Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets
Abstract
1. Introduction
2. Results
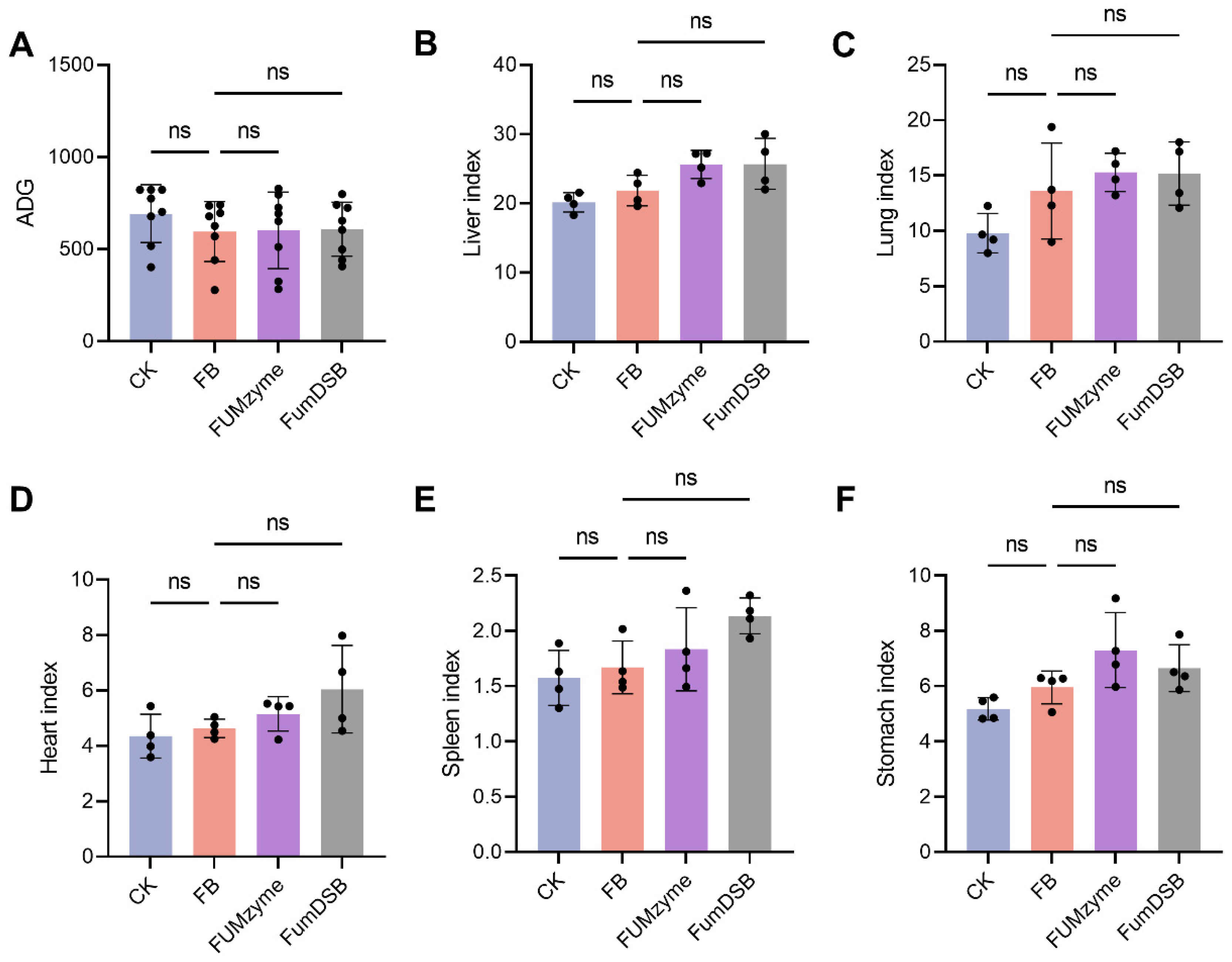
2.1. Effect of FUMzyme® and FumDSB Supplementation on Growth Performance of Piglets Fed Fumonisin-Contaminated Diets
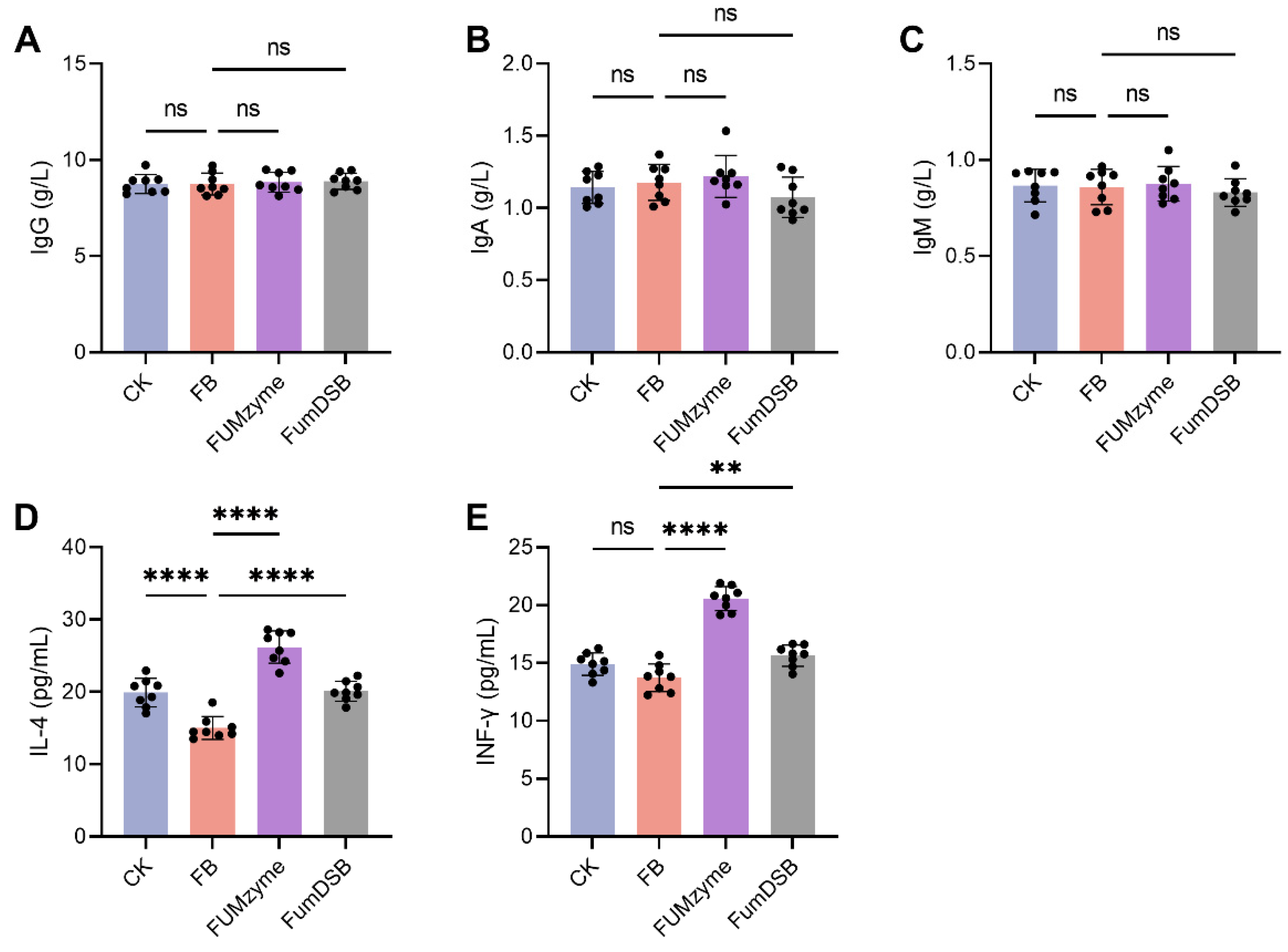
2.2. Effect of FUMzyme® and FumDSB on Serum Biochemical Indices in Piglets
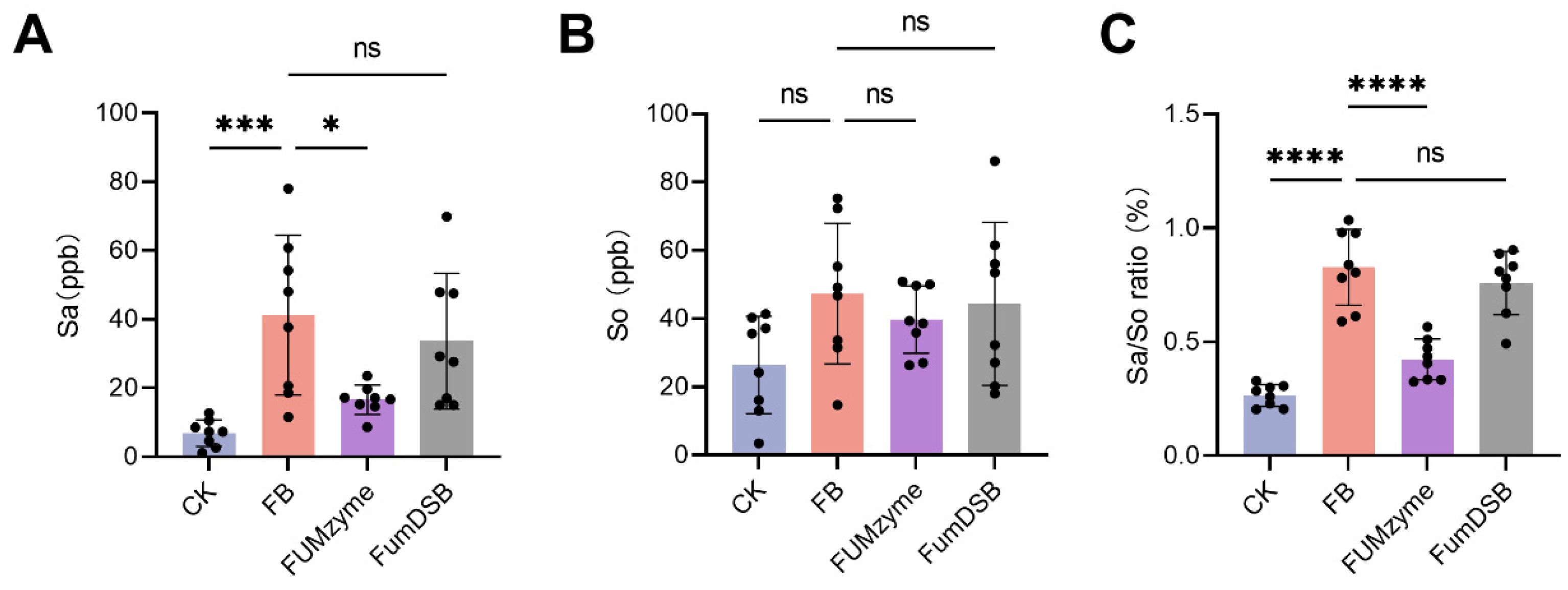
2.3. Effect of FUMzyme® and FumDSB on the Sa, So, and Sa/So Ratio in the Serum of Piglets
3. Discussion
4. Methods
4.1. Source of Fumonisin Detoxification Enzymes FUMzyme® and FumDSB
4.2. Animal Feeding and Sampling
4.3. Growth Performance and Organ Index
4.4. Measurement of Serum Biochemical Indicators
4.5. HPLC-MS/MS Analysis of Serum Sa and So
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voss, K.A.; Chamberlain, W.J.; Riley, R.T.; Bacon, C.W.; Norred, W.P. In vitro and in vivo effects of fumonisins: Toxicity and mechanism of action. JSM Mycotoxins 2009, 1994, 1–12. [Google Scholar] [CrossRef][Green Version]
- Rheeder; John, P.; Marasas; Walter, F.O.; Vismer; Hester, F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Li, X.; Cao, C.; Zhu, X.; Li, X.; Wang, K. Fumonisins B1 exposure triggers intestinal tract injury via activating nuclear xenobiotic receptors and attracting inflammation response. Environ. Pollut. 2020, 267, 115461. [Google Scholar] [CrossRef]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Gbore, F.A. Growth performance and puberty attainment in growing pigs fed dietary fumonisin B(1). J. Anim. Physiol. Anim. Nutr. 2010, 93, 761–767. [Google Scholar] [CrossRef]
- Constable, P.D.; Riley, R.T.; Waggoner, A.L.; Hsiao, S.H.; Haschek, W.M. Serum sphingosine-1-phosphate and sphinganine-1-phosphate are elevated in horses exposed to fumonisin B1. In AOAC International Midwest Section Final Program; 2005; pp. 63–64. Available online: https://www.researchgate.net/publication/286418060_Serum_Sphingosine-1-Phosphate_and_Sphinganine-1-Phosphate_Are_Elevated_in_Horses_Exposed_to_Fumonisin_B1 (accessed on 10 December 2023).
- Jovanovic, M.; Nesic, S.; Marinkovic, D.; Kukolj, V.; Trailovic, D. Fumonisin toxicosis in horses. J. Comp. Pathol. 2015, 152, 51. [Google Scholar] [CrossRef]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: An overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar]
- Xietong-Xin, Z.H. Contamination of fungi and mycotoxins in foodstuffs in high risk area of esophageal cancer. Biomed. Environ. Sci. 1998, 11, 140–146. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- Liu, Y.; Tan, L.P.; Xing, F.G.; Chen, Z.J. Reduction of fumonisins in maize using extrusion-cooking and nixtamalization method. Sci. Technol. Food Ind. 2010, 31, 86–89. [Google Scholar]
- Zhang, J.; Tang, X.; Cai, Y.; Zhou, W.W. Mycotoxin contamination status of cereals in China and potential microbial decontamination methods. Metabolites 2023, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic mechanism and biological detoxification of fumonisins. Toxins 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sărăndan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal degradation of fumonisin B₁ by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in Turkey and swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.D.; Grabherr, R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of fumonisin esterase from Komagataella phaffii DSM 32159 as a feed additive for all animal species. EFSA J. 2020, 18, e06207. [Google Scholar]
- Li, Z.; Wang, Y.; Liu, Z.; Jin, S.; Pan, K.; Liu, H.; Liu, T.; Li, X.; Zhang, C.; Luo, X.; et al. Biological detoxification of fumonisin by a novel carboxylesterase from Sphingomonadales bacterium and its biochemical characterization. Int. J. Biol. Macromol. 2021, 169, 18–27. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Huang, L.; Chen, W.; Li, Z.; Wang, C. FumDSB can reduce the toxic effects of fumonisin B(1) by regulating several brain-gut peptides in both the hypothalamus and jejunum of growing pigs. Toxins 2021, 13, 874. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance for the preparation of dossiers for technological additives. EFSA J. 2012, 10, 2528. [Google Scholar] [CrossRef]
- Jager, A.V.; Tonin, F.G.; Baptista, G.Z.; Souto, P.C.; Oliveira, C.A. Assessment of aflatoxin exposure using serum and urinary biomarkers in Sao Paulo, Brazil: A pilot study. Int. J. Hyg. Environ. Health 2016, 219, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Westhuizen, L.V.D.; Sewram, V. Biomarkers of exposure to fumonisin mycotoxins: A review. Food Addit. Contam. 2007, 24, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregrd, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 6, e05242. [Google Scholar]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Rao, Z.X.; Tokach, M.D.; Woodworth, J.C.; Derouchey, J.M.; Dritz, S.S. Effects of fumonisin-contaminated corn on growth performance of 9 to 28 kg nursery pigs. Toxins 2020, 12, 604. [Google Scholar] [CrossRef]
- Li, Y.C.; Ledoux, D.R.; Bermudez, A.J.; Fritsche, K.L.; Rottinghaus, G.E. The individual and combined effects of fumonisin B1 and moniliformin on performance and selected immune parameters in turkey poults. Poult. Sci. 2000, 79, 871–878. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Bouhet, S.; Pascale, F.; Bailly, J.D.; Miller, J.D.; Pinton, P.; Oswald, I.P. Mycotoxin fumonisin B1 alters the cytokine profile and decreases the vaccinal antibody titer in pigs. Toxicol. Sci. 2005, 84, 301–307. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Y. Fumonisin B(1) induces immunotoxicity and apoptosis of chicken splenic lymphocytes. Front. Vet. Sci. 2022, 9, 898121. [Google Scholar] [CrossRef]
- Dilkin, P.; Direito, G.; Simas, M.M.S.; Mallmann, C.A.; Corrêa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chem.-Biol. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Terciolo, C.; Bracarense, A.P.; Souto, P.C.M.C.; Cossalter, A.M.; Dopavogui, L.; Loiseau, N.; Oliveira, C.A.F.; Pinton, P.; Oswald, I.P. Fumonisins at doses below EU regulatory limits induce histological alterations in piglets. Toxins 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
| Group | Content of Mycotoxin (mg/kg, ppm) | Enzyme Additives (%) | |||
|---|---|---|---|---|---|
| FB1 + FB2 | Zearalenone | Aflatoxin B1 | Deoxynivalenol | ||
| CK | 0.62 ± 0.3 | 0.02 ± 0.001 | <0.5 | <10 | - |
| FBs | 4.41 ± 0.5 | 0.05 ± 0.015 | 0.004 ± 0.001 | <10 | - |
| FUMzyme® | 4.41 ± 0.5 | 0.05 ± 0.015 | 0.004 ± 0.001 | <10 | 0.2 |
| FumDSB | 4.41 ± 0.5 | 0.05 ± 0.015 | 0.004 ± 0.001 | <10 | 0.2 |
| Ingredients a | % | Nutrition Levels | |
|---|---|---|---|
| Maize | 30.00 | DE/(MJ·kg−1) | 12.79 |
| Concentrate | 70.00 | Crude protein (%) | 17.40 |
| Calcium (%) | 0.78 | ||
| Total phosphorus (%) | 0.52 | ||
| Lysine (%) | 0.96 | ||
| Methionine + cysteine (%) | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lv, Z.; Czabany, T.; Nagl, V.; Krska, R.; Wang, X.; Han, B.; Tao, H.; Liu, J.; Wang, J. Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets. Toxins 2024, 16, 3. https://doi.org/10.3390/toxins16010003
Wang Z, Lv Z, Czabany T, Nagl V, Krska R, Wang X, Han B, Tao H, Liu J, Wang J. Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets. Toxins. 2024; 16(1):3. https://doi.org/10.3390/toxins16010003
Chicago/Turabian StyleWang, Zhenlong, Zonghao Lv, Tibor Czabany, Veronika Nagl, Rudolf Krska, Xiumin Wang, Bing Han, Hui Tao, Jie Liu, and Jinquan Wang. 2024. "Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets" Toxins 16, no. 1: 3. https://doi.org/10.3390/toxins16010003
APA StyleWang, Z., Lv, Z., Czabany, T., Nagl, V., Krska, R., Wang, X., Han, B., Tao, H., Liu, J., & Wang, J. (2024). Comparison Study of Two Fumonisin-Degrading Enzymes for Detoxification in Piglets. Toxins, 16(1), 3. https://doi.org/10.3390/toxins16010003







