Aflatoxin B1 Degradation by Ery4 Laccase: From In Vitro to Contaminated Corn
Abstract
1. Introduction
2. Results
2.1. Aflatoxin B1 Degradation in Buffer Solution Using Different LMSs
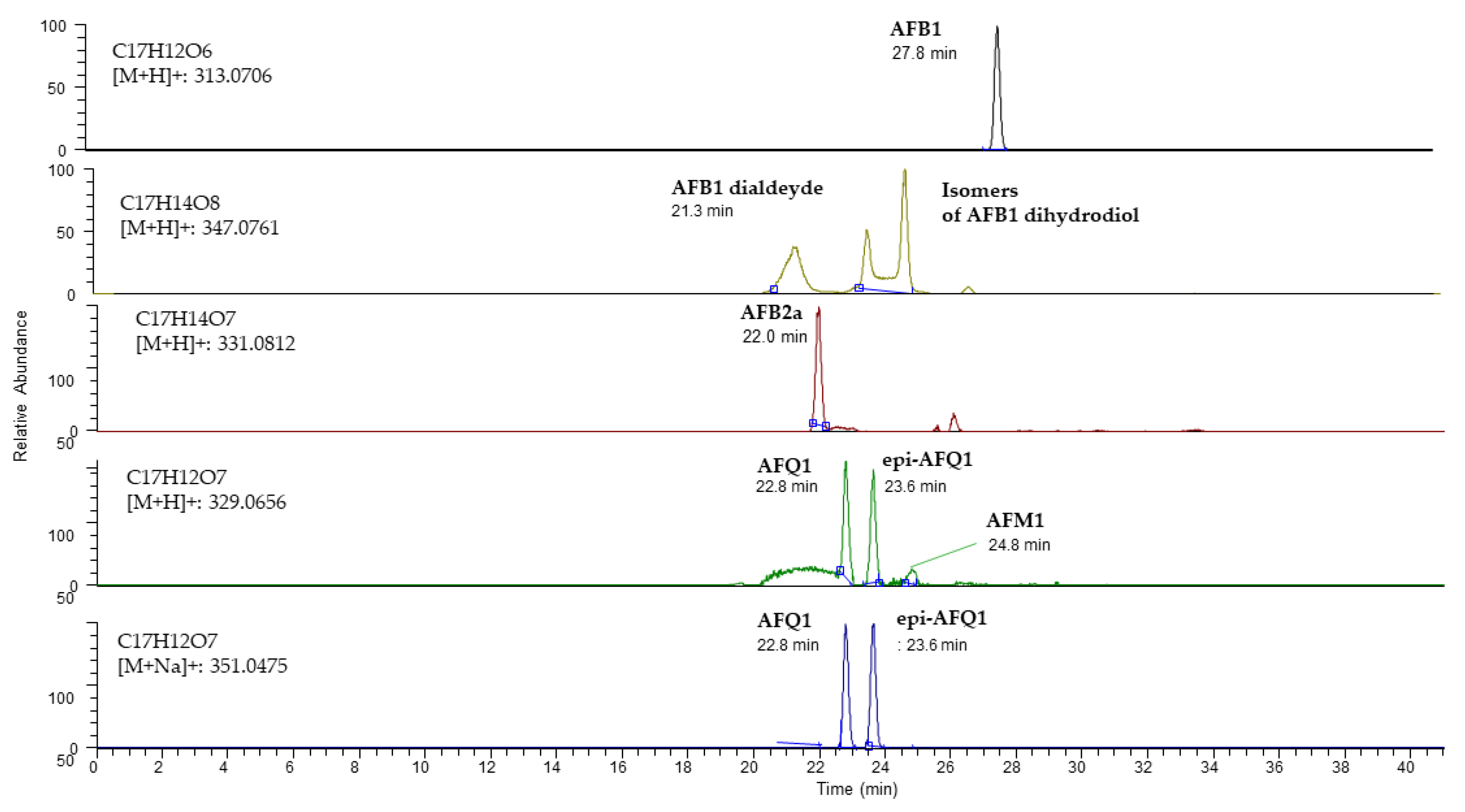
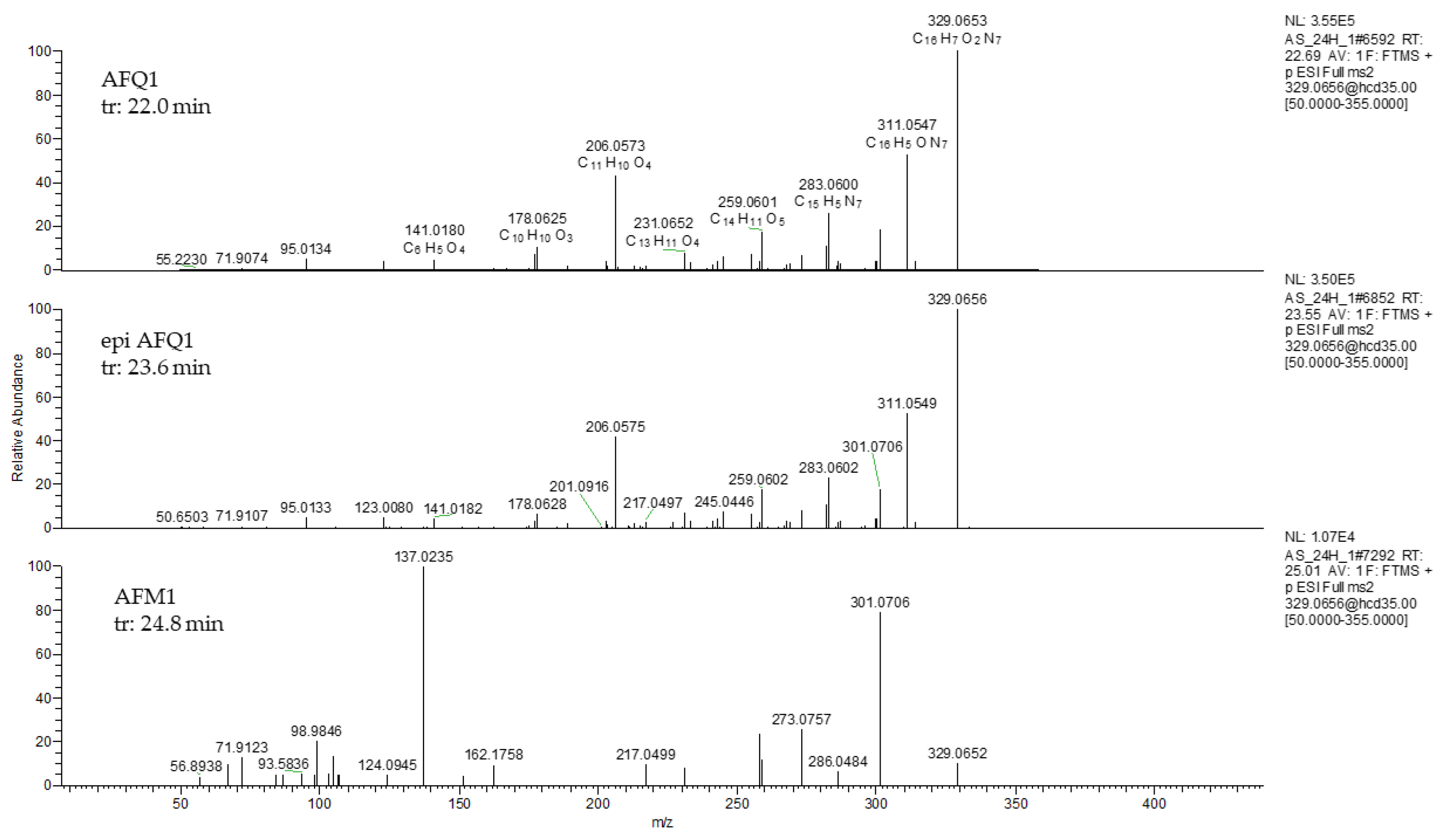
2.2. In Vitro Study of Aflatoxin B1 Degradation Products
2.3. Aflatoxin B1 Degradation in Corn
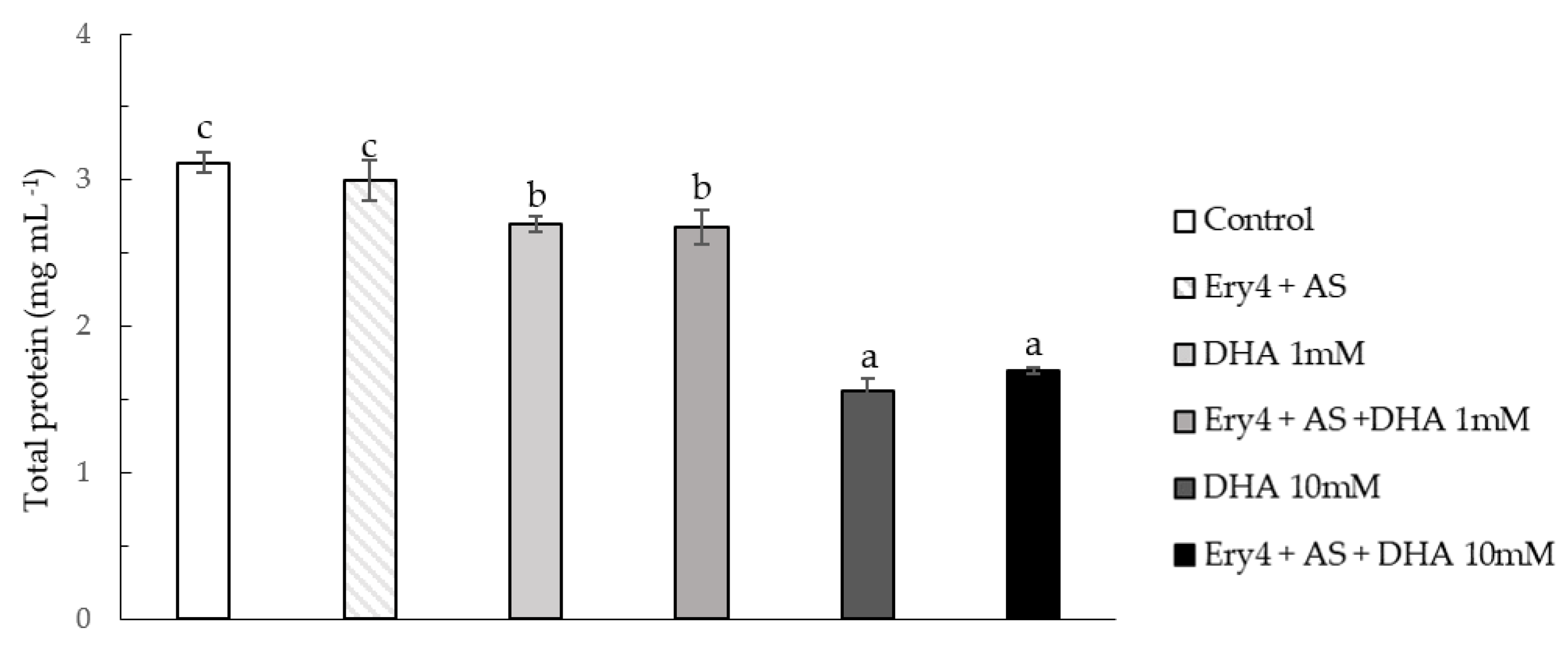
2.4. Protein Content
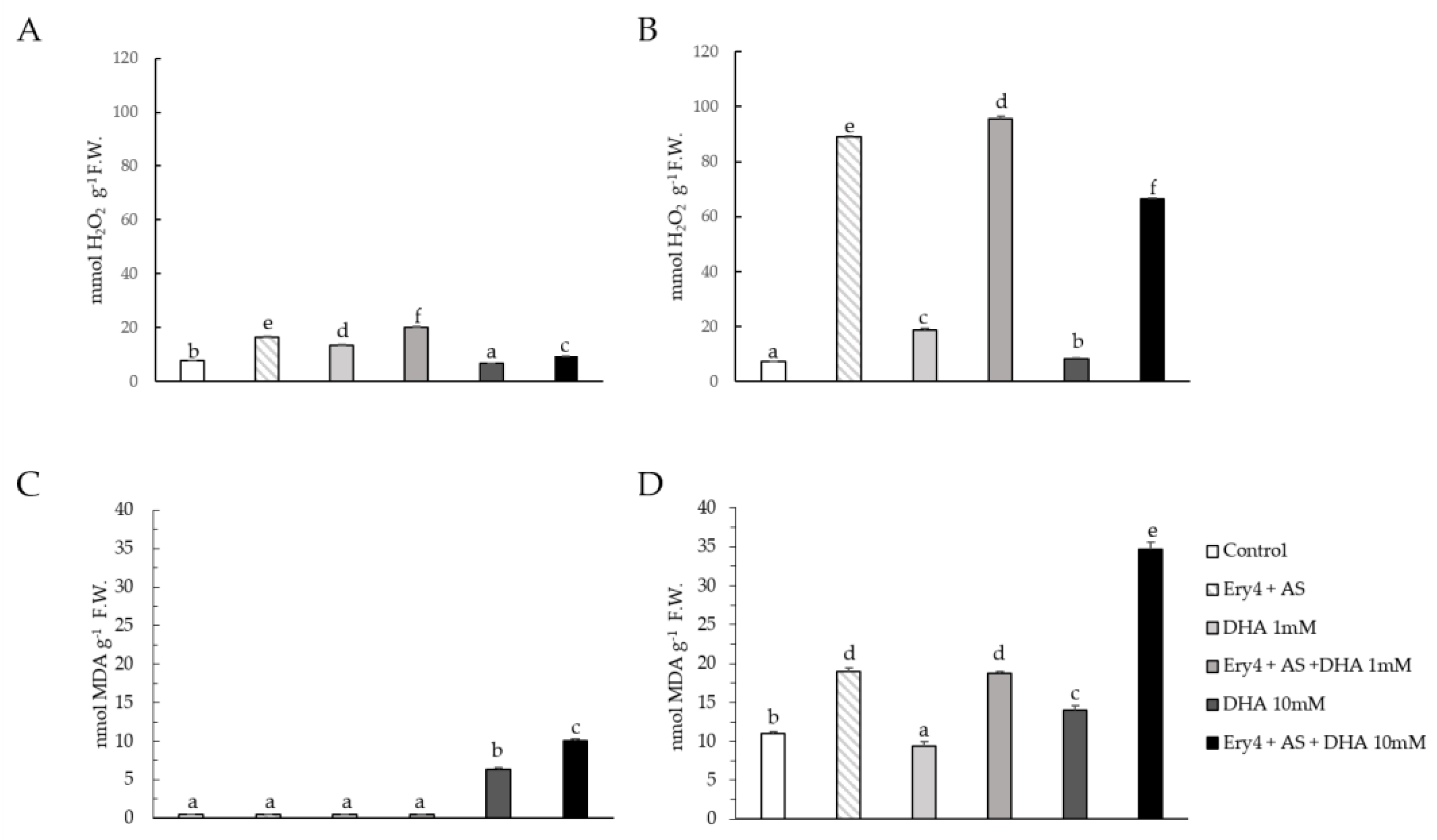
2.5. Lipid Peroxidation and H2O2 Content
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals, Reagents, and Corn Kernels
5.2. Preparation of Standards
5.3. Laccase Production and Purification
5.4. Laccase Activity Assay
5.5. Aflatoxin B1 Degradation In Vitro
5.6. In Vitro Study of Aflatoxin B1 Degradation Products
5.7. UHPLC-HRMS Analysis
5.8. Aflatoxin B1 Degradation in Corn
5.9. Aflatoxin Extraction and Chemical Analyses
5.9.1. Corn Samples Clean-Up
5.9.2. HPLC Analyses
5.10. Lipid Peroxidation and H2O2 Content
5.11. Protein Content
5.12. Statistical Analyses
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [PubMed]
- Aflatoxins. IARC Monographs; International Agency for the Research on Cancer: Lyon, France, 2012; Volume 100F. [Google Scholar]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- RASFF Annual Report 2021. Available online: https://food.ec.europa.eu/system/files/2022-07/acn_annual-report_2021-final.pdf (accessed on 2 December 2022).
- Loi, M.; Logrieco, A.F.; Pusztahelyi, T.; Hornok, L.; Pócsi, I. Advanced Mycotoxin Control and Decontamination Techniques In View Of An Increased Aflatoxin Risk In Europe Due To Climate Change. Front. Microbiol. 2023, 13, 5258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef]
- Loi, M.; Renaud, J.B.; Rosini, E.; Pollegioni, L.; Vignali, E.; Haidukowski, M.; Sumarah, M.W.; Logrieco, A.F.; Mule, G. Enzymatic transformation of aflatoxin B1 by Rh_DypB peroxidase and characterization of the reaction products. Chemosphere 2020, 250, 126296. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Cimmarusti, M.T.; Mirabelli, V.; Haidukowski, M.; Logrieco, A.F.; Caliandro, R.; Mule, G. In vitro single and combined mycotoxins degradation by Ery4 laccase from Pleurotus eryngii and redox mediators. Food Control 2018, 90, 401–406. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2015/786 of 19 May 2015 Defining Acceptability Criteria for Detoxification Processes Applied to Products Intended for Animal Feed as Provided for in Directive 2002/32/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/reg/2015/786 (accessed on 14 November 2022).
- Wang, L.; Huang, Q.; Wu, J.; Wanying, W.; Jiang, J.; Yan, H.; Huang, J.; Sun, Y.; Deng, Y. The metabolism and biotransformation of AFB1: Key enzymes and pathways. Biochem. Pharmacol. 2022, 199, 115005. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res./Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Mahuku, G.; Nzioki, H.S.; Mutegi, C.; Kanampiu, F.; Narrod, C.; Makumbi, D. Pre-harvest management is a critical practice for minimizing aflatoxin contamination of maize. Food Control 2019, 96, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Mohammadi Shad, Z.; Venkitasamy, C.; Wen, Z. Maize distillers dried grains with solubles: Production, properties, and potential uses. Cereal Chem. 2021, 98, 999–1019. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Swain, M.R. Bioethanol production from maize and wheat: Food, fuel, and future. In Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Academic Press: London, UK, 2019; pp. 45–59. [Google Scholar] [CrossRef]
- Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Blandino, M. Fate of regulated, masked, emerging mycotoxins and secondary fungal metabolites during different large-scale maize dry-milling processes. Food Res. Int. 2021, 140, 109861. [Google Scholar] [CrossRef]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin distribution in maize dry-milling products and by-products: Impact of two industrial degermination systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef]
- Deepak, T.S.; Jayadeep, P.A. Prospects of Maize (Corn) Wet Milling By-Products as a Source of Functional Food Ingredients and Nutraceuticals. Food Technol. Biotechnol. 2022, 60, 109–120. [Google Scholar] [CrossRef]
- Parsons, H.T.; Fry, S.C. Oxidation of dehydroascorbic acid and 2,3-diketogulonate under plant apoplastic conditions. Phytochemistry 2012, 75, 41–49. [Google Scholar] [CrossRef]
- Vega, V.A.; Young, M.; Todd, S. Laboratory Information Bulletin: Quantitation of Aflatoxin M1 in Bovine Milk by Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2016, 99, 174–179. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, J.; Qin, W.; Li, J.; Zhou, Y.; Gao, Y. Enhanced transformation of organic pollutants by mild oxidants in the presence of synthetic or natural redox mediators: A review. Water Res. 2021, 189, 116667. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Fidal Kumar, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. The role of natural laccase redox mediators in simultaneous dye decolorization and power production in microbial fuel cells. Energies 2018, 11, 3455. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Y.; Ma, Q.; Ji, C.; Zhao, L. Detoxification of the mycoestrogen zearalenone by Bacillus licheniformis spore CotA laccase and application of immobilized laccase in contaminated corn meal. LWT 2022, 163, 113548. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of four major mycotoxins by eight manganese peroxidases in presence of a dicarboxylic acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Obleser, K.; Kalaus, H.; Seidl, B.; Kozich, M.; Stanetty, C.; Mihovilovic, M.D. An Organic Chemist’s Guide to Mediated Laccase Oxidation. ChemBioChem 2022, 23, e202200411. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.; Aguila, S.; Baratto, M.C.; Martorana, A.; Basosi, R.; Alderete, J.B.; Vazquez-Duhalt, R. Prediction model based on decision tree analysis for laccase mediators. Enzym. Microb. 2013, 52, 68–76. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase–mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Tripathi, R.P.; Singh, B.; Bisht, S.S.; Pandey, J. L-Ascorbic Acid in Organic Synthesis: An Overview. Curr. Org. Chem. 2009, 13, 99–122. [Google Scholar] [CrossRef]
- Matsui, T.; Kitagawa, Y.; Okumura, M.; Shigeta, Y. Accurate standard hydrogen electrode potential and applications to the redox potentials of vitamin C and NAD/NADH. J. Phys. Chem. A 2015, 119, 369–376. [Google Scholar] [CrossRef]
- Paciolla, C.; Paradiso, A.; de Pinto, M.C. Cellular redox homeostasis as central modulator in plant stress. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–23. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Vignault, A.; Gombau, J.; Jourdes, M.; Moine, V.; Canals, J.M.; Fermaud, M.; Roudet, J.; Zamora, F.; Teissedre, P.L. Oenological tannins to prevent Botrytis cinerea damage in grapes and musts: Kinetics and electrophoresis characterization of laccase. Food Chem. 2020, 316, 126334. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef] [PubMed]
- Okwara, P.C.; Afolabi, I.S.; Ahuekwe, E.F. Application of laccase in aflatoxin B1 degradation: A review. IOP Conf. Ser. Mater. Eng. 2021, 1107, 012178. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, R.; Ng, T.B.; Lai, Y.; Yang, J.; Ye, X. A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B1. Toxins 2020, 12, 476. [Google Scholar] [CrossRef]
- Qin, X.; Xin, Y.; Zou, J.; Su, X.; Wang, X.; Wang, Y.; Zhang, J.; Tu, T.; Yao, B.; Luo, H.; et al. Efficient degradation of aflatoxin B1 and zearalenone by laccase-like multicopper oxidase from Streptomyces thermocarboxydus in the presence of mediators. Toxins 2021, 13, 754. [Google Scholar] [CrossRef]
- Johnson, W.W.; Harris, T.M.; Guengerich, F.P. Kinetics and mechanism of hydrolysis of aflatoxin B1 exo-8, 9-epoxide and rearrangement of the dihydrodiol. J. Am. Chem. Soc. 1996, 118, 8213–8220. [Google Scholar] [CrossRef]
- Mykkänen, H.; Zhu, H.; Salminen, E.; Juvonen, R.O.; Ling, W.; Ma, J.; Polychronaki, N.; Kemiläinen, H.; Mykkänen, O.; Salminen, S.; et al. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N7-guanine) in young Chinese males. Int. J. Cancer 2005, 115, 879–884. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, J.; Wang, W.; Ni, D.; Jiang, H. Dehydroascorbic acid affects the stability of catechins by forming conjunctions. Molecules 2020, 25, 4076. [Google Scholar] [CrossRef]
- Chavan, S.N.; De Kesel, J.; Desmedt, W.; Degroote, E.; Singh, R.R.; Nguyen, G.T.; Demeestere, K.; De Meyer, T.; Kyndt, T. Dehydroascorbate induces plant resistance in rice against root-knot nematode Meloidogyne graminicola. Mol. Plant Pathol. 2022, 23, 1303–1319. [Google Scholar] [CrossRef]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Nardai, G.; Braun, L.; Csala, M.; Csermely, P.; Benedetti, A.; Mandl, J.; Banhegyi, G. Protein disulfide isomerase and protein thiol dependent dehydroascorbate reduction and ascorbate accumulation in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2001, 276, 8825–8828. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; De Sanctis, R.; Scarlatti, F.; Vallorani, L.; De Bellis, R.; Serafini, G.; Bianchi, M.; Stocchi, V. Dehydroascorbic acid irreversibly inhibits hexokinase activity. Mol. Cell. Biochem. 2000, 209, 145–153. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Zhang, B.; Sanchez, R.; Golde, D.W. Vitamin C is a kinase inhibitor: Dehydroascorbic acid inhibits IkappaBalpha kinase beta. Mol. Cell Biol. 2004, 24, 6645–6652. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef]
- Kumar, V.; Bahuguna, A.; Ramalingam, S.; Dhakal, G.; Shim, J.J.; Kim, M. Recent technological advances in mechanism, toxicity, and food perspectives of enzyme-mediated aflatoxin degradation. CritiCal Rev. Food Sci. Nutr. 2022, 62, 5395–5412. [Google Scholar] [CrossRef]
- Alberts, J.F.; Davids, I.; Moll, W.-D.; Schatzmayr, G.; Burger, H.-M.; Shephard, G.S.; Gelderblom, W.C.A. Enzymatic Detoxification of the Fumonisin Mycotoxins during Dry Milling of Maize. Food Control 2021, 123, 107726. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Reduction of the Fusarium Mycotoxins: Deoxynivalenol, Nivalenol and Zearalenone by Selected Non-Conventional Yeast Strains in Wheat Grains and Bread. Molecules 2022, 27, 1578. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, F.; Chitrakar, B.; Wei, G.; Wang, X.; Sang, Y. Three recombinant peroxidases as a degradation agent of aflatoxin M1 applied in milk and beer. Food Res. Int. 2022, 166, 112352. [Google Scholar] [CrossRef]
- Yang, P.; Xiao, W.; Lu, S.; Jiang, S.; Zheng, Z.; Zhang, D.; Zhan, M.; Jiang, S.; Jiang, S. Recombinant Expression of Trametes versicolor Aflatoxin B1-Degrading Enzyme (TV-AFB1D) in Engineering Pichia pastoris GS115 and Application in AFB1 Degradation in AFB1-Contaminated Peanuts. Toxins 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Alborino, V.; Lanzuise, S.; Lombardi, N.; Marra, R.; Balestrieri, A.; Ritieni, A.; Woo, S.L.; Vinale, F. Trichoderma Enzymes for Degradation of Aflatoxin B1 and Ochratoxin A. Molecules 2022, 27, 3959. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Garcia, S.; Sibaja, K.V.M.; Nogueira, W.V.; Feltrin, A.C.P.; Pinheiro, D.F.A.; Cerqueira, M.B.R.; Furlong, E.; Garda-Buffon, J. Peroxidase as a simultaneous degradation agent of ochratoxin A and zearalenone applied to model solution and beer. Food Res. Int. 2020, 131, 109039. [Google Scholar] [CrossRef] [PubMed]
- Marimón Sibaja, K.V.; de Oliveira Garcia, S.; Feltrin, A.C.P.; Diaz Remedi, R.; Cerqueira, M.B.R.; Badiale-Furlong, E.; Garda-Buffon, J. Aflatoxin biotransformation by commercial peroxidase and its application in contaminated food. J. Chem. Technol. Biotechnol. 2019, 94, 1187–1194. [Google Scholar] [CrossRef]
- Loi, M.; Quintieri, L.; Fanelli, F.; Caputo, L.; Mulè, G. Application of a recombinant laccase-chlorogenic acid system in protein crosslink and antioxidant properties of the curd. Food Res. Int. 2018, 106, 763–770. [Google Scholar] [CrossRef]
- Loi, M.; Quintieri, L.; De Angelis, E.; Monaci, L.; Logrieco, A.F.; Caputo, L.; Mule, G. Yield improvement of the Italian fresh Giuncata cheese by laccase–induced protein crosslink. Int. Dairy J. 2020, 100, 104555. [Google Scholar] [CrossRef]
- AOAC 971.22-1988; Association of Official Analytical Chemists. Standards for Aflatoxins. Thin-Layer Chromatographic Method. 2000. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=625 (accessed on 29 September 2022).
- Ciasca, B.; Lanubile, A.; Marocco, A.; Pascale, M.; Logrieco, A.F.; Lattanzio, V.M.T. Application of an Integrated and Open Source Workflow for LC-HRMS Plant Metabolomics Studies. Case-Control Study: Metabolic Changes of Maize in Response to Fusarium verticillioides Infection. Front. Plant Sci. 2020, 11, 664. [Google Scholar] [CrossRef]
- AOAC 991.31; Association of Official Analytical Chemists. Aflatoxins in Corn, Raw Peanuts, and Peanut Butter. Immunoaffinity Column (Aflatest). 2002. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1723 (accessed on 29 September 2022).
- Villani, A.; Tommasi, F.; Paciolla, C. The arbuscular mycorrhizal fungus Glomus viscosum improves the tolerance to verticillium wilt in artichoke by modulating the antioxidant defense systems. Cells 2021, 10, 1944. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant-Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitaton of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

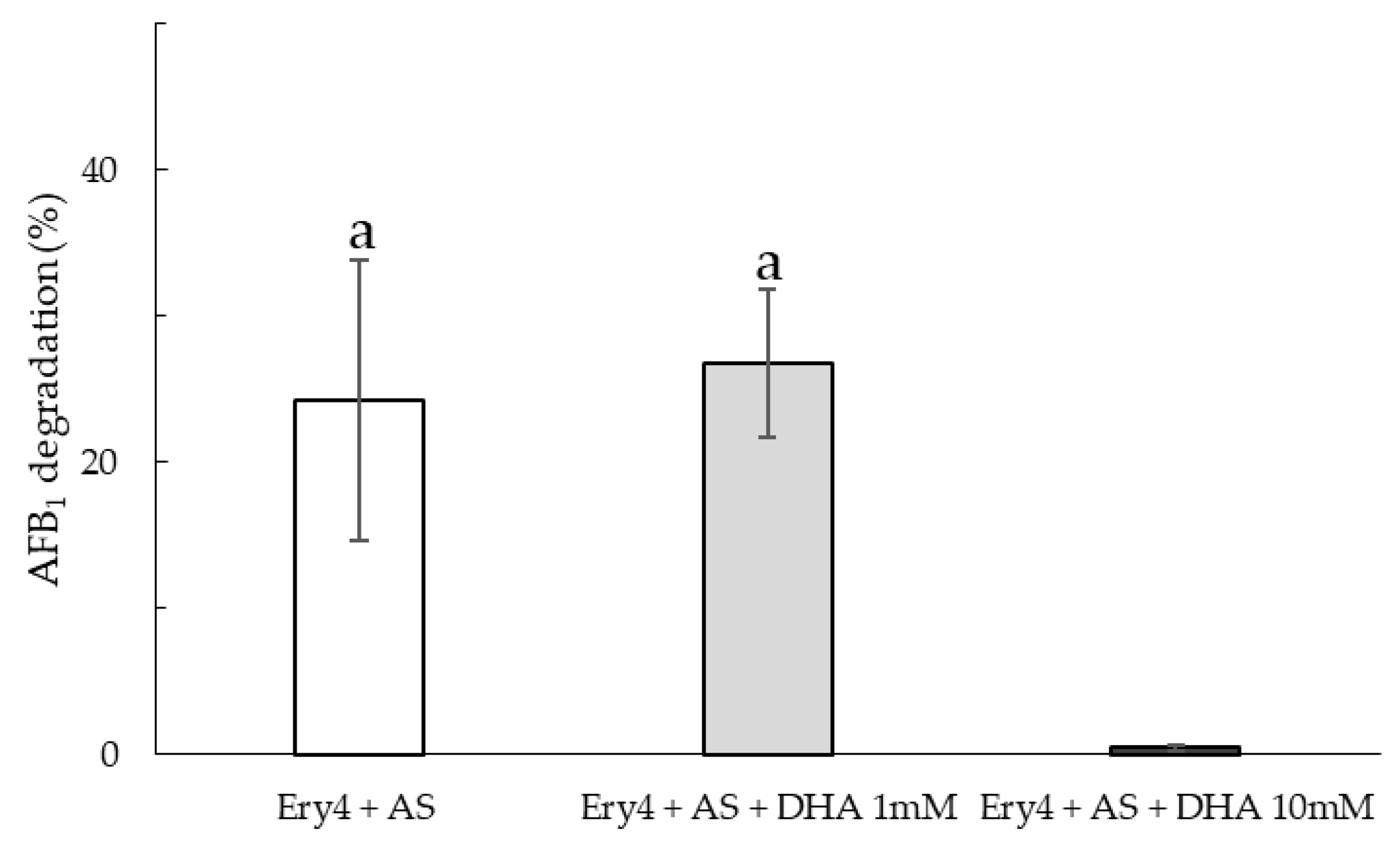
| Time (h) | Ery4 + AS | Ery4 + AS + DHA 1 mM | Ery4 + AS + DHA 10 mM |
|---|---|---|---|
| 1 | 100 | 84.1 ± 3.5 | 6.1 ± 1.0 |
| 2 | 100 | 90.3 ± 0.8 | 8.6 ± 6.5 |
| 3 | 100 | 93.2 ± 2.7 | 8.4 ± 4.2 |
| 6 | 100 | 95.7 ± 1.9 | 11.8 ± 0.3 |
| 24 | 100 | 100 | 16.6 ± 1.6 |
| 48 | 100 | 100 | 20.3 ± 1.8 |
| Proposed Product | Molecular Formula | Mass Exact [M+H]+/[M+Na]+ | Error (ppm) | Retention Time (min) | Fragments |
|---|---|---|---|---|---|
| AFB1-dialdehyde/AFB1-dihydrodiol | C17H14O8 | 347.0761 | 1.6 | 21.3 | No data |
| AFB2a/P1 | C17H14O7 | 331.0812 | 1.2 | 22.0 | 303.0861, 299.0550, 284.0316, 267.0288, 239.0338 |
| AFQ1 | C17H12O7 | 329.0656/351.0475 | 2.2 | 22.8 | 311.0343, 283.0601, 259.0601, 247.0602 |
| Unidentified peak 1 | C17H14O8 | 347.0761 | 1.3 | 23.6 | No data |
| epi-AFQ1 | C17H12O7 | 329.0656/351.0475 | 1.9 | 23.6 | 311.0343, 283.0601, 259.0601, 247.0603 |
| Unidentified peak 2 | C17H14O8 | 347.0761 | 1.3 | 24.7 | No data |
| AFM1 | C17H12O7 | 329.0656/351.0475 | 3.2 | 24.8 | 329.0656, 301.07, 273.05 |
| Unidentified peak3 | C17H14O8 | 347.0761 | 2.3 | 26.7 | No data |
| AFB1 | C17H12O6 | 313.0706 | 1.0 | 27.8 | 285.0575, 270.0522, 243.0652, 201.0912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loi, M.; De Leonardis, S.; Ciasca, B.; Paciolla, C.; Mulè, G.; Haidukowski, M. Aflatoxin B1 Degradation by Ery4 Laccase: From In Vitro to Contaminated Corn. Toxins 2023, 15, 310. https://doi.org/10.3390/toxins15050310
Loi M, De Leonardis S, Ciasca B, Paciolla C, Mulè G, Haidukowski M. Aflatoxin B1 Degradation by Ery4 Laccase: From In Vitro to Contaminated Corn. Toxins. 2023; 15(5):310. https://doi.org/10.3390/toxins15050310
Chicago/Turabian StyleLoi, Martina, Silvana De Leonardis, Biancamaria Ciasca, Costantino Paciolla, Giuseppina Mulè, and Miriam Haidukowski. 2023. "Aflatoxin B1 Degradation by Ery4 Laccase: From In Vitro to Contaminated Corn" Toxins 15, no. 5: 310. https://doi.org/10.3390/toxins15050310
APA StyleLoi, M., De Leonardis, S., Ciasca, B., Paciolla, C., Mulè, G., & Haidukowski, M. (2023). Aflatoxin B1 Degradation by Ery4 Laccase: From In Vitro to Contaminated Corn. Toxins, 15(5), 310. https://doi.org/10.3390/toxins15050310







