y.; Kong, Y.; Pan, L.; Liao, Q.; Rao, Z.; Xie, L.; Yao, C.; Li, S.; et al. NF-ΚB Pathway Activation during Endothelial-to-Mesenchymal Transition in a Rat Model of Doxorubicin-Induced Cardiotoxicity. Biomed. Pharmacother. 2020, 130, 110525. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, H.; Dong, H.; Qi, F.; Zhang, B.; Yu, Q.; Lin, B.; Jiang, H.; Du, H.; Liu, Y.; et al. A Preliminary Study of T-2 Toxin That Cause Liver Injury in Rats via the NF-KB and NLRP3-Mediated Pyroptosis Pathway. Toxicon 2024, 249, 108060. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Qiu, M.; Sun, L.; Wang, X.; Li, C.; Xu, D.; Gooneratne, R. Cytotoxicity of T-2 and Modified T-2 Toxins: Induction of JAK/STAT Pathway in RAW264.7 Cells by Hepatopancreas and Muscle Extracts of Shrimp Fed with T-2 Toxin. Toxicol. Res. 2017, 6, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Huang, T.; Chen, Y.; Song, W.; Chen, F.; Jiang, Y.; Zhang, C.; Yang, X. Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin. Toxics 2023, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, W.; Chu, F.; Wang, S.; Ji, Y.; Liu, Z.; Yu, H.; Qin, S.; Sun, D.; Jiao, Z.; et al. Hesperetin Attenuates T-2 Toxin-Induced Chondrocyte Injury by Inhibiting the P38 MAPK Signaling Pathway. Nutrients 2024, 16, 3107. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, X.; Zhang, L.; Wang, B.; Li, P.; Cheng, B.; Liang, C.; Ma, M.; Guo, X.; Zhang, F.; et al. An Integrative Analysis of DNA Methylation and Transcriptome Showed the Dysfunction of MAPK Pathway Was Involved in the Damage of Human Chondrocyte Induced by T-2 Toxin. BMC Mol. Cell Biol. 2022, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, W.; Zhang, K.; Wang, Y.; Chen, F.; Chen, Y.; Huang, T.; Jiang, Y.; Wang, X.; Zhang, C. P38 Mediates T-2 Toxin-Induced Leydig Cell Testosterone Synthesis Disorder. Ecotoxicol. Environ. Saf. 2023, 253, 114695. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Wang, F.; Chen, Z. Bakuchiol Induces Apoptosis in Human Hepatocellular Carcinoma Cells HepG2 via Enhancing Bcl-2/Bax/Cyc-t/Caspase-3 Signaling Pathway. Int. J. Pharmacol. 2024, 20, 862–873. [Google Scholar] [CrossRef]
- Wu, D.; Tan, H.; Gao, B.; Tang, X.; Dai, Y.; Li, Y. Effects of Dexmedetomidine on Proliferation, Invasion, Migration and Angiogenesis of Hypoxia-Induced HepG2 Liver Cancer Cells. Int. J. Pharmacol. 2024, 20, 1125–1134. [Google Scholar] [CrossRef]
- Qiao, H.; Ren, H.; Liu, Q.; Jiang, Y.; Wang, Q.; Zhang, H.; Gan, L.; Wang, P.; Cui, Y.; Wang, J.; et al. Anti-Inflammatory Effects of Rehmannia Glutinosa Polysaccharide on LPS-Induced Acute Liver Injury in Mice and Related Underlying Mechanisms. J. Ethnopharmacol. 2025, 351, 120099. [Google Scholar] [CrossRef]
- Li, B.; Gao, Q.; Wu, Y.; Lei, H.; Ma, S.; Wang, W.; Sun, H.; Ma, W.; Zheng, J.; Yuan, C.; et al. Broad-Spectrum Anti-Inflammatory and Antioxidant Therapy of Inflammatory-Storm Actuated Diseases via Programmable Self-Derived Cryo-Dead Neutrophils. Chem. Eng. J. 2025, 507, 160643. [Google Scholar] [CrossRef]
- Tiam, S.K.; Comte, K.; Dalle, C.; Duval, C.; Pancrace, C.; Gugger, M.; Marie, B.; Yéprémian, C.; Bernard, C. Development of a New Extraction Method Based on High-Intensity Ultra-Sonication to Study RNA Regulation of the Filamentous Cyanobacteria Planktothrix. PLoS ONE 2019, 14, e0222029. [Google Scholar] [CrossRef]
- Al-Adsani, A.M.; Barhoush, S.A.; Bastaki, N.K.; Al-Bustan, S.A.; Al-Qattan, K.K. Comparing and Optimizing RNA Extraction from the Pancreas of Diabetic and Healthy Rats for Gene Expression Analyses. Genes 2022, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ávila, J.A.; De La Puente, R.; Catalina, P.; Rejón, J.D.; Espín-Vallejo, L.; Valdivieso, V.; Aguilar-Quesada, R. Evaluation of RNA Purification Methods by Using Different Blood Stabilization Tubes: Identification of Key Features for Epidemiological Studies. BMC Res. Notes 2020, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Sabat, J.; Subhadra, S.; Rath, S.; Ho, L.M.; Kanungo, S.; Panda, S.; Mandal, M.C.; Dash, S.; Pati, S.; Turuk, J. Yielding Quality Viral RNA by Using Two Different Chemistries: A Comparative Performance Study. Biotechniques 2021, 71, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zuñiga, L.D.; Rodriguez-Anaya, L.Z.; Gonzalez-Galaviz, J.R.; Cruz-Mendívil, A.; Lares-Villa, F.; Lares-Jiménez, L.F. Evaluation and Standardization of RNA Extractions with Quality for RNA-Seq for Balamuthia Mandrillaris. Parasitologia 2024, 4, 199–208. [Google Scholar] [CrossRef]
- Rabilloud, T. Optimization of the cydex blue assay: A one-step colorimetric protein assay using cyclodextrins and compatible with detergents and reducers. PLoS ONE 2018, 13, e0195755. [Google Scholar] [CrossRef]
| RefSeq Accession (NM_) | Gene Symbol | Gene Name | Optimum Tª (°C) | Forward Primer/Revers Primer |
|---|---|---|---|---|
| NM_000576.3 | IL-1β | Interleukin-1 β | 60 | 5′-GGAGAATGACCTGAGCACCT-3′ 3′-GGAGGTGGAGAGCTTTCAGT-5′ |
| NM_00600.5 | IL-6 | Interleukin-6 | 60 | 5′-AGTCCTGATCCAGTTCCTGC-3′ 3′-CTACATTTGCCGAAGAGCCC-5′ |
| NM_00594.4 | TNF-α | Tumor Necrosis Factor-α | 58 | 5′-CCGACTATCTCGACTTTGCC-3′ 3′-ACAGGGCAATGATCCCAAAG-5′ |
| NM_001145412.3 | Nrf2 | Nuclear factor erythroid type 2 | 62 | 5′-TCCAGTCAGAAACCAGTGGAT-3′ 3′-GAATGTCTGCGCCAAAAGCTG-5′ |
| NM_002133.3 | HO-1 | Heme oxygenase-1 | 60 | 5′-AAGACTGCGTTCCTGCTCAAC-3′ 3′-AAAGCCCTACAGCAACTGTCG-5′ |
| NM_022551.3 | rS18 | Ribosomal S18 | 58 | 5′-CGGCTACCACATCCAAGGAA-3′ 3′-GCTGGAATTACCGCGGCT-5′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taroncher, M.; Franco-Campos, F.; Rodríguez-Carrasco, Y.; Ruiz, M.-J. T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways. Toxins 2025, 17, 397. https://doi.org/10.3390/toxins17080397
Taroncher M, Franco-Campos F, Rodríguez-Carrasco Y, Ruiz M-J. T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways. Toxins. 2025; 17(8):397. https://doi.org/10.3390/toxins17080397
Chicago/Turabian StyleTaroncher, Mercedes, Felipe Franco-Campos, Yelko Rodríguez-Carrasco, and María-José Ruiz. 2025. "T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways" Toxins 17, no. 8: 397. https://doi.org/10.3390/toxins17080397
APA StyleTaroncher, M., Franco-Campos, F., Rodríguez-Carrasco, Y., & Ruiz, M.-J. (2025). T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways. Toxins, 17(8), 397. https://doi.org/10.3390/toxins17080397






