Onabotulinumtoxin-A for Chronic Migraine in Children and Adolescents: A Narrative Review of Current Evidence and Clinical Perspectives
Abstract
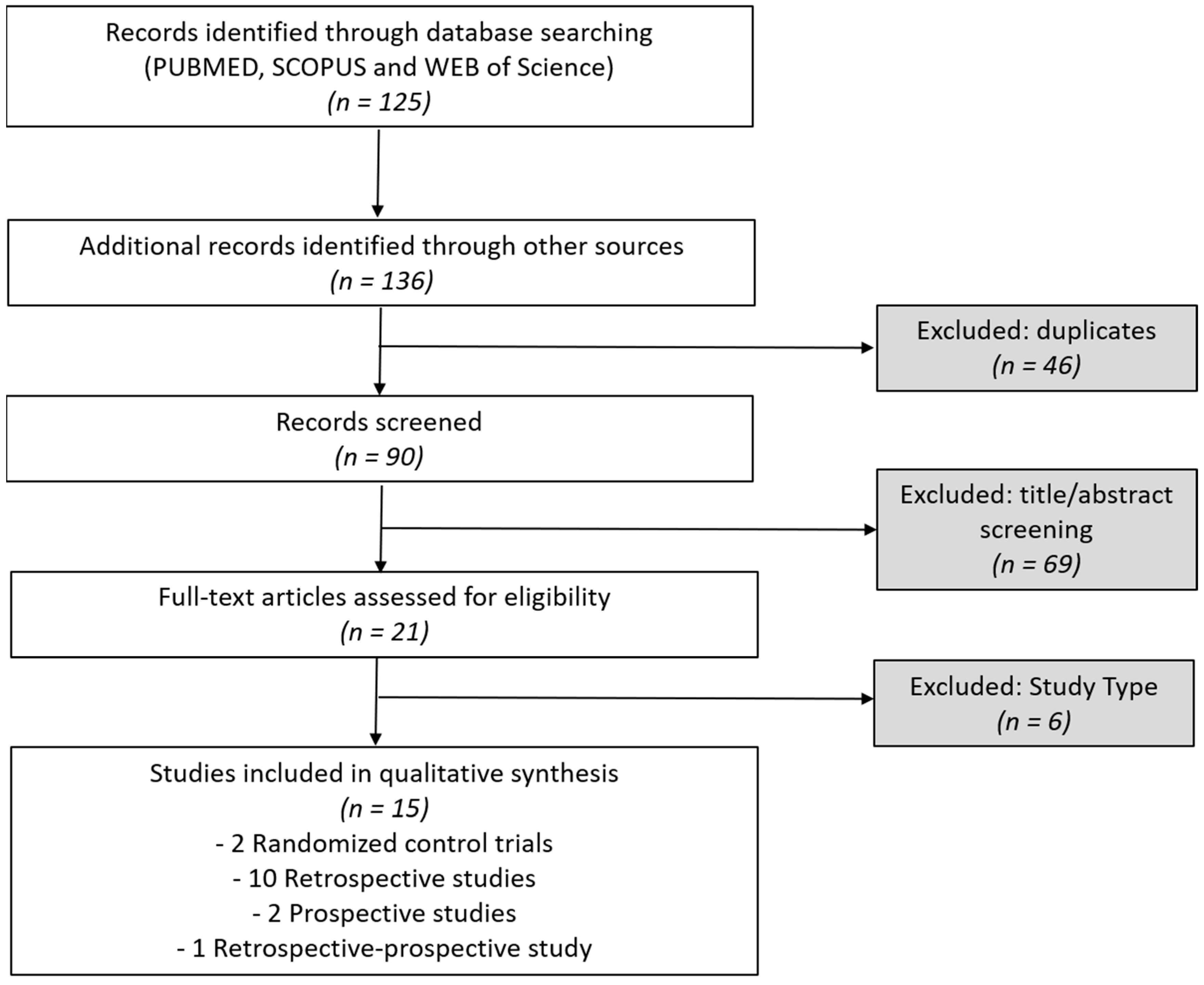
1. Introduction
2. Results
2.1. Results from Randomized Controlled Trials
2.2. Results from Non-Randomized Prospective Studies
2.3. Results from Retrospective Studies
3. Discussion
3.1. Practical Clinical Considerations
3.1.1. Dosing
3.1.2. Treatment Duration
3.1.3. Safety and Tolerability
3.1.4. Concomitant Therapy
3.1.5. Ethical and Regulatory Aspects
3.1.6. Research Gaps
3.1.7. Limitations
4. Conclusions
5. Materials and Methods
5.1. Eligibility Criteria
5.2. Information Sources
5.3. Search Strategy
5.4. Selection and Data Collection Process
5.5. Risk of Bias, Effect Measures, and Synthesis Approach
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ONA | Onabotulinumtoxin-A |
| ICHD-3 | International Classification of Headache Disorders, 3rd edition |
| FDA | Food and Drug Administration (FDA) |
| CM | Chronic Migraine |
| RCT | Randomized Controlled Trial |
| CGRP | Calcitonin Gene-Related Peptide |
| PREEMPT | Phase 3 Research Evaluating Migraine Prophylaxis Therapy |
| NSR | Numeric Rating Scale |
| PedMIDAS | Pediatric Migraine Disability Assessment |
| MHD | Monthly headache days |
| MMD | Monthly migraine days |
| AMDM | Acute medication days/month |
| GAD | Generalized Anxiety Disorder |
| PHQ-9 | Patient Health Questionnaire 9-item scale |
| VAS | Visual analog scale |
| HIT 3 | Headache Impact Test |
| AEs | Adverse events |
| Sjbs | Subjects |
| Cy | Cycle |
| Min | Minimum |
| Max | Maximum |
| EMA | European medicines agency |
References
- Raggi, A.; Leonardi, M.; Arruda, M.; Caponnetto, V.; Castaldo, M.; Coppola, G.; Della Pietra, A.; Fan, X.; Garcia-Azorin, D.; Gazerani, P.; et al. Hallmarks of primary headache: Part 1—Migraine. J. Headache Pain 2024, 25, 189. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A.; Pensato, U.; Rosignoli, C.; Wells-Gatnik, W.; Stanyer, E.; Ornello, R.; Chen, H.Z.; De Santis, F.; Torrente, A.; Mikulenka, P.; et al. European Headache Federation School of Advanced Studies (EHF-SAS). Primary headache epidemiology in children and adolescents: A systematic review and meta-analysis. J. Headache Pain 2023, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Torriero, R.; Capuano, C.; Mariani, R.; Frusciante, R.; Tarantino, S.; Papetti, L.; Vigevano, F.; Valeriani, M. Diagnosis of primary headache in children younger than 6 years: A clinical challenge. Cephalalgia 2017, 37, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Salfa, I.; Battan, B.; Moavero, R.; Termine, C.; Bartoli, B.; Di Nunzio, F.; Tarantino, S.; Alaimo Di Loro, P.; Vigevano, F.; et al. Features of Primary Chronic Headache in Children and Adolescents and Validity of Ichd 3 Criteria. Front. Neurol. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Frattale, I.; Ferilli, M.A.N.; Ursitti, F.; Sforza, G.; Monte, G.; Proietti Checchi, M.; Tarantino, S.; Mazzone, L.; Valeriani, M.; Papetti, L. Unsatisfactory response to acute medications does not affect the medication overuse headache development in pediatric chronic migraine. J. Headache Pain 2024, 25, 61. [Google Scholar] [CrossRef]
- Tarantino, S.; Proietti Checchi, M.; Papetti, L.; Ursitti, F.; Sforza, G.; Ferilli, M.A.N.; Moavero, R.; Monte, G.; Capitello, T.G.; Vigevano, F.; et al. Interictal Cognitive Performance in Children and Adolescents With Primary Headache: A Narrative Review. Front. Neurol. 2022, 13, 898626. [Google Scholar] [CrossRef]
- Papetti, L.; Ursitti, F.; Moavero, R.; Ferilli, M.A.N.; Sforza, G.; Tarantino, S.; Vigevano, F.; Valeriani, M. Prophylactic Treatment of Pediatric Migraine: Is There Anything New in the Last Decade? Front. Neurol. 2019, 10, 771. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). TOPAMAX® (topiramate) Tablets and Sprinkle Capsules, for Oral Use: Prescribing Information; Silver Spring: Montgomery, MD, USA, 2014. Available online: https://www.fda.gov/media/100137/download (accessed on 22 August 2025).
- Özge, A.; Peres, M.F.P.; Burstein, R. Pediatric migraine care: Bridging gaps, overcoming barriers, and advancing solutions. Eur. J. Pediatr. 2025, 184, 362. [Google Scholar] [CrossRef]
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendtsen, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MaassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.D.; et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J. Headache Pain 2022, 23, 67. [Google Scholar] [CrossRef]
- Oliveira, R.; Gil-Gouveia, R.; Puledda, F. CGRP-targeted medication in chronic migraine—Systematic review. J. Headache Pain 2024, 25, 51. [Google Scholar] [CrossRef]
- Lindsay, R.; Kalifa, A.; Kuziek, J.; Kabbouche, M.; Hershey, A.D.; Orr, S.L. The safety and efficacy of onabotulinumtoxinA injections for children and adolescents with chronic migraine: A systematic review and meta-analysis. Headache 2024, 64, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinAfor treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. PREEMPT 2 Chronic Migraine Study Group Collaborators. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Shah, S.; Calderon, M.D.; Crain, N.; Pham, J.; Rinehart, J. Effectivenessof onabotulinumtoxinA (BOTOX) in pediatric patients experienc-ing migraines: A randomized, double- blinded, placebo-controlledcrossover study in the pediatric pain population. Reg. Anesth. Pain Med. 2021, 46, 41–48. [Google Scholar] [CrossRef]
- Winner, P.K.; Kabbouche, M.; Yonker, M.; Wangsadipura, V.; Lum, A.; Brin, M.F. A randomized trial to evaluate onabotulinumtoxinA for preven-tion of headaches in adolescents with chronic migraine. Headache 2020, 60, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Mavridi, A.; Redmond, A.; Archontakis-Barakakis, P.; Bogdanova-Mihaylova, P.; Deligianni, C.I.; Mitsikostas, D.D.; Mavridis, T. Onabotulinumtoxina in the prevention of migraine in pediatric population: A systematic review. Toxins 2024, 16, 295. [Google Scholar] [CrossRef]
- Gómez-Dabó, L.; Caronna, E.; Mas-de-Les-Valls, R.; Gallardo, V.J.; Alpuente, A.; Torres-Ferrus, M.; Pozo-Rosich, P. Effectiveness and Safety of OnabotulinumtoxinA in Adolescent Patients with Chronic Migraine. Toxins 2024, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Frattale, I.; Ursitti, F.; Sforza, G.; Monte, G.; Ferilli, M.A.D.; Tarantino, S.; Proietti Checchi, M.; Valeriani, M. Real life data on onabotulinumtoxinA for treatment of chronic migraine in pediatric age. J. Clin. Med. 2023, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.; Liu, C. Experience of Botulinum Toxin A Injections for Chronic Migraine Headaches in a Pediatric Chronic Pain Clinic. J. Pediatr. Pharmacol. Ther. 2021, 26, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.E.; Shields, J.M.; Young, W.W.C.; Eye, P.G. Botulinum Toxin for Pediatric Migraine: A Retrospective Multisite Cohort Study. Pediatr. Neurol. 2023, 147, 68–71. [Google Scholar] [CrossRef]
- Karian, V.; Morton, H.; Schefter, Z.J.; Smith, A.; Rogan, H.; Morse, B.; LeBel, A. OnabotulinumtoxinA for pediatric migraine. Pain Manag. Nurs. 2023, 24, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Bragin, I.; Rende, E.; Mejico, L.; Werner, K.E. Further evidence that onabotulinum toxin is a viable treatment option for pediatric chronic migraine patients. Cureus 2019, 11, e4343. [Google Scholar] [CrossRef]
- Akbar, A.; Ford, J.; Tripathi, S. The Use of Botulinum Toxin Type A in Medically Refractory Pediatric Patients With Chronic Daily Headaches and Its Impact on the Quality of Life. J. Child Neurol. 2024, 39, 55–60. [Google Scholar] [CrossRef]
- Goenka, A.; Yu, S.G.; George, M.C.; Chikkannaiah, M.; MacDonald, S.; Stolfi, A.; Kumar, G. Is Botox Right for Me: When to Assess the Efficacy of the Botox Injection for Chronic Migraine in Pediatric Population. Neuropediatrics 2022, 53, 344–350. [Google Scholar] [CrossRef]
- Goenka, A.; Yu, S.G.; Chikkannaiah, M.; George, M.C.; MacDonald, S.; Stolfi, A.; Kumar, G. Generalized Anxiety Disorder: A Predictor for Poor Responsiveness to Botulinum Toxin Type A Therapy for Pediatric Migraine. Pediatr. Neurol. 2022, 130, 21–27. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Huss, K.; Blaschek, A.; Koerte, I.K.; Zeycan, B.; Roser, T.; Langhagen, T.; Schwerin, A.; Berweck, S.; Reilich, P.; et al. Ten-year follow-up in a case series of integrative botulinum toxin intervention in adolescents with chronic daily headache and associated muscle pain. Neuropediatrics 2012, 43, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.; McCabe, E.J.; MacGregor, D.L. Botox treatment for migraine and chronic daily headache in adolescents. J. Neurosci. Nurs. 2009, 41, 235–243. [Google Scholar] [CrossRef]
- Ahmed, K.; Oas, K.H.; Mack, K.J.; Garza, I. Experience with botulinum toxin type A in medically intractable pediatric chronic daily headache. Pediatr. Neurol. 2010, 43, 316–319. [Google Scholar] [CrossRef]
- Shah, S.; Calderon, M.D.; Wu, W.D.; Grant, J.; Rinehart, J. Onabotulinumtoxin A (BOTOX®) for prophylactic treatment of pediatric migraine: A retrospective longitudinal analysis. J. Child Neurol. 2018, 33, 580–586. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. BOTOX® (OnabotulinumtoxinA) Prescribing Information. Silver Spring, MD: FDA; Revised 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf (accessed on 22 August 2025).
- Kabbouche, M.; O’Brien, H.; Hershey, A.D. OnabotulinumtoxinA in pediatric chronic daily headache. Curr. Neurol. Neurosci. Rep. 2012, 12, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, R.; Freund, B. The Efficacy of Botulinum Toxin in Pediatric Chronic Migraine: A Literature Review. J. Child Neurol. 2020, 35, 844–851. [Google Scholar] [CrossRef]
- Pieniak, M.; Höfer, B.; Knipping, J.; Faria, V.; Richter, M.; Schriever, V.A.; Haehner, A.; Gossrau, G. Children and adolescents with primary headaches exhibit altered sensory profiles—A multi-modal investigation. J. Headache Pain 2024, 25, 111. [Google Scholar] [CrossRef]
- Magdy, R.; Hassan, A.; Mohammed, Z.; Abdeltwab, M.A.; Ghaffar, N.F.A.; Hussein, M. Validity and reliability of Arabic version of pediatric migraine disability assessment scale (Child Self-Report versus Parent Proxy-Report): A multi-center study. J. Headache Pain 2024, 25, 15. [Google Scholar] [CrossRef]
- European Medicines Agency. Botox: EPAR—Product Information; EPAR: London, UK, 2022; Available online: https://www.ema.europa.eu/en/documents/psusa/botulinum-toxin-cmdh-scientific-conclusions-and-grounds-variation-amendments-product-information-and-timetable-implementation-psusa00000426202112_en.pdf (accessed on 22 August 2025).
- Dimitrova, R.; McCusker, E.; Gormley, M.; Fehlings, D.; Alter, K.E.; Greaves, S.; Liu, C.; Brin, M.F. Efficacy and safety of onabotulinumtoxinA with standardized occupational therapy for treatment of pediatric upper limb spasticity: Phase III placebo-controlled randomized trial. NeuroRehabilitation 2021, 49, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Berweck, S.; Bonikowski, M.; Kim, H.; Althaus, M.; Flatau-Baqué, B.; Mueller, D.; Banach, M.D. Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI. Neurology 2021, 97, e1425–e1436. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Rosich, P.; Alpuente, A.; Silberstein, S.D.; Burstein, R. Insights from 25 years of onabotulinumtoxinA in migraine—Mechanisms and management. Nat. Rev. Neurol. 2024, 20, 555–568. [Google Scholar] [CrossRef]
- Bendtsen, B.; Sacco, S.; Ashina, M.; Mitsikostas, D.; Ahmed, F.; Pozo-Rosich, P.; Martelletti, P. Guideline on the use of onabotulinumtoxinA in chronic migraine: A consensus statement from the European Headache Federation. J. Headache Pain 2018, 19, 91. [Google Scholar] [CrossRef]
- Engel, S.J.; Afifi, A.M.; Zins, J.E. Botulinum toxin injection pain relief using a topical anesthetic skin refrigerant. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1443–1446. [Google Scholar] [CrossRef]
- Alpuente, A.; Gallardo, V.J.; Torres-Ferrús, M.; Santos-Lasaosa, S.; Guerrero, A.L.; Laínez, J.M.; Viguera, J.; Gago-Veiga, A.; Irimia, P.; del Río, M.S.; et al. Evaluation of the concomitant use of oral preventive treatments and onabotulinumtoxinA in chronic migraine: The PREVENBOX study. Eur. J. Neurol. 2020, 27, 2102–2108. [Google Scholar] [CrossRef]
- Hepp, Z.; Rosen, N.L.; Gillard, P.G.; Varon, S.F.; Mathew, N.; Dodick, D.W. Comparative effectiveness of onabotulinumtoxinA versus oral migraine prophylactic medications on headache-related resource utilization in the management of chronic migraine: Retrospective analysis of a US-based insurance claims database. Cephalalgia 2016, 36, 862–874. [Google Scholar] [CrossRef]
- Domínguez, C.; Pozo-Rosich, P.; Torres-Ferrús, M.; Hernández-Beltrán, N.; Jurado-Cobo, C.; González-Oria, C.; Santos, S.; Monzón, M.J.; Latorre, G.; Álvaro, L.C.; et al. OnabotulinumtoxinA in chronic migraine: Predictors of response. A prospective multicentre descriptive study. Eur. J. Neurol. 2018, 25, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, S.; Papetti, L.; Di Stefano, A.; Messina, V.; Ursitti, F.; Ferilli, M.A.N.; Sforza, S.; Moavero, R.; Vigevano, F.; Gentile, S.; et al. Anxiety, Depression, and Body Weight in Children and Adolescents with Migraine. Front. Psychol. 2020, 11, 530911. [Google Scholar] [CrossRef] [PubMed]
- Moavero, R.; Stornelli, M.; Papetti, L.; Ursitti, F.; Ferilli, M.A.N.; Balestri, M.; Sforza, G.; Tarantino, S.; Vigevano, F.; Valeriani, M. Medication Overuse Withdrawal in Children and Adolescents Does Not Always Improve Headache: A Cross-Sectional Study. Front. Neurol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Negro, A.; Curto, M.; Lionetto, L.; Crialesi, D.; Martelletti, P. OnabotulinumtoxinA 155 U in medication overuse headache: A two years prospective study. Springerplus 2015, 30, 826. [Google Scholar] [CrossRef] [PubMed]
- Liberman, R. An analysis of the placebo phenomenon. J. Chronic Dis. 1962, 15, 761–783. [Google Scholar] [CrossRef]
- Abu-Arafeh, I.; Hershey, A.D.; Diener, H.C.; Tassorelli, C. Guidelines Update: Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine in children and adolescents, 1st edition—An experience-based update. Cephalalgia 2023, 43, 3331024231178239. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Author (Year) | Design | N° Patients (Age in Years); Weight or BMI | Protocol (Dose/Injections of ONA) | Main Outcomes | Efficacy | Concomitant Therapies | Adverse Events |
|---|---|---|---|---|---|---|---|
| Shah et al. (2021) [17] | Randomized crossover trial | 15 (8–17) | Fixed 31 site PREEMPT (155 U vs. placebo) 3 cycles | Baseline vs. ONA vs. Placebo period (median values): * MMD: 28 vs. 20 vs. 28 * Intensity Score: 8 vs. 5 vs. 7 * PedMIDAS: 4 vs. 3 vs. 4 Duration in hours: 24 vs. 10 vs. 24 | + | 87% of subjects Median: 3 | No serious AEs |
| Winner et al. (2020) [18] | Randomized controlled trial | 125 (12–17) | Fixed 31 site PREEMPT (155 U or 74 U vs. placebo) 1 cycle | After 12 weeks: 155 U vs. 74 U vs. placebo (mean) MHD: −6.3 vs. −6.4 vs. −6.8 | 0 | Not included | 155 U vs. 74 U vs. placebo treatment-related AE: 10% vs. 7% vs. 4% Serious AE: 1% vs. 2% vs. 0% (no treatment related) |
| Gómez-Dabó et al. (2024) [20] | Prospective study | 20 (14–17) | Fixed 31-site PREEMPT protocol + follow-the-pain (195 U in 39 sites) 1–2 cycles | After 6 and 12 months: * MHD (mean): −20; −17.5; Sbjs ≥ 50% of reduction in the attacks: 55%; 57.1%. | + | 20% (beta blockers; antidepressants) | No AEs reported |
| Papetti et al. (2023) [21] | Prospective study | 43 (12–17) | Fixed 31-site PREEMPT protocol + follow-the-pain (155–195 U) 1–4 cycles | MHD (mean): −5.25 (from baseline to cy 1); −4.3 (from cy 1 to cy 2); −2 (from cy 2 to cy3), -0.65 (from cy 3 to cy4) sbjs ≥ 50% of reduction in the attacks: 55.8 (after 3 cycles) | + | 65% of non-responders and 54% of responders (1 medication) | 32% AES: pruritus (4%); headache (5%); neck muscle weakness (1%); and neck pain (1%). 19.5% of patients discontinued the treatment because the injections were painful |
| Karian et al. (2023) [24] | Retrospective case series | 32 (13–17) | Fixed 31 site PREEMPT (155 U) 2 cycles | After 1 and 2 cy: * MHD (mean): −6.5; −7.3; * Headache severity: −0.72; −1.37; Headache Duration: −2.14; −9.04. | + | Not included | 47% AES: worsening pain (14%); fever/flu-like symptoms (8.9%); fatigue (5%); neck stiffness (3.8%); nausea (2.5%); dizziness (1.3%); dysphagia (1.3%); ptosis (1.3%) |
| Horvat et al. (2023) [23] | Retrospective case series | 51 (13–17) | ONA or INCO: Fixed 31 site PREEMPT (155 U) ± follow the pain (195 U) or Modified PREEMPT (100 U) Min 1 cycle, max not specified. | After 16.6 weeks: Sbjs ≥ 50% of reduction in the attacks: Fixed site PREEMPT: 100% Follow the pain: 59% Modified PREEMPT: 69% No differences between ONA and INCO groups | + | 63% | 4% AES: neck soreness (2%) headache (2%) |
| Akbar et al. (2024) [26] | Retrospective study | 24 (12–17.5) | Fixed 31 site PREEMPT protocol + follow the pain (155–195 U) 1–3 cycles | After 6 months: PedMIDAS (mean): −50.8 HIT 3 (mean): −19.6 | + | Not included | 20% AEs Not specified neurological manifestations (4%), Gastrointestinal symptoms (12%) and renal symptoms 12%); Injection site reaction (20%) |
| Goenka et al. (a). (2022) [27] | Retrospective and prospective | 34 (13–21) | Fixed 31-site PREEMPT protocol (155 U) 1–4 cycles | MHD (mean): −2.9 (from baseline to cy 1); −3.3 (from cy 1 to cy 2); −3.9 (from cy 2 to cy3), −1.2 (from cy 3 to cy4) Headache Severity (mean): −1.4 (from baseline to cy 1); −2.2 (from cy 1 to cy 2); −2.1 (from cy 2 to cy3), −0.6 (from cy 3 to cy4) | + | Unspecified but permitted | 5% AEs: Lateral eyebrow elevation (5.8%); pain (1.7%). |
| Goenka et al. (b). (2022) [28] | Retrospective | 34 (13–21) | Fixed 31-site PREEMPT protocol (155 U) 4 cycles | After 9 months: Sbjs ≥ 50% of reduction in the attacks: 75% * MHD (mean): −8.6 * Headache Severity (mean): −3.7 | + | Unspecified but permitted | 39% discontinuation; 5% AEs: lateral eyebrow elevation (5.8%); pain (1.7%). |
| Santana & Liu (2021) [22] | Retrospective case series | 65 (11–18) Range: 33–158 kg, mean ± SD: 62.8 ± 23.4 | Fixed 31-site PREEMPT + follow the pain (median dose 175 U; mean ± SD adjusted for weight was 2.8 ± 1.1 units/kg) 1 cycle | After 6 weeks: VAS (mean): −5.2 MHD (mean): −12 | + | Not included | 3%AES: dizziness (1.5%); fever (1.5%). |
| Shah et al. (2018) [32] | Retrospective case series | 10 (8–17) | Fixed 31-site PREEMPT + follow the pain (155–215 U) 1–11 cycles | After a mean of 2.5 cycles: MMD (median): −11.5; Headache duration (median): −7 Headache Intensity (median): −2 | + | 40% (>3 medication) | 8/35 Injections: 3/8 lower extremity weakness; 1 nausea; 1 monocular vision loss. |
| Ali et al. (2016) [25] | Retrospective case series | 30 (mean: 16.5 ± 1.83) | Fixed 31 site PREEMPT + follow the pain (155–185 U) Average 1–2 cycles | After 12 months: * MMD (mean): −9.9 * VAS (mean): −3.2 | + | Not included | 3% AEs: nausea. |
| Schroeder at al., (2012) [29] | Retrospective case series | 5 (10–16) | Personalized follow the pain pattern with ultrasound guidance (20–90 U) 1–4 cycles | After 4 weeks from the last injection: MMD (mean): −15 VAS (mean): −4.2 | + | Bio-behavioral and complementary therapies | No severe AEs |
| Ahmed et al. (2010) [31] | Retrospective case series | 10 (11–17) | Fixed 31 site PREEMPT (100 U) 3 cycles | After 1 cycle: 40% Reduction in headache frequency and intensity | + | Not included | 30% AEs: flu-like symptoms (20%); arm paraesthesia; (10%). |
| Chan et al. (2009) [30] | Retrospective case series | 12 (14–18) | Fixed 31 site PREEMPT (100 U) 1–9 cycles | After long term follow up (non-specified): 40% had a reduction in frequency and intensity and improvement of quality of life | + | 33% | 33% mild ptosis (8%): blurred vision (8%); burning sensations (8%), hematoma injection site (8%) |
| Key Point | Practical Guidance |
|---|---|
| Patient selection | Children/adolescents with CM who failed ≥ 2 preventive therapies and have significant school/daily impairment. |
| Baseline documentation | Record monthly headache days (MHD), attack severity, PedMIDAS/quality-of-life, analgesic use, and comorbidities before starting. |
| Starting dose | 155 U per cycle (PREEMPT paradigm) is the most commonly used dose, reported as tolerable even from age ≥ 8 years. |
| Dose escalation | Consider 195 U case-by-case based on pain topography and response to 155 U; reassess benefit–risk each cycle. |
| Protocol | Start with PREEMPT fixed-site injections, with follow-the-pain modifications considered as needed. |
| Treatment duration | Expect cumulative efficacy with repeated cycles; benefit may emerge after 2–3 administrations. |
| Stopping rule (inefficacy) | Do not judge failure after one cycle; evaluate after 2–3 cycles. Discontinuation should be considered if no clinically meaningful improvement (≥30% reduction in headache days) is achieved. |
| Continuation (responders) | Continue long-term in responders with periodic review; consider spacing or de-escalation only after sustained control. |
| Safety/tolerability | Generally well tolerated; most AEs are mild/transient (injection-site pain, occasional neck discomfort); serious AEs rare. |
| Improving tolerability | Use topical anesthetic, child-friendly setting, and clear procedural explanations. |
| Monitoring | Track MHD, PedMIDAS/quality-of-life, acute medication use, school attendance; review adverse events each cycle. |
| Concomitant preventives | Maintain stable preventives initially; adjust only after clear ONA response pattern to avoid attribution bias; no major safety issues reported, but efficacy as monotherapy vs. combination remains unclear. |
| Ethical/regulatory | Pediatric migraine use is off-label; obtain informed consent/assent and document rationale and uncertainties. |
| Reimbursement/access | Policies vary by country and payer; anticipate prior authorization or case-by-case approval. Provide detailed clinical justification. |
| Research gaps | Future studies should assess predictors of response (e.g., anxiety, depression), clarify the role of body weight/BMI, and evaluate efficacy in children with medication overuse headache. RCTs in children and adoelscents should incorporate measures to minimize the confounding effect of a high placebo response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papetti, L.; Martelletti, P.; Valeriani, M. Onabotulinumtoxin-A for Chronic Migraine in Children and Adolescents: A Narrative Review of Current Evidence and Clinical Perspectives. Toxins 2025, 17, 476. https://doi.org/10.3390/toxins17100476
Papetti L, Martelletti P, Valeriani M. Onabotulinumtoxin-A for Chronic Migraine in Children and Adolescents: A Narrative Review of Current Evidence and Clinical Perspectives. Toxins. 2025; 17(10):476. https://doi.org/10.3390/toxins17100476
Chicago/Turabian StylePapetti, Laura, Paolo Martelletti, and Massimiliano Valeriani. 2025. "Onabotulinumtoxin-A for Chronic Migraine in Children and Adolescents: A Narrative Review of Current Evidence and Clinical Perspectives" Toxins 17, no. 10: 476. https://doi.org/10.3390/toxins17100476
APA StylePapetti, L., Martelletti, P., & Valeriani, M. (2025). Onabotulinumtoxin-A for Chronic Migraine in Children and Adolescents: A Narrative Review of Current Evidence and Clinical Perspectives. Toxins, 17(10), 476. https://doi.org/10.3390/toxins17100476






