Anticancer Activity of Snake Venom Against Breast Cancer: A Scoping Review
Abstract
1. Introduction
2. Results
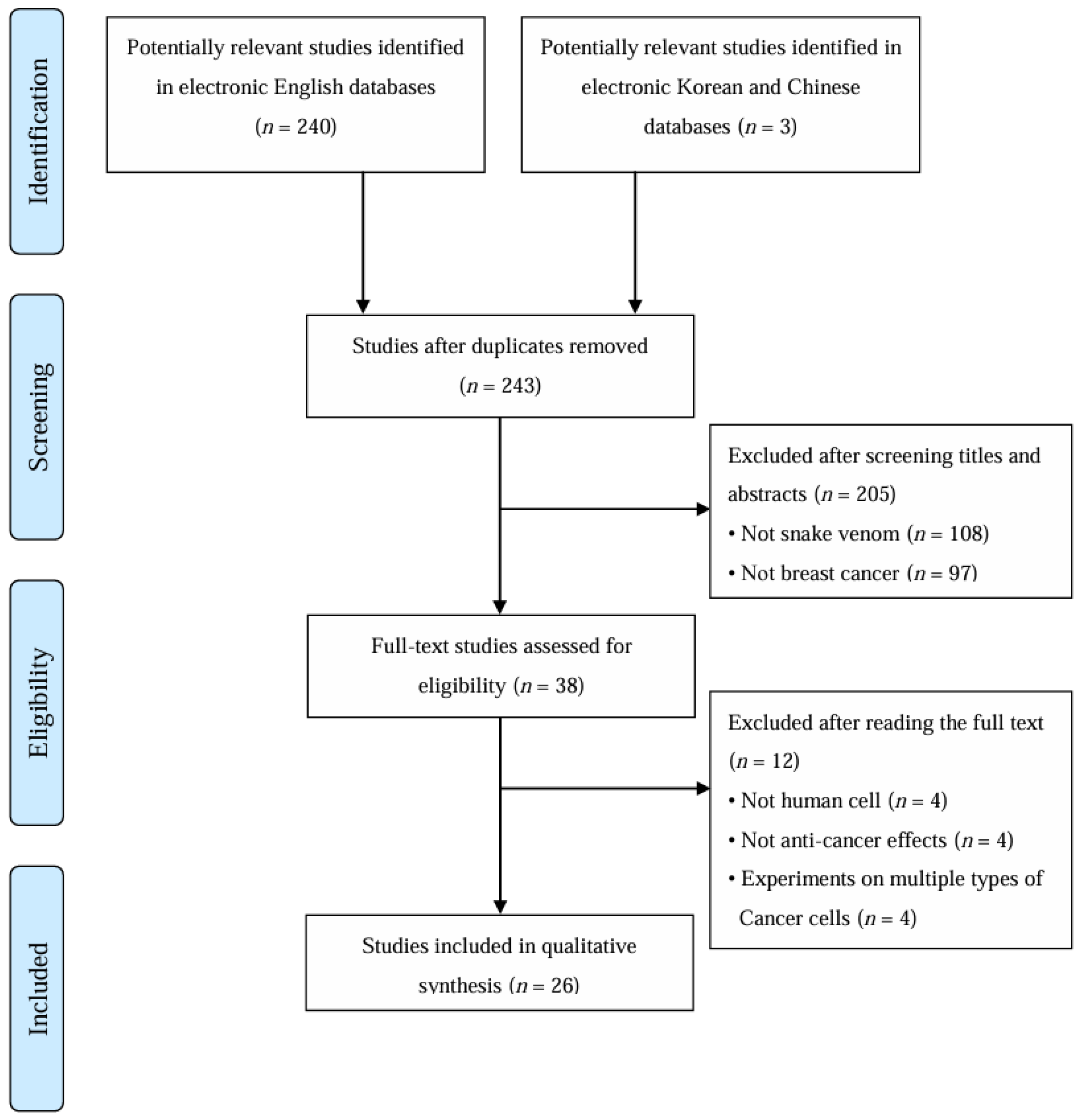
2.1. Study Description
2.2. Analysis of Experimental Methods
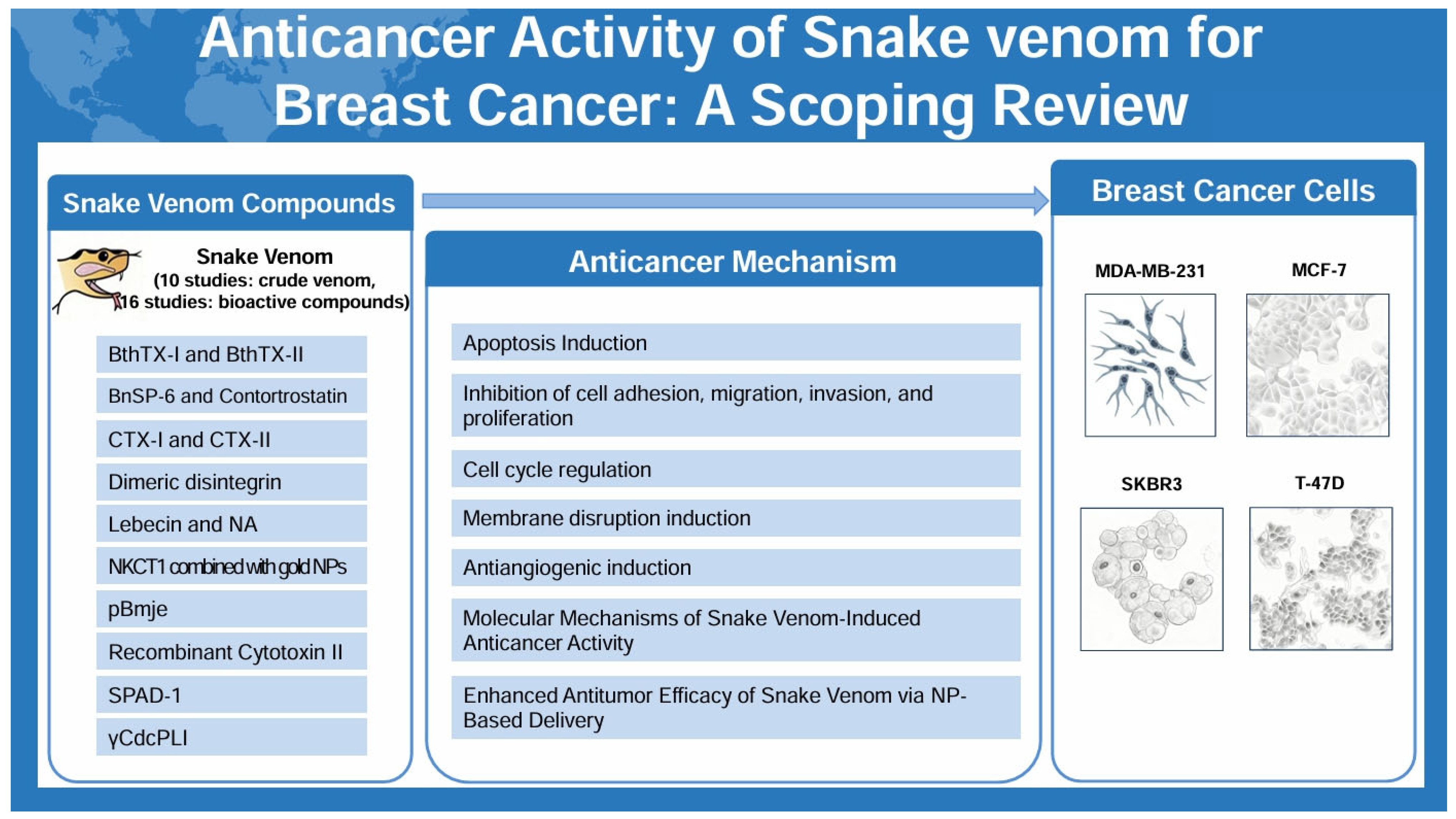
2.3. Analysis of Snake Venom
2.4. Anticancer Activity
2.5. Anticancer Mechanism
2.5.1. Apoptosis Induction
2.5.2. Inhibition of Cell Adhesion, Migration, Invasion, and Proliferation
2.5.3. Cell Cycle Regulation
2.5.4. Membrane Disruption Induction
2.5.5. Antiangiogenic Induction
2.5.6. Molecular Mechanisms of Snake Venom-Induced Anticancer Activity
2.5.7. Enhanced Antitumor Efficacy of Snake Venom via NP-Based Delivery
3. Discussion
3.1. Main Finding and Its Implication
3.2. Study Strength and Limitation
3.3. Future Perspective
4. Conclusions
5. Materials and Methods
5.1. Study Design and Registration
5.2. Data Sources and Searches
5.3. Study Selection
5.4. Data Extraction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HUVEC | Human umbilical vein endothelial cell |
| SPAD-1 | Serine Proteinase-Associated Disintegrin-1 |
| NP | Nanoparticle |
| LDH | Lactate dehydrogenase |
| MMP | Matrix metalloproteinase |
| ROS | Reactive oxygen species |
| CFSE | Carboxyfluorescein succinimidyl ester |
| bFGF | Basic fibroblast growth factor |
| EMT | Epithelial–mesenchymal transition |
| VEGF | Vascular endothelial growth factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, K.; Shirazi, F.H.; Vatanpour, H.; Zare, A.; Kobarfard, F.; Rabiei, H. Anticancer Activity of Cobra Venom Polypeptide, Cytotoxin-II, against Human Breast Adenocarcinoma Cell Line (MCF-7) via the Induction of Apoptosis. J. Breast Cancer 2014, 17, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R.; Esteva, F.J. HER2 Therapy: Molecular Mechanisms of Trastuzumab Resistance. Breast Cancer Res. 2006, 8, 215. [Google Scholar] [CrossRef]
- Lee, K.L.; Kuo, Y.C.; Ho, Y.S.; Huang, Y.H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.; Zuliani, J.P.; et al. Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. BioMed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef]
- Offor, B.C.; Piater, L.A. Snake Venom Toxins: Potential Anticancer Therapeutics. J. Appl. Toxicol. 2024, 44, 666–685. [Google Scholar] [CrossRef]
- Ramesh, D.; Bakkannavar, S.M.; Kumar, G.; Bhat, V.R. Exploring Snake Venom-Derived Molecules for Cancer Treatment: Challenges and Opportunities. J. Appl. Pharm. Sci. 2025, 15, 8–22. [Google Scholar] [CrossRef]
- Guo, X.; Fu, Y.; Peng, J.; Fu, Y.; Dong, S.; Ding, R.B.; Qi, X.; Bao, J. Emerging Anticancer Potential and Mechanisms of Snake Venom Toxins: A Review. Int. J. Biol. Macromol. 2024, 269 Pt 1, 131990. [Google Scholar] [CrossRef]
- Abdallah, S.; Abdel-Halim, K.Y.; Alm-Eldeen, A. Anticancer potency of Egyptian venom snakes on MCF-7 and HepG2 carcinoma cells. Environ. Anal. Health Toxicol. 2024, 39, e2024001. [Google Scholar] [CrossRef]
- Marinho, A.D.; da Silva, E.L.; Portilho, A.J.S.; de Oliveira, L.L.B.; Bezerra, C.A.; Nogueira, M.D.; Leitão-Araújo, M.; Machado-Alves, M.L.; Neto, C.C.; Ferreira, R.S., Jr.; et al. Three Snake Venoms from Bothrops Genus Induced Apoptosis and Cell Cycle Arrest in K562 Human Leukemic Cell Line. Toxicon 2024, 238, 107547. [Google Scholar] [CrossRef]
- Ghodeif, S.K.; El-Fahla, N.A.; Abdel-Rahman, M.A.; El-Shenawy, N.S. Arthropod venom peptides: Pioneering nanotechnology in cancer treatment and drug delivery. Cancer Pathog. Ther. 2025. [Google Scholar] [CrossRef]
- da Silva, J.R.; Castro-Amorim, J.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. The Application of Snake Venom in Anticancer Drug Discovery: An Overview of the Latest Developments. Expert Opin. Drug Discov. 2025, 20, 317–335. [Google Scholar] [CrossRef]
- Hboub, H.; Ben Mrid, R.; Bouchmaa, N.; Oukkache, N.; El Fatimy, R. An in-depth exploration of snake venom-derived molecules for drug discovery in advancing antiviral therapeutics. Heliyon 2024, 10, e37321. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.K.; Brahmbhatt, K.; Bhatt, H.; Parmar, U. Therapeutic Potential of Snake Venom in Cancer Therapy: Current Perspectives. Asian Pac. J. Trop. Biomed. 2013, 3, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Riyasdeen, A.; Al-Shahrani, M.H.; Islam, M. Snake Venom Causes Apoptosis by Increasing the Reactive Oxygen Species in Colorectal and Breast Cancer Cell Lines. OncoTargets Ther. 2016, 9, 6485–6498. [Google Scholar] [CrossRef]
- Al-Sadoon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; Badr, G. Induction of Apoptosis and Growth Arrest in Human Breast Carcinoma Cells by a Snake (Walterinnesia aegyptia) Venom Combined with Silica Nanoparticles: Crosstalk between Bcl2 and Caspase 3. Cell. Physiol. Biochem. 2012, 30, 653–665. [Google Scholar] [CrossRef]
- Bezerra, P.H.A.; Ferreira, I.M.; Franceschi, B.T.; Bianchini, F.; Ambrósio, L.; Cintra, A.C.O.; Sampaio, S.V.; de Castro, F.A.; Torqueti, M.R. BthTX-I from Bothrops jararacussu Induces Apoptosis in Human Breast Cancer Cell Lines and Decreases Cancer Stem Cell Subpopulation. J. Venom. Anim. Toxins Trop. Dis. 2019, 25, e20190010. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Kolvekar, N.; Mondal, S.; Sarkar, A.; Chakrabarty, D. SPAD-1, a Serine Proteinase Associated Disintegrin from Russell’s Viper Venom Disrupts Adhesion of MCF7 Human Breast Cancer Cells. Toxicon 2023, 221, 106979. [Google Scholar] [CrossRef]
- Bhowmik, T.; Gomes, A. Down-Regulation of Cyclin-Dependent Kinase-4 and MAPK through Estrogen Receptor Mediated Cell Cycle Arrest in Human Breast Cancer Induced by Gold Nanoparticle Tagged Toxin Protein NKCT1. Chem. Biol. Interact. 2017, 268, 119–128. [Google Scholar] [CrossRef]
- Derakhshani, A.; Silvestris, N.; Hajiasgharzadeh, K.; Mahmoudzadeh, S.; Fereidouni, M.; Paradiso, A.V.; Brunetti, O.; Atarod, D.; Safarpour, H.; Baradaran, B. Expression and Characterization of a Novel Recombinant Cytotoxin II from Naja naja oxiana Venom: A Potential Treatment for Breast Cancer. Int. J. Biol. Macromol. 2020, 162, 1283–1292. [Google Scholar] [CrossRef]
- Erlista, G.P.; Ahmed, N.; Swasono, R.T.; Raharjo, S.; Raharjo, T.J. Proteome of Monocled Cobra (Naja kaouthia) Venom and Potent Anti-Breast Cancer Peptide from Trypsin Hydrolyzate of the Venom Protein. Saudi Pharm. J. 2023, 31, 1115–1124. [Google Scholar] [CrossRef]
- Gallego-Londoño, V.; Santa-González, G.A.; Giraldo-Lorza, J.M.; Rojas, M.; Wisman, G.B.A.; de Jong, S.; Manrique-Moreno, M. Crotalicidin and NA-CATH-ATRA-1-ATRA-1 Peptide-Induced Membrane Disruption in Human Breast Cancer Cells. Biochim. Biophys. Acta Biomembr. 2025, 1867, 184429. [Google Scholar] [CrossRef]
- Gimenes, S.N.C.; Lopes, D.S.; Alves, P.T.; Azevedo, F.V.P.V.; Vecchi, L.; Goulart, L.R.; Rodrigues, T.C.S.; Santos, A.L.Q.; Brites, V.L.C.; Teixeira, T.L.; et al. Antitumoral Effects of γCdcPLI, a PLA2 Inhibitor from Crotalus durissus collilineatus via PI3K/Akt Pathway on MDA-MB-231 Breast Cancer Cell. Sci. Rep. 2017, 7, 7077. [Google Scholar] [CrossRef] [PubMed]
- Hiu, J.J.; Yap, M.K.K. The Effects of Naja sumatrana Venom Cytotoxin, sumaCTX, on Alteration of the Secretome in MCF-7 Breast Cancer Cells Following Membrane Permeabilization. Int. J. Biol. Macromol. 2021, 184, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Jebali, J.; Fakhfekh, E.; Morgen, M.; Srairi-Abid, N.; Majdoub, H.; Gargouri, A.; El Ayeb, M.; Luis, J.L.; Marrakchi, N.; Sarray, S. Lebecin, a New C-Type Lectin-Like Protein from Macrovipera lebetina Venom with Anti-Tumor Activity Against the Breast Cancer Cell Line MDA-MB231. Toxicon 2014, 86, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Canale, J.; Fernández-Quiroz, D.; Teran-Saavedra, N.G.; Diaz-Galvez, K.R.; Gallegos-Tabanico, A.; Burgara-Estrella, A.J.; Sarabia-Sainz, H.M.; Guzman-Partida, A.M.; Robles-Burgueño, M.R.; Vazquez-Moreno, L.; et al. Cytotoxic Activity of Snake Venom-Loaded Chitosan Nanoparticles Against T-47D Breast Carcinoma Cells. Acta Biochim. Pol. 2022, 69, 233–243. [Google Scholar] [CrossRef]
- Kisaki, C.Y.; Arcos, S.S.S.; Montoni, F.; da Silva Santos, W.; Calacina, H.M.; Lima, I.F.; Cajado-Carvalho, D.; Ferro, E.S.; Nishiyama-Jr, M.Y.; Iwai, L.K. Bothrops jararaca Snake Venom Modulates Key Cancer-Related Proteins in Breast Tumor Cell Lines. Toxins 2021, 13, 519. [Google Scholar] [CrossRef]
- Latinović, Z.; Leonardi, A.; Petan, T.; Žlajpah, M.; Križaj, I. Disintegrins from the Venom of Vipera ammodytes ammodytes Efficiently Inhibit Migration of Breast Cancer Cells. Acta Chim. Slov. 2017, 64, 555–559. [Google Scholar] [CrossRef][Green Version]
- Malekara, E.; Pazhouhi, M.; Rashidi, I.; Jalili, C. Anti-Proliferative and Cytotoxic Effect of Iranian Snake (Vipera raddei kurdistanica) Venom on Human Breast Cancer Cells via Reactive Oxygen Species-Mediated Apoptosis. Res. Pharm. Sci. 2020, 15, 76–86. [Google Scholar] [CrossRef]
- Silva, M.A.; Lopes, D.S.; Teixeira, S.C.; Gimenes, S.N.C.; Azevedo, F.V.P.V.; Polloni, L.; Borges, B.C.; da Silva, M.S.; Barbosa, M.J.; de Oliveira Júnior, R.J.; et al. Genotoxic Effects of BnSP-6, a Lys-49 Phospholipase A2 (PLA2) Homologue from Bothrops pauloensis Snake Venom, on MDA-MB-231 Breast Cancer Cells. Int. J. Biol. Macromol. 2018, 118 Pt A, 311–319. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Fu, Y.-S.; Chang, L.-S.; Lin, S.-R. Taiwan Cobra Cardiotoxin III Suppresses EGF/EGFR-Mediated Epithelial-to-Mesenchymal Transition and Invasion of Human Breast Cancer MDA-MB-231 Cells. Toxicon 2016, 111, 108–120. [Google Scholar] [CrossRef]
- Azevedo, F.V.P.V.; Lopes, D.S.; Gimenes, S.N.C.; Achê, D.C.; Vecchi, L.; Alves, P.T.; Guimarães, D.O.; Rodrigues, R.S.; Goulart, L.R.; Rodrigues, V.M.; et al. Human Breast Cancer Cell Death Induced by BnSP-6, a Lys-49 PLA2 Homologue from Bothrops pauloensis Venom. Int. J. Biol. Macromol. 2016, 82, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.V.P.V.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R.; et al. Antitumor and Antimetastatic Effects of PLA2-BthTX-II from Bothrops jararacussu Venom on Human Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.V.P.V.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; de Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. [Google Scholar] [CrossRef]
- Badr, G.; Al-Sadoon, M.K.; Rabah, D.M. Therapeutic Efficacy and Molecular Mechanisms of Snake (Walterinnesia aegyptia) Venom-Loaded Silica Nanoparticles in the Treatment of Breast Cancer- and Prostate Cancer-Bearing Experimental Mouse Models. Free Radic. Biol. Med. 2013, 65, 175–189. [Google Scholar] [CrossRef]
- Soliman, N.A.; Shalaby, A.A.; Mohamed, H.A.; Alashqar, S.M.A.; Ammar, M.A. Robust Anticancer Efficacy of Naja haje Venom-Loaded Silica Nanoparticles against Triple-Negative Breast Cancer Xenografts in a Preclinical Rat Model. Open Vet. J. 2024, 14, 3552–3562. [Google Scholar] [CrossRef]
- Swenson, S.; Costa, F.; Minea, R.; Sherwin, R.P.; Ernst, W.; Fujii, G.; Yang, D.; Markland, F.S., Jr. Intravenous Liposomal Delivery of the Snake Venom Disintegrin Contortrostatin Limits Breast Cancer Progression. Mol. Cancer Ther. 2004, 3, 499–511. [Google Scholar] [CrossRef]
- Badr, G.; Sayed, D.; Maximous, D.; Mohamed, A.O.; Gul, M. Increased Susceptibility to Apoptosis and Growth Arrest of Human Breast Cancer Cells Treated by a Snake Venom-Loaded Silica Nanoparticles. Cell. Physiol. Biochem. 2014, 34, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Jokhio, R.; Shaikh, D.M. Effect of Snake Venom on Nucleic Acids and Total Proteins in Various Normal and Cancerous Animal Tissues. Pak. J. Physiol. 2005, 1, 1–2. [Google Scholar]
- Peña-Carrillo, M.S.; Pinos-Tamayo, E.A.; Domínguez-Borbor, C.; Mendes, B.; Proaño-Bolaños, C.; Miguel, D.C.; Almeida, J.R. Dissection of Phospholipases A2 Reveals Multifaceted Peptides Targeting Cancer Cells, Leishmania and Bacteria. Bioorg. Chem. 2021, 114, 105041. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Yu, C.S.; Kim, T.; Yoo, K.H.; Kang, K. The T47D Cell Line Is an Ideal Experimental Model to Elucidate the Progesterone-Specific Effects of a Luminal A Subtype of Breast Cancer. Biochem. Biophys. Res. Commun. 2017, 486, 752–758. [Google Scholar] [CrossRef]
- Kunte, S.; Abraham, J.; Montero, A.J. Novel HER2-Targeted Therapies for HER2-Positive Metastatic Breast Cancer. Cancer 2020, 126, 4278–4288. [Google Scholar] [CrossRef]
- Barratt, G. Colloidal Drug Carriers: Achievements and Perspectives. Cell. Mol. Life Sci. 2003, 60, 21–37. [Google Scholar] [CrossRef]
- Saha, P.P.; Bhowmik, T.; Dasgupta, A.K.; Gomes, A. In Vivo and In Vitro Toxicity of Nanogold Conjugated Snake Venom Protein Toxin GNP-NKCT1. Toxicol. Rep. 2014, 1, 74–84. [Google Scholar] [CrossRef]
- Bhowmik, T.; Saha, P.P.; Dasgupta, A.; Gomes, A. Antileukemic Potential of PEGylated Gold Nanoparticle Conjugated with Protein Toxin (NKCT1) Isolated from Indian Cobra (Naja kaouthia) Venom. Cancer Nanotechnol. 2013, 4, 39–55. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers, Composites Renewable Resources; Gandini, A., Belgacem, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- Heiser, L.M.; Sadanandam, A.; Kuo, W.L.; Benz, S.C.; Goldstein, T.C.; Ng, S.; Gibb, W.J.; Wang, N.J.; Ziyad, S.; Tong, F.; et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, U.; Singh, R.; Sharma, S.; Kumar, S.; Singh, S. Nanoparticle-Based Drug Delivery Systems: A Review of Nanocarrier Technologies for Precision Medicine in Oncology. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Zhang, D.; He, X.; Wang, X.; Han, H.; Qin, Y. Nanoparticle-Based Drug Delivery Systems to Enhance Cancer Immunotherapy in Solid Tumors. Front. Immunol. 2023, 14, 1230893. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-Based Peptide Therapy: Insights into Anti-Cancer Mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef]
- Tan, C.H. Snake Venomics: Fundamentals, Recent Updates, and a Look to the Next Decade. Toxins 2022, 14, 247. [Google Scholar] [CrossRef]
- Jhade, S.K.; Kalidoss, K.; Pathak, P.K.; Shrivastava, R. Proteogenomic Approaches for Snake Venom Protein-Based Drug Development: Current Trends and Challenges. Transl. R. Soc. Trop. Med. Hyg. 2025, 119, 638–647. [Google Scholar] [CrossRef]
- Rakshit, T.; Pal, S. Extracellular Vesicles for Drug Delivery and Theranostics In Vivo. JACS Au 2024, 4, 318–327. [Google Scholar] [CrossRef]
- Alves, Á.E.F.; Barros, A.B.C.; Silva, L.C.F.; Carvalho, L.M.M.; Pereira, G.M.A.; Uchôa, A.F.C.; Barbosa-Filho, J.M.; Silva, M.S.; Luna, K.P.O.; Soares, K.S.R.; et al. Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview. Pharmaceutics 2025, 17, 204. [Google Scholar] [CrossRef]
- Dudda, L.A.; Kozula, M.; Ross-Hellauer, T.; Kormann, E.; Spijker, R.; DeVito, N.; Gopalakrishna, G.; Van den Eynden, V.; Onghena, P.; Naudet, F.; et al. Scoping Review and Evidence Mapping of Interventions Aimed at Improving Reproducible and Replicable Science: Protocol. Open Res. Eur. 2024, 3, 179. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
| Author (Year) Countries | Snake Venom | Target Cell, Animal Model | Mechanism | Main Results |
|---|---|---|---|---|
| Al-Asmari et al. (2016) [18] Saudi Arabia | Bitis arietans Cerastes gasperettii Echis coloratus Echis pyramidum | MDA-MB-231 | Apoptotic effects by increasing the ROS Anti-proliferative effects |
|
| Al-Sadoon et al. (2012) [19] Saudi Arabia | Walterinnesia aegyptia | MDA-MB-231 MCF-7 | Apoptotic effects Induction of growth arrest |
|
| Bezerra et al. (2019) [20] Brazil | Bothrops jararacussu | MDA-MB-231 MCF-7 SK-BR-3 | Apoptotic effects Autophagy effects Reduction in cancer stem cells subpopulation |
|
| Bhattacharya et al. (2023) [21] India | Russell’s viper | MCF-7 | Cytotoxic effects Inhibition of adhesion |
|
| Bhowmik et al. (2017) [22] India | Naja kaouthia | MDA-MB-231 MCF-7 | Apoptotic effects Anti-metastatic effects in MCF7 cells through estrogen receptor-mediated cell cycle arrest via MAPK pathway inhibition |
|
| Derakhshani et al. (2020) [23] Iran | Naja naja oxiana | MCF-7 | Cytotoxic effects Apoptotic effects Anti-proliferative effects Induction of cell cycle arrest |
|
| Erlista et al. (2023) [24] Indonesia | Naja kaouthia | MCF-7 | Characterize and identify peptides from the snake venom of Naja kaouthia as anticancer |
|
| Gallego-Londoño et al. (2025) [25] Colombia | Crotalus durissus Naja atra | MDA-MB-231 MCF-7 | Cytotoxic effects Induction of membrane disruption effects |
|
| Gimenes et al. (2017) [26] Brazil | Crotalus durissus collilineatus | MDA-MB-231 MCF-7 HUVEC | Cytotoxic effects Anti-metastatic effects Anti-angiogenic effects Anti-tumoral effects via PI3K/Akt pathway |
|
| BALB/c mice (6 wk) Aortic fragments | Anti-angiogenic effects |
| ||
| Hiu et al. (2021) [27] Malaysia | Naja sumatrana | MCF-7 | Necroptosis effects Induction of membrane permeabilization and loss of membrane integrity |
|
| Jebali et al. (2014) [28] Tunisia | Macrovipera lebetina | MDA-MB-231 | Anti-proliferative effects Inhibition of adhesion and migration |
|
| Jimenez-Canale et al. (2022) [29] Mexico | Crotalus molossus molossus | T-47D | Cytotoxic effects |
|
| Kisaki et al. (2021) [30] Brazil | Bothrops Jararaca | MDA-MB-231 MCF-7 | Describe the quantitative changes in proteomics of MCF7 and MDA-MB-231 cell lines treatment with Bothrops jararaca snake venom |
|
| Latinovi et al. (2017) [31] Slovenia | Vipera ammodytes ammodytes | MDA-MB-231 | Inhibition of migration Anti-metastatic effects |
|
| Malekara et al. (2020) [32] Iran | Vipera raddei kurdistanica | MDA-MB-231 MCF-7 | Cytotoxic effects and Anti-proliferation effects via ROS mediated apoptosis |
|
| Silva et al. (2018) [33] Brazil | Bothrops pauloensis | MDA-MB-231 MCF-7 | Cytotoxic effects Genotoxic effects Anti-proliferative effects Induction of cell cycle arrest |
|
| Tsai et al. (2016) [34] Taiwan | Naja naja atra | MDA-MB-231 | Inhibition of EGF/EGFR-mediated EMT and invasion |
|
| Van Petten de Vasconcelos Azevedo et al. (2016) [35] Brazil | Bothrops jararacussu | MDA-MB-231 | Cytotoxic effects Apoptosis effects Autophagy effects Inhibition of adhesion and migration Anti-angiogenic effects Anti-metastatic effects |
|
| Van Petten de Vasconcelos Azevedo et al. (2019) [36] Brazil | Bothrops jararacussu | MDA-MB-231 | Apoptosis effects Autophagy effects Induction of cell cycle arrest Anti-metastatic effects |
|
| Van Petten de Azevedo et al. (2022) [37] Brazil | Bothrops jararacussu | MDA-MB-231 HUVEC | Anti-angiogenic effects |
|
| Chick embryos (3 d) MDA-MB-231 |
| |||
| BALB/c mice (6 wk) Aortic fragments |
| |||
| Badr et al. (2013) [38] Saudi Arabia | Walterinnesia aegyptia | [Xenograft] BALB/c mice (10 wk, 22–25 g) MDA-MB-231 | Cytotoxic effects Apoptotic effects Anti-proliferative effects |
|
| Soliman et al. (2024) [39] Egypt | Naja haje | [Xenograft] Albino Wistar rats (7–9 wk, 100–120 g) MDA-MB-231 | Anti-cancer efficacy |
|
| Swenson et al. (2004) [40] United States | Agkistrodon contortrix contortrix | [Xenograft] Mice (5 wk) MDA-MB-231 | Anti-angiogenic effects Anti-proliferative effects |
|
| Badr et al. (2014) [41] Egypt | Walterinnesia aegyptia | Human Breast cancer tissue samples | Anti-proliferative effects |
|
| Jokhio et al. (2005) [42] Pakistan | Cobra | Human Breast cancerous tissues | Anti-proliferative effects |
|
| Peña-Carrillo et al. (2021) [43] Ecuador | Bothrops marajoensis | MCF-7 | Cytotoxic effects |
|
| Author (Year) | Species | Target Cell, Animal Model | IC50 [LC50] |
|---|---|---|---|
| Al-Asmari et al. (2016) [18] | Bitis arietans Cerastes gasperettii Echis coloratus Echis pyramidum | MDA-MB-231 | NR |
| Al-Sadoon et al. (2012) [19] | Walterinnesia aegyptia | MDA-MB-231 MCF-7 | 50 ng/mL (12 h) |
| Walterinnesia aegyptia combined with silica NPs | 20 ng/mL (12 h) | ||
| Badr et al. (2013) [38] | Walterinnesia aegyptia | [Xenograft] BALB/c mice (10 wk, 22–25 g) MDA-MB-231 | NR |
| Badr et al. (2014) [41] | Walterinnesia aegyptia | Human Breast cancer tissue samples | 50 ng/mL (12 h) |
| Walterinnesia aegyptia combined with silica NPs | 20 ng/mL (12 h) | ||
| Erlista et al. (2023) [24] | Naja kaouthia | MCF-7 | 4.17 μg/mL (25% methanol peptide fraction) |
| Jimenez-Canale et al. (2022) [29] | Crotalus molossus molossus | T-47D | 15.45 μg/mL |
| Jokhio et al. (2005) [42] | Cobra | Human Breast cancerous tissues | NR |
| Kisaki et al. (2021) [30] | Bothrops Jararaca | MDA-MB-231 | [4.76 μg/mL] |
| MCF-7 | [4.50 μg/mL] | ||
| Malekara et al. (2020) [32] | Vipera raddei kurdistanica | MDA-MB-231 | 20.29 μg/mL (24 h) 11.01 μg/mL (48 h) 5.99 μg/mL (72 h) 1.27 μg/mL (96 h) |
| MCF-7 | 18.53 μg/mL (24 h) 8.96 μg/mL (48 h) 2.14 μg/mL (72 h) 0.98 μg/mL (96 h) | ||
| Soliman et al. (2024) [39] | Naja haje | [Xenograft] Albino Wistar rats (7–9 wk, 100–120 g) MDA-MB-231 | [0.568 mg/kg] |
| Author (Year) | Species | Main Compound | Target cell | IC50 |
|---|---|---|---|---|
| Bezerra et al. (2019) [20] | Bothrops jararacussu | BthTX-I | MDA-MB-231 | >409 ± 5.34 μg/mL |
| MCF-7 | 104.35 ± 13.21 μg/mL | |||
| SKBR3 | 81.20 ± 8.58 μg/mL | |||
| Bhattacharya et al. (2023) [21] | Russell’s viper | SPAD-1 | MCF-7 | 0.41 μM (24 h) 0.21 μM (48 h) |
| Bhowmik et al. (2017) [22] | Naja kaouthia | NKCT1 combined with gold NPs | MDA-MB-231 MCF-7 | NR |
| Derakhshani et al. (2020) [23] | Naja naja oxiana | Recombinant Cytotoxin II | MCF-7 | 3.66 μg/mL |
| Gallego-Londoño et al. (2025) [25] | Crotalus durissus | Crotalicidin | MDA-MB-231 | 21.3 μM/mL |
| MCF-7 | 58.9 μM/mL | |||
| Naja atra | NA | MDA-MB-231 | 6.4 μM/mL | |
| MCF-7 | 13.4 μM/mL | |||
| Gimenes et al. (2017) [26] | Crotalus durissus collilineatus | γCdcPLI | MDA-MB-231 | 25 ± 1.72 μM/mL |
| MCF-7 | 28 ± 4.1 μM/mL | |||
| HUVEC | NR | |||
| Hiu et al. (2021) [27] | Naja sumatrana | CTX-I | MCF-7 | 29.80 ± 2.3 μg/mL (4 h) 19.33 ± 0.6 μg/mL (8 h) 8.15 ± 0.1 μg/mL (16 h) 9.99 ± 1.2 μg/mL (24 h) |
| Jebali et al. (2014) [28] | Macrovipera lebetina | Lebecin | MDA-MB-231 | NR |
| Latinovi et al. (2017) [31] | Vipera ammodytes ammodytes | Dimeric disintegrin | MDA-MB-231 | NR |
| Silva et al. (2018) [33] | Bothrops pauloensis | BnSP-6 | MDA-MB-231 | 52.24 μg/mL |
| MCF-7 | NR | |||
| Swenson et al. (2004) [40] United states | Agkistrodon contortrix contortrix | Contortrostatin | [Xenograft] Mice (5 wk) MDA-MB-231 | NR |
| Tsai et al. (2016) [34] | Naja naja atra | CTX-III | MDA-MB-231 | NR |
| Van Petten de Vasconcelos Azevedo et al. (2016) [35] | Bothrops pauloensis | BnSP-6 | MDA-MB-231 | NR |
| Van Petten de Vasconcelos Azevedo et al. (2019) [36] | Bothrops jararacussu | BthTX-II | MDA-MB-231 | NR |
| Van Petten de Vasconcelos Azevedo et al. (2022) [37] | Bothrops jararacussu | BthTX-II | MDA-MB-231 HUVEC | NR |
| Peña-Carrillo et al. (2021) [43] | Bothrops marajoensis | pBmje | MCF-7 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-J.; Park, J.-K.; Sung, S.-H.; Sung, H.-K. Anticancer Activity of Snake Venom Against Breast Cancer: A Scoping Review. Toxins 2025, 17, 477. https://doi.org/10.3390/toxins17100477
Kim E-J, Park J-K, Sung S-H, Sung H-K. Anticancer Activity of Snake Venom Against Breast Cancer: A Scoping Review. Toxins. 2025; 17(10):477. https://doi.org/10.3390/toxins17100477
Chicago/Turabian StyleKim, Eun-Jin, Jang-Kyung Park, Soo-Hyun Sung, and Hyun-Kyung Sung. 2025. "Anticancer Activity of Snake Venom Against Breast Cancer: A Scoping Review" Toxins 17, no. 10: 477. https://doi.org/10.3390/toxins17100477
APA StyleKim, E.-J., Park, J.-K., Sung, S.-H., & Sung, H.-K. (2025). Anticancer Activity of Snake Venom Against Breast Cancer: A Scoping Review. Toxins, 17(10), 477. https://doi.org/10.3390/toxins17100477





