The Cloning and Characterization of a Three-Finger Toxin Homolog (NXH8) from the Coralsnake Micrurus corallinus That Interacts with Skeletal Muscle Nicotinic Acetylcholine Receptors
Abstract
1. Introduction
2. Results
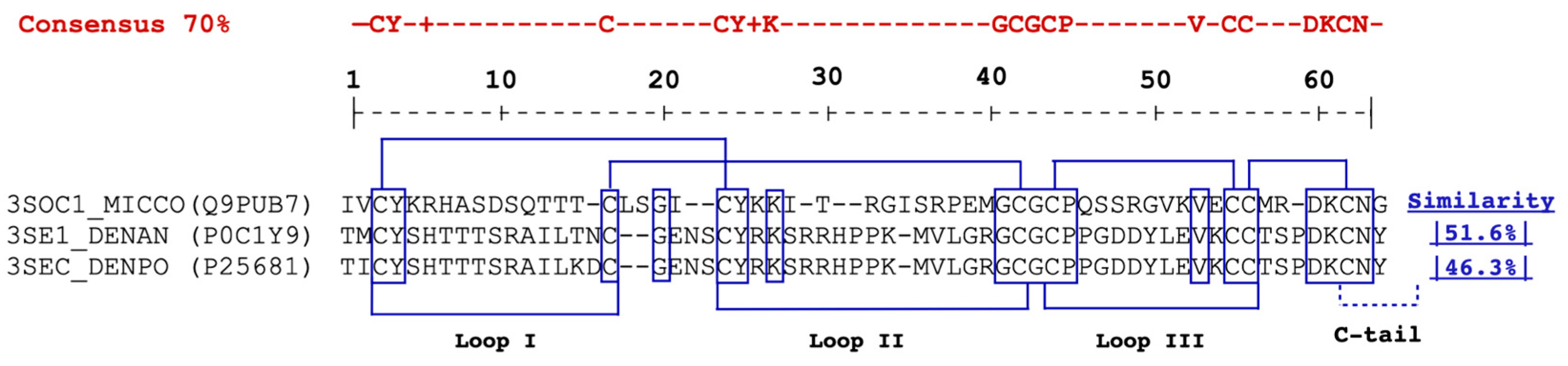
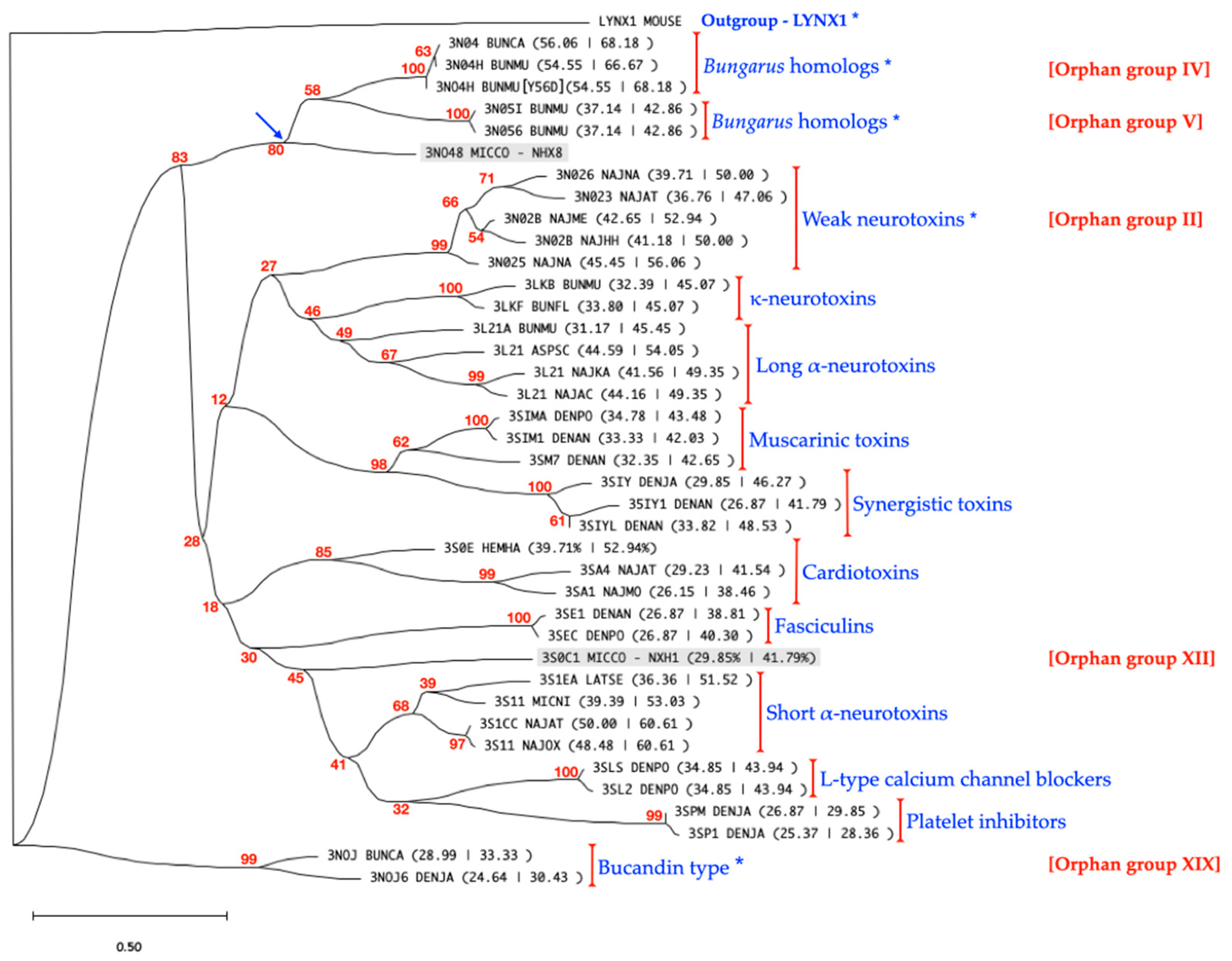
2.1. cDNA and Protein Sequence Analysis
2.2. Recombinant Expression of NXH8
2.3. Cross-Reactivity of Anti-rNXH8 with Other Elapid and Non-Elapid Venoms
2.4. Components of M. corallinus Coralsnake Venom Bind to nAChR in Muscle-Membrane Preparations and Are Inhibited by Anti-NXH8 Antiserum
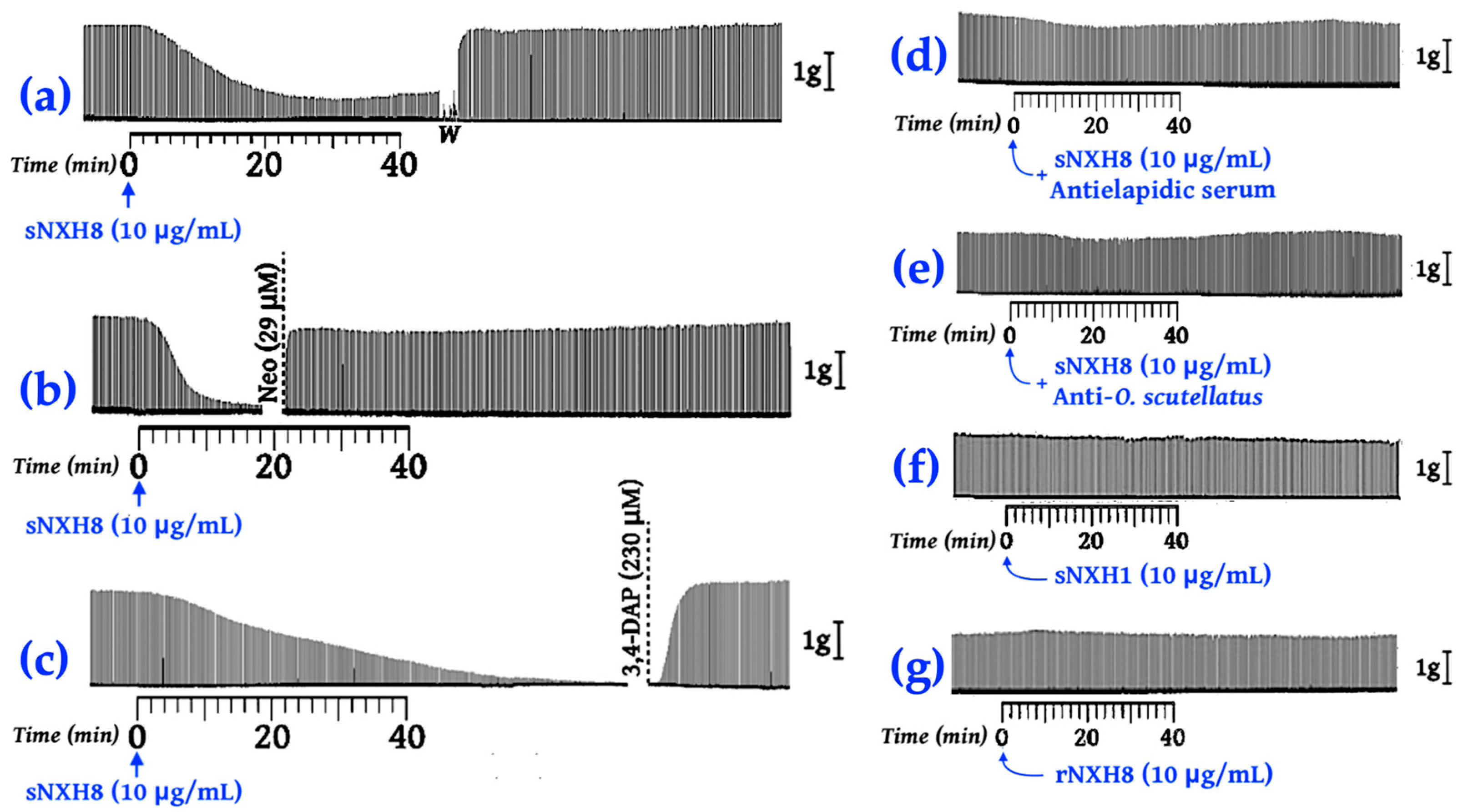
2.5. Chemically Synthesized NXH8 Causes Reversible Neuromuscular Blockades in Isolated Phrenic Nerve–Diaphragm Preparations
3. Discussion
4. Materials and Methods
4.1. Venoms, Toxins, and Antivenom
4.2. cDNA Cloning, Recombinant Protein Expression, and Metal Ion Affinity Purification
4.2.1. Cloning of nxh8
4.2.2. DNA Sequencing
4.2.3. Recombinant Expression of NXH8
4.2.4. Cell Lysis and Recombinant Protein Solubilization
4.2.5. Solubilization of Inclusion Bodies
4.2.6. Renaturation of Recombinant Proteins
4.2.7. Metal Ion Affinity Chromatography Purification
4.2.8. Protein Dialysis
4.3. Peptide Synthesis, Disulfide Bond Formation, and Protein Purification
4.4. Sequences, Alignments, and Analysis
4.5. Antibody Production and Western Blotting
4.6. Acetylcholine Receptor Binding Assay
4.7. Twitch Tension Experiments
4.8. Ethics Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. [FACT SHEET] Snakebite Envenoming. Available online: https://web.archive.org/web/20240303212634/https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 3 March 2024).
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Roze, J.A. Coral Snakes of the Americas: Biology, Identification and Venoms; Krieger: Malabar, FL, USA, 1996; 328p. [Google Scholar]
- Carvalho, A.V.; David, C.F.; Pessoa, A.d.M.; da Silva, N.J., Jr. A study on venom yield of Brazilian coralsnakes and its use in the evaluation of antielapidic serum. Sci. Medica 2014, 2, 142–149. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Capitani, E.M.; Vieira, R.J.; Rodrigues, C.K.; Zannin, M.; Da Silva, N.J., Jr.; Casais-e-Silva, L.L.; Hyslop, S. Coral snake bites (Micrurus spp.) in Brazil: A review of literature reports. Clin. Toxicol. 2016, 54, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; Hyslop, S.; Vieira, R.J.; Toledo, A.S.; Madureira, P.R.; de Capitani, E.M. Bites by coral snakes (Micrurus spp.) in Campinas, State of Sao Paulo, Southeastern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.R.; da Silva Junior, N.J.; Seebart, C.; Casais e Silva, L.L.; Schmidt, J.J.; Kaiser, I.I. Toxins isolated from the venom of the Brazilian coral snake (Micrurus frontalis frontalis) include hemorrhagic type phospholipases A2 and postsynaptic neurotoxins. Toxicon 1997, 35, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Hessel, M.M.; McAninch, S.A. Coral Snake Toxicity; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vital Brazil, O. Coral snake venoms: Mode of action and pathophysiology of experimental envenomation (1). Rev. Inst. Med. Trop. Sao Paulo 1987, 29, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vital Brazil, O.; Fontana, M.D. Ações pré-juncionais e pós-juncionais da peçonha da cobra coral Micrurus corallinus na junção neuromuscular. Mem. Inst. Butantan 1983, 47, 13–26. [Google Scholar]
- Cecchini, A.L.; Marcussi, S.; Silveira, L.B.; Borja-Oliveira, C.R.; Rodrigues-Simioni, L.; Amara, S.; Stabeli, R.G.; Giglio, J.R.; Arantes, E.C.; Soares, A.M. Biological and enzymatic activities of Micrurus sp. (Coral) snake venoms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 140, 125–134. [Google Scholar] [CrossRef]
- Arce-Bejarano, R.; Lomonte, B.; Gutierrez, J.M. Intravascular hemolysis induced by the venom of the Eastern coral snake, Micrurus fulvius, in a mouse model: Identification of directly hemolytic phospholipases A2. Toxicon 2014, 90, 26–35. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rojas, G.; da Silva Junior, N.J.; Nunez, J. Experimental myonecrosis induced by the venoms of South American Micrurus (coral snakes). Toxicon 1992, 30, 1299–1302. [Google Scholar] [CrossRef]
- de Roodt, A.R.; Lago, N.R.; Stock, R.P. Myotoxicity and nephrotoxicity by Micrurus venoms in experimental envenomation. Toxicon 2012, 59, 356–364. [Google Scholar] [CrossRef]
- Reis, L.P.G.; Botelho, A.F.M.; Novais, C.R.; Fiuza, A.T.L.; Barreto, M.S.O.; Ferreira, M.G.; Bonilla, C.; Chavez-Olortegui, C.; Melo, M.M. Cardiotoxic Effects of Micrurus surinamensis (Cuvier, 1817) Snake Venom. Cardiovasc. Toxicol. 2021, 21, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Lempek, M.R.; Botelho, A.F.M.; Fernandes, P.B.U.; Ribeiro, V.M.; Olortegui, C.C.D.; Melo, M.M. In Vivo Cardiotoxic Potential of Micrurus frontalis Venom. Cardiovasc. Toxicol. 2022, 22, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.L.; Pfalzgraf, R.R.; Laing, G. Death following coral snake bite in the United States—First documented case (with ELISA confirmation of envenomation) in over 40 years. Toxicon 2009, 53, 693–697. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Committee on the Selection of Essential Drugs; World Health Organization. The Selection of Essential Drugs: Report of a WHO Expert Committee. In Proceedings of the WHO Expert Committee on the Selection of Essential Drugs, Geneva, Switzerland, 17–21 October 1977. [Google Scholar]
- Mannel, R.; Gross, O.A.P.; Gross, G.A. Coral Snake Antivenin’s Deadly Deadline. Toxicon 2012, 60, 225–226. [Google Scholar] [CrossRef]
- Lewis-Younger, C.R.; Bernstein, J.N.; Schauben, J. Critical Shortage of Coral Snake Antivenom is Impacting Patient Care. Toxicon 2012, 60, 224–225. [Google Scholar] [CrossRef]
- de Roodt, A.R.; Dolab, J.A.; Galarce, P.P.; Gould, E.; Litwin, S.; Dokmetjian, J.C.; Segre, L.; Vidal, J.C. A study on the venom yield of venomous snake species from Argentina. Toxicon 1998, 36, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Serapicos, E.; Merusse, J. Variação de peso e sobrevida de Micrurus corallinus sob diferentes condições de alimentação em biotério (Serpentes, Elapidae). Iheringia Série Zool. 2002, 92, 105–109. [Google Scholar] [CrossRef][Green Version]
- Ho, P.L.; Yamagushi, I.K.; Tambourgi, V. Immunology of coralsnake venoms and antivenom production. In Advances in Coralsnake Biology: With an Emphasis on South America; Silva, N.J., Porras, L.W., Aird, S.D., Prudente, A.L.C., Eds.; Eagle Mountain Publishing LC.: Salt Lake City, UT, USA, 2021; pp. 637–650. [Google Scholar]
- Leao, L.I.; Ho, P.L.; Junqueira-de-Azevedo Ide, L. Transcriptomic basis for an antiserum against Micrurus corallinus (coral snake) venom. BMC Genom. 2009, 10, 112. [Google Scholar] [CrossRef]
- Correa-Netto, C.; Junqueira-de-Azevedo Ide, L.; Silva, D.A.; Ho, P.L.; Leitao-de-Araujo, M.; Alves, M.L.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J. Proteom. 2011, 74, 1795–1809. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Batista, C.V.; Restano-Cassulini, R.; Pando, V.; Villa-Hernandez, O.; Zavaleta-Martinez-Vargas, A.; Salas-Arruz, M.C.; Rodriguez de la Vega, R.C.; Becerril, B.; Possani, L.D. Proteomic analysis of the venom from the fish eating coral snake Micrurus surinamensis: Novel toxins, their function and phylogeny. Proteomics 2008, 8, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Ciscotto, P.H.; Rates, B.; Silva, D.A.; Richardson, M.; Silva, L.P.; Andrade, H.; Donato, M.F.; Cotta, G.A.; Maria, W.S.; Rodrigues, R.J.; et al. Venomic analysis and evaluation of antivenom cross-reactivity of South American Micrurus species. J. Proteom. 2011, 74, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; da Silva, N.J., Jr. Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. B 1991, 99, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Rey-Suarez, P.; Fernandez, J.; Sasa, M.; Pla, D.; Vargas, N.; Benard-Valle, M.; Sanz, L.; Correa-Netto, C.; Nunez, V.; et al. Venoms of Micrurus coral snakes: Evolutionary trends in compositional patterns emerging from proteomic analyses. Toxicon 2016, 122, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; da Silva, N.J.; Qiu, L.; Villar-Briones, A.; Saddi, V.A.; Pires de Campos Telles, M.; Grau, M.L.; Mikheyev, A.S. Coralsnake Venomics: Analyses of Venom Gland Transcriptomes and Proteomes of Six Brazilian Taxa. Toxins 2017, 9, 187. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais, E.S.L.L.; Correa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New insights into the phylogeographic distribution of the 3FTx/PLA(2) venom dichotomy across genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef]
- Menez, A.; Bontems, F.; Roumestand, C.; Gilquin, B.; Toma, F. Structural Basis for Functional Diversity of Animal Toxins. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1992, 99, 83–103. [Google Scholar] [CrossRef]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Tsetlin, V. Snake venom alpha-neurotoxins and other ‘three-finger’ proteins. Eur. J. Biochem. 1999, 264, 281–286. [Google Scholar] [CrossRef]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Nirthanan, S. Snake three-finger alpha-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem. Pharmacol. 2020, 181, 114168. [Google Scholar] [CrossRef]
- Radic, Z.; Duran, R.; Vellom, D.C.; Li, Y.; Cervenansky, C.; Taylor, P. Site of fasciculin interaction with acetylcholinesterase. J. Biol. Chem. 1994, 269, 11233–11239. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Tamiya, N. Current view on the structure-function relationship of postsynaptic neurotoxins from snake venoms. Pharmacol. Ther. 1987, 34, 403–451. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Konshina, A.G.; Dubovskii, P.V.; Efremov, R.G. Structure and dynamics of cardiotoxins. Curr. Protein Pept. Sci. 2012, 13, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Utkin, Y.N. Antiproliferative activity of cobra venom cytotoxins. Curr. Top. Med. Chem. 2015, 15, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Dufton, M.J.; Hider, R.C. Structure and pharmacology of elapid cytotoxins. Pharmacol. Ther. 1988, 36, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Adem, A.; Karlsson, E. Muscarinic receptor subtype selective toxins. Life Sci. 1997, 60, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Blanchet, G.; Mourier, G.; Marquer, C.; Marcon, E.; Fruchart-Gaillard, C. Muscarinic toxins. Toxicon 2011, 58, 455–463. [Google Scholar] [CrossRef]
- McDowell, R.S.; Dennis, M.S.; Louie, A.; Shuster, M.; Mulkerrin, M.G.; Lazarus, R.A. Mambin, a potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor structurally related to the short neurotoxins. Biochemistry 1992, 31, 4766–4772. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Deuis, J.R.; Dashevsky, D.; Dobson, J.; Jackson, T.N.; Brust, A.; Xie, B.; Koludarov, I.; Debono, J.; Hendrikx, I.; et al. The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus). Toxins 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Torres, I.O.; Jin, T.B.; Cadene, M.; Chait, B.T.; Poget, S.F. Discovery and characterisation of a novel toxin from Dendroaspis angusticeps, named Tx7335, that activates the potassium channel KcsA. Sci. Rep. 2016, 6, 23904. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.I.; Rash, L.D.; Vila-Farres, X.; Rosengren, K.J.; Mobli, M.; King, G.F.; Alewood, P.F.; Craik, D.J.; Durek, T. Chemical synthesis, 3D structure, and ASIC binding site of the toxin mambalgin-2. Angew. Chem. Int. Ed. Engl. 2014, 53, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- de Weille, J.R.; Schweitz, H.; Maes, P.; Tartar, A.; Lazdunski, M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl. Acad. Sci. USA 1991, 88, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Winckler-Dietrich, V.; Hu, H.Y.; Kessler, P.; Drevet, P.; Bertrand, D.; Menez, A. Only snake curaremimetic toxins with a fifth disulfide bond have high affinity for the neuronal alpha7 nicotinic receptor. J. Biol. Chem. 1997, 272, 24279–24286. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, V.A.; Weaver, W.R.; McLane, K.E.; Conti-Fine, B.M.; Fiordalisi, J.J.; Grant, G.A. Binding of native kappa-neurotoxins and site-directed mutants to nicotinic acetylcholine receptors. Toxicon 1996, 34, 1243–1256. [Google Scholar] [CrossRef]
- Grant, G.A.; Al-Rabiee, R.; Xu, X.L.; Zhang, Y. Critical interactions at the dimer interface of kappa-bungarotoxin, a neuronal nicotinic acetylcholine receptor antagonist. Biochemistry 1997, 36, 3353–3358. [Google Scholar] [CrossRef]
- Carlsson, F.H. Snake venom toxins. The primary structure of protein S4C11. A neurotoxin homologue from the venom of forest cobra (Naja melanoleuca). Biochim. Biophys. Acta 1975, 400, 310–321. [Google Scholar] [CrossRef]
- Joubert, F.J.; Taljaard, N. Snake venoms. The amino acid sequences of two Melanoleuca-type toxins. Hoppe Seylers Z. Physiol. Chem. 1980, 361, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.C.; Fan, C.Y.; Gong, Y.; Yang, S.L. cDNA sequence analysis and expression of four long neurotoxin homologues from Naja naja atra. Biochim. Biophys. Acta 1998, 1443, 233–238. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Kryukova, E.V.; Chiodini, F.; Bertrand, D.; Methfessel, C.; Tsetlin, V.I. “Weak toxin” from Naja kaouthia is a nontoxic antagonist of alpha 7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 2001, 276, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Huang, H.B.; Wu, B.N.; Chang, L.S. Characterization and cloning of long neurotoxin homolog from Naja naja atra. Biochem. Mol. Biol. Int. 1998, 46, 1211–1217. [Google Scholar] [CrossRef]
- Shafqat, J.; Siddiqi, A.R.; Zaidi, Z.H.; Jornvall, H. Extensive multiplicity of the miscellaneous type of neurotoxins from the venom of the cobra Naja naja naja and structural characterization of major components. FEBS Lett. 1991, 284, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Charpantier, E.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Cheah, L.S.; Kini, R.M.; Bertrand, D. Neuromuscular effects of candoxin, a novel toxin from the venom of the Malayan krait (Bungarus candidus). Br. J. Pharmacol. 2003, 139, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Charpantier, E.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Cheah, L.S.; Bertrand, D.; Kini, R.M. Candoxin, a novel toxin from Bungarus candidus, is a reversible antagonist of muscle (alphabetagammadelta) but a poorly reversible antagonist of neuronal alpha 7 nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 17811–17820. [Google Scholar] [CrossRef]
- Parvathy, V.R.; Chary, K.V.R.; Kini, R.M.; Govil, G. Sequence-specific C-13 NMR assignments in a neurotoxin (candoxin) from Bungarus candidus. Magn. Reson. Chem. 2001, 39, 577–580. [Google Scholar] [CrossRef]
- Aird, S.D.; Womble, G.C.; Yates, J.R., 3rd; Griffin, P.R. Primary structure of gamma-bungarotoxin, a new postsynaptic neurotoxin from venom of Bungarus multicinctus. Toxicon 1999, 37, 609–625. [Google Scholar] [CrossRef]
- Chang, L.S.; Lin, J. cDNA sequence analysis of a novel neurotoxin homolog from Taiwan banded krait. Biochem. Mol. Biol. Int. 1997, 43, 347–354. [Google Scholar] [CrossRef]
- Danse, J.M.; Garnier, J.M.; Kempf, J. A cDNA sequence encoding a neurotoxin-homolog from Bungarus multicinctus. Nucleic Acids Res. 1990, 18, 1045. [Google Scholar] [CrossRef][Green Version]
- Chang, C.C.; Lee, C.Y. Isolation of Neurotoxins from the Venom of Bungarus Multicinctus and Their Modes of Neuromuscular Blocking Action. Arch. Int. Pharmacodyn. Ther. 1963, 144, 241–257. [Google Scholar]
- da Silva, D.C.; de Medeiros, W.A.; Batista Ide, F.; Pimenta, D.C.; Lebrun, I.; Abdalla, F.M.; Sandoval, M.R. Characterization of a new muscarinic toxin from the venom of the Brazilian coral snake Micrurus lemniscatus in rat hippocampus. Life Sci. 2011, 89, 931–938. [Google Scholar] [CrossRef]
- Foo, C.S.; Jobichen, C.; Hassan-Puttaswamy, V.; Dekan, Z.; Tae, H.S.; Bertrand, D.; Adams, D.J.; Alewood, P.F.; Sivaraman, J.; Nirthanan, S.; et al. Fulditoxin, representing a new class of dimeric snake toxins, defines novel pharmacology at nicotinic ACh receptors. Br. J. Pharmacol. 2020, 177, 1822–1840. [Google Scholar] [CrossRef]
- Rey-Suarez, P.; Floriano, R.S.; Rostelato-Ferreira, S.; Saldarriaga-Cordoba, M.; Nunez, V.; Rodrigues-Simioni, L.; Lomonte, B. Mipartoxin-I, a novel three-finger toxin, is the major neurotoxic component in the venom of the redtail coral snake Micrurus mipartitus (Elapidae). Toxicon 2012, 60, 851–863. [Google Scholar] [CrossRef]
- Alape-Giron, A.; Stiles, B.; Schmidt, J.; Giron-Cortes, M.; Thelestam, M.; Jornvall, H.; Bergman, T. Characterization of multiple nicotinic acetylcholine receptor-binding proteins and phospholipases A2 from the venom of the coral snake Micrurus nigrocinctus. FEBS Lett. 1996, 380, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kleiz-Ferreira, J.M.; Bernaerts, H.; Pinheiro-Junior, E.L.; Peigneur, S.; Zingali, R.B.; Tytgat, J. Pharmacological Screening of Venoms from Five Brazilian Micrurus Species on Different Ion Channels. Int. J. Mol. Sci. 2022, 23, 7714. [Google Scholar] [CrossRef]
- Kleiz-Ferreira, J.M.; Cirauqui, N.; Trajano, E.A.; Almeida, M.S.; Zingali, R.B. Three-Finger Toxins from Brazilian Coral Snakes: From Molecular Framework to Insights in Biological Function. Toxins 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suarez, P.; Saldarriaga-Cordoba, M.; Torres, U.; Marin-Villa, M.; Lomonte, B.; Nunez, V. Novel three-finger toxins from Micrurus dumerilii and Micrurus mipartitus coral snake venoms: Phylogenetic relationships and characterization of Clarkitoxin-I-Mdum. Toxicon 2019, 170, 85–93. [Google Scholar] [CrossRef]
- Ho, P.L.; Soares, M.B.; Yamane, T.; Raw, I. Reverse Biology Applied to Micrurus-Corallinus, a South-American Coral Snake. J. Toxicol.-Toxin Rev. 1995, 14, 327–337. [Google Scholar] [CrossRef]
- Ho, P.L.; Soares, M.B.; Maack, T.; Gimenez, I.; Puorto, G.; Furtado, M.F.; Raw, I. Cloning of an unusual natriuretic peptide from the South American coral snake Micrurus corallinus. Eur. J. Biochem. 1997, 250, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Silveira de Oliveira, J.; Rossan de Brandao Prieto da Silva, A.; Soares, M.B.; Stephano, M.A.; de Oliveira Dias, W.; Raw, I.; Ho, P.L. Cloning and characterization of an alpha-neurotoxin-type protein specific for the coral snake Micrurus corallinus. Biochem. Biophys. Res. Commun. 2000, 267, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.R.; Junqueira-de-Azevedo Ide, L.; Novo, J.B.; Castro, K.; Duarte, C.G.; Machado-de-Avila, R.A.; Chavez-Olortegui, C.; Ho, P.L. A Heterologous Multiepitope DNA Prime/Recombinant Protein Boost Immunisation Strategy for the Development of an Antiserum against Micrurus corallinus (Coral Snake) Venom. PLoS Negl. Trop. Dis. 2016, 10, e0004484. [Google Scholar] [CrossRef] [PubMed]
- Pillet, L.; Tremeau, O.; Ducancel, F.; Drevet, P.; Zinn-Justin, S.; Pinkasfeld, S.; Boulain, J.C.; Menez, A. Genetic engineering of snake toxins. Role of invariant residues in the structural and functional properties of a curaremimetic toxin, as probed by site-directed mutagenesis. J. Biol. Chem. 1993, 268, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Tremeau, O.; Lemaire, C.; Drevet, P.; Pinkasfeld, S.; Ducancel, F.; Boulain, J.C.; Menez, A. Genetic engineering of snake toxins. The functional site of Erabutoxin a, as delineated by site-directed mutagenesis, includes variant residues. J. Biol. Chem. 1995, 270, 9362–9369. [Google Scholar] [CrossRef]
- Ackermann, E.J.; Ang, E.T.; Kanter, J.R.; Tsigelny, I.; Taylor, P. Identification of pairwise interactions in the alpha-neurotoxin-nicotinic acetylcholine receptor complex through double mutant cycles. J. Biol. Chem. 1998, 273, 10958–10964. [Google Scholar] [CrossRef]
- Osaka, H.; Malany, S.; Kanter, J.R.; Sine, S.M.; Taylor, P. Subunit interface selectivity of the alpha-neurotoxins for the nicotinic acetylcholine receptor. J. Biol. Chem. 1999, 274, 9581–9586. [Google Scholar] [CrossRef]
- Antil, S.; Servent, D.; Menez, A. Variability among the sites by which curaremimetic toxins bind to torpedo acetylcholine receptor, as revealed by identification of the functional residues of alpha-cobratoxin. J. Biol. Chem. 1999, 274, 34851–34858. [Google Scholar] [CrossRef]
- Antil-Delbeke, S.; Gaillard, C.; Tamiya, T.; Corringer, P.J.; Changeux, J.P.; Servent, D.; Menez, A. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha 7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 29594–29601. [Google Scholar] [CrossRef]
- Moise, L.; Piserchio, A.; Basus, V.J.; Hawrot, E. NMR structural analysis of alpha-bungarotoxin and its complex with the principal alpha-neurotoxin-binding sequence on the alpha 7 subunit of a neuronal nicotinic acetylcholine receptor. J. Biol. Chem. 2002, 277, 12406–12417. [Google Scholar] [CrossRef]
- Zeng, H.; Moise, L.; Grant, M.A.; Hawrot, E. The solution structure of the complex formed between alpha-bungarotoxin and an 18-mer cognate peptide derived from the alpha 1 subunit of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 2001, 276, 22930–22940. [Google Scholar] [CrossRef]
- Sprules, T.; Green, N.; Featherstone, M.; Gehring, K. Nickel-induced oligomerization of proteins containing 10-histidine tags. Biotechniques 1998, 25, 20–22. [Google Scholar] [CrossRef]
- Prieto da Silva, A.R.; Yamagushi, I.K.; Morais, J.F.; Higashi, H.G.; Raw, I.; Ho, P.L.; Oliveira, J.S. Cross reactivity of different specific Micrurus antivenom sera with homologous and heterologous snake venoms. Toxicon 2001, 39, 949–953. [Google Scholar] [CrossRef]
- Floriano, R.S.; Carregari, V.C.; de Abreu, V.A.; Kenzo-Kagawa, B.; Ponce-Soto, L.A.; da Cruz-Hofling, M.A.; Hyslop, S.; Marangoni, S.; Rodrigues-Simioni, L. Pharmacological study of a new Asp49 phospholipase A(2) (Bbil-TX) isolated from Bothriopsis bilineata smargadina (forest viper) venom in vertebrate neuromuscular preparations. Toxicon 2013, 69, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; de Cassia de, O.C.R.; Villalta, M.; Segura, A.; Vargas, M.; Wright, C.E.; Paiva, O.K.; Matainaho, T.; Jensen, S.D.; Leon, G.; et al. Neutralization of the neuromuscular inhibition of venom and taipoxin from the taipan (Oxyuranus scutellatus) by F(ab’)2 and whole IgG antivenoms. Toxicol. Lett. 2016, 241, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Floriano, R.S.; Schezaro-Ramos, R.; Silva, N.J., Jr.; Bucaretchi, F.; Rowan, E.G.; Hyslop, S. Neurotoxicity of Micrurus lemniscatus lemniscatus (South American coralsnake) venom in vertebrate neuromuscular preparations in vitro and neutralization by antivenom. Arch. Toxicol. 2019, 93, 2065–2086. [Google Scholar] [CrossRef]
- Ramos, H.R.; Vassao, R.C.; de Roodt, A.R.; Santos, E.S.E.C.; Mirtschin, P.; Ho, P.L.; Spencer, P.J. Cross neutralization of coral snake venoms by commercial Australian snake antivenoms. Clin. Toxicol. 2017, 55, 33–39. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Maslennikov, I.V.; Eletsky, A.V.; Starkov, V.G.; Weise, C.; Franke, P.; Hucho, F.; Tsetlin, V.I. First tryptophan-containing weak neurotoxin from cobra venom. Toxicon 2001, 39, 921–927. [Google Scholar] [CrossRef]
- Hannan, S.; Mortensen, M.; Smart, T.G. Snake neurotoxin alpha-bungarotoxin is an antagonist at native GABA(A) receptors. Neuropharmacology 2015, 93, 28–40. [Google Scholar] [CrossRef]
- Kudryavtsev, D.S.; Shelukhina, I.V.; Son, L.V.; Ojomoko, L.O.; Kryukova, E.V.; Lyukmanova, E.N.; Zhmak, M.N.; Dolgikh, D.A.; Ivanov, I.A.; Kasheverov, I.E.; et al. Neurotoxins from snake venoms and alpha-conotoxin ImI inhibit functionally active ionotropic gamma-aminobutyric acid (GABA) receptors. J. Biol. Chem. 2015, 290, 22747–22758. [Google Scholar] [CrossRef] [PubMed]
- Rosso, J.P.; Schwarz, J.R.; Diaz-Bustamante, M.; Ceard, B.; Gutierrez, J.M.; Kneussel, M.; Pongs, O.; Bosmans, F.; Bougis, P.E. MmTX1 and MmTX2 from coral snake venom potently modulate GABAA receptor activity. Proc. Natl. Acad. Sci. USA 2015, 112, E891–E900. [Google Scholar] [CrossRef]
- Naimuddin, M.; Kobayashi, S.; Tsutsui, C.; Machida, M.; Nemoto, N.; Sakai, T.; Kubo, T. Directed evolution of a three-finger neurotoxin by using cDNA display yields antagonists as well as agonists of interleukin-6 receptor signaling. Mol. Brain 2011, 4, 2. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Folch, B.; Letourneau, M.; Truong, N.H.; Doucet, N.; Fournier, A.; Chatenet, D. Design of a truncated cardiotoxin-I analogue with potent insulinotropic activity. J. Med. Chem. 2014, 57, 2623–2633. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Folch, B.; Letourneau, M.; Vaudry, D.; Truong, N.H.; Doucet, N.; Chatenet, D.; Fournier, A. Cardiotoxin-I: An unexpectedly potent insulinotropic agent. Chembiochem 2012, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, T.M.; Al Khoury, S.; Jaquillard, L.; Triquigneaux, M.; Martinez, G.; Bourgoin-Voillard, S.; Seve, M.; Arnoult, C.; Beroud, R.; De Waard, M. Actiflagelin, a new sperm activator isolated from Walterinnesia aegyptia venom using phenotypic screening. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Deacon, A.M.; Comsa, D.S.; Rajaseger, G.; Kini, R.M.; Uson, I.I.; Kolatkar, P.R. The atomic resolution structure of bucandin, a novel toxin isolated from the malayan krait, determined by direct methods. erratum. Acta Crystallogr. D Biol. Crystallogr. 2000, 56 Pt 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Kini, R.M.; Selvanayagam, N.; Kuchel, P.W. NMR structure of bucandin, a neurotoxin from the venom of the Malayan krait (Bungarus candidus). Biochem. J. 2001, 360, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lin, S.; Wang, J.; Hu, W.P.; Wu, B.; Huang, H. Structure-function studies on Taiwan cobra long neurotoxin homolog. Biochim. Biophys. Acta 2000, 1480, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S. Biochemical and Pharmacological Studies on Candoxin, a Novel Toxin from the Venom of the Malayan Krait Bungarus candidus; National University of Singapore: Singapore, 2002. [Google Scholar]
- Mordvitsev, D.Y.; Polyak, Y.L.; Kuzmin, D.A.; Levtsova, O.V.; Tourleigh, Y.V.; Utkin, Y.N.; Shaitan, K.V.; Tsetlin, V.I. Computer modeling of binding of diverse weak toxins to nicotinic acetylcholine receptors. Comput. Biol. Chem. 2007, 31, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Kini, R.M. Non-conventional toxins from Elapid venoms. Toxicon 2003, 41, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Chung, C.; Wu, B.N.; Yang, C.C. Characterization and gene organization of Taiwan banded krait (Bungarus multicinctus) gamma-bungarotoxin. J. Protein Chem. 2002, 21, 223–229. [Google Scholar] [CrossRef]
- Joubert, F.J.; Taljaard, N. Naja haje haje (Egyptian cobra) venom. Some properties and the complete primary structure of three toxins (CM-2, CM-11 and CM-12). Eur. J. Biochem. 1978, 90, 359–367. [Google Scholar] [CrossRef]
- Poh, S.L.; Mourier, G.; Thai, R.; Armugam, A.; Molgo, J.; Servent, D.; Jeyaseelan, K.; Menez, A. A synthetic weak neurotoxin binds with low affinity to Torpedo and chicken alpha7 nicotinic acetylcholine receptors. Eur. J. Biochem. 2002, 269, 4247–4256. [Google Scholar] [CrossRef]
- Ogay, A.Y.; Rzhevsky, D.I.; Murashev, A.N.; Tsetlin, V.I.; Utkin, Y.N. Weak neurotoxin from Naja kaouthia cobra venom affects haemodynamic regulation by acting on acetylcholine receptors. Toxicon 2005, 45, 93–99. [Google Scholar] [CrossRef]
- Kolbe, H.V.; Huber, A.; Cordier, P.; Rasmussen, U.B.; Bouchon, B.; Jaquinod, M.; Vlasak, R.; Delot, E.C.; Kreil, G. Xenoxins, a family of peptides from dorsal gland secretion of Xenopus laevis related to snake venom cytotoxins and neurotoxins. J. Biol. Chem. 1993, 268, 16458–16464. [Google Scholar] [CrossRef] [PubMed]
- Macleod, R.J.; Lembessis, P.; James, S.; Bennett, H.P. Isolation of a member of the neurotoxin/cytotoxin peptide family from Xenopus laevis skin which activates dihydropyridine-sensitive Ca2+ channels in mammalian epithelial cells. J. Biol. Chem. 1998, 273, 20046–20051. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, B.; Driscoll, P.C.; Campbell, I.D.; Willis, A.C.; van der Merwe, P.A.; Davis, S.J. Three-dimensional solution structure of the extracellular region of the complement regulatory protein CD59, a new cell-surface protein domain related to snake venom neurotoxins. Biochemistry 1994, 33, 4471–4482. [Google Scholar] [CrossRef] [PubMed]
- Gumley, T.P.; McKenzie, I.F.; Sandrin, M.S. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol. Cell Biol. 1995, 73, 277–296. [Google Scholar] [CrossRef]
- Ploug, M.; Ellis, V. Structure-function relationships in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom alpha-neurotoxins. FEBS Lett. 1994, 349, 163–168. [Google Scholar] [CrossRef]
- Chou, J.H.; Bargmann, C.I.; Sengupta, P. The Caenorhabditis elegans odr-2 gene encodes a novel Ly-6-related protein required for olfaction. Genetics 2001, 157, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Miwa, J.M.; Ibanez-Tallon, I.; Crabtree, G.W.; Sanchez, R.; Sali, A.; Role, L.W.; Heintz, N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 1999, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The three-finger toxin fold: A multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017, 142 (Suppl. S2), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Antil-Delbeke, S.; Gaillard, C.; Corringer, P.J.; Changeux, J.P.; Menez, A. Molecular characterization of the specificity of interactions of various neurotoxins on two distinct nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2000, 393, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nys, M.; Zarkadas, E.; Brams, M.; Mehregan, A.; Kambara, K.; Kool, J.; Casewell, N.R.; Bertrand, D.; Baenziger, J.E.; Nury, H.; et al. The molecular mechanism of snake short-chain alpha-neurotoxin binding to muscle-type nicotinic acetylcholine receptors. Nat. Commun. 2022, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Raw, I.; Guidolin, R.; Higashi, H.; Kelen, E. Antivenins in Brazil: Preparation. In Handbook of Natural Toxins: Reptile Venoms and Toxins; Tu, A.T., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; Volume 5. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; Volume 3, 1890p. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Schuler, G.D.; Altschul, S.F.; Lipman, D.J. A workbench for multiple alignment construction and analysis. Proteins 1991, 9, 180–190. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-Phylogeny Inference Package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- Harlow, E.; Lane, D. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1988. [Google Scholar]
- de Almeida-Paula, L.D.; Costa-Lotufo, L.V.; Silva Ferreira, Z.; Monteiro, A.E.; Isoldi, M.C.; Godinho, R.O.; Markus, R.P. Melatonin modulates rat myotube-acetylcholine receptors by inhibiting calmodulin. Eur. J. Pharmacol. 2005, 525, 24–31. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman-Ramos, H.; Prieto-da-Silva, Á.R.B.; Dellê, H.; Floriano, R.S.; Dias, L.; Hyslop, S.; Schezaro-Ramos, R.; Servent, D.; Mourier, G.; de Oliveira, J.L.; et al. The Cloning and Characterization of a Three-Finger Toxin Homolog (NXH8) from the Coralsnake Micrurus corallinus That Interacts with Skeletal Muscle Nicotinic Acetylcholine Receptors. Toxins 2024, 16, 164. https://doi.org/10.3390/toxins16040164
Roman-Ramos H, Prieto-da-Silva ÁRB, Dellê H, Floriano RS, Dias L, Hyslop S, Schezaro-Ramos R, Servent D, Mourier G, de Oliveira JL, et al. The Cloning and Characterization of a Three-Finger Toxin Homolog (NXH8) from the Coralsnake Micrurus corallinus That Interacts with Skeletal Muscle Nicotinic Acetylcholine Receptors. Toxins. 2024; 16(4):164. https://doi.org/10.3390/toxins16040164
Chicago/Turabian StyleRoman-Ramos, Henrique, Álvaro R. B. Prieto-da-Silva, Humberto Dellê, Rafael S. Floriano, Lourdes Dias, Stephen Hyslop, Raphael Schezaro-Ramos, Denis Servent, Gilles Mourier, Jéssica Lopes de Oliveira, and et al. 2024. "The Cloning and Characterization of a Three-Finger Toxin Homolog (NXH8) from the Coralsnake Micrurus corallinus That Interacts with Skeletal Muscle Nicotinic Acetylcholine Receptors" Toxins 16, no. 4: 164. https://doi.org/10.3390/toxins16040164
APA StyleRoman-Ramos, H., Prieto-da-Silva, Á. R. B., Dellê, H., Floriano, R. S., Dias, L., Hyslop, S., Schezaro-Ramos, R., Servent, D., Mourier, G., de Oliveira, J. L., Lemes, D. E., Costa-Lotufo, L. V., Oliveira, J. S., Menezes, M. C., Markus, R. P., & Ho, P. L. (2024). The Cloning and Characterization of a Three-Finger Toxin Homolog (NXH8) from the Coralsnake Micrurus corallinus That Interacts with Skeletal Muscle Nicotinic Acetylcholine Receptors. Toxins, 16(4), 164. https://doi.org/10.3390/toxins16040164







