Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells
Abstract
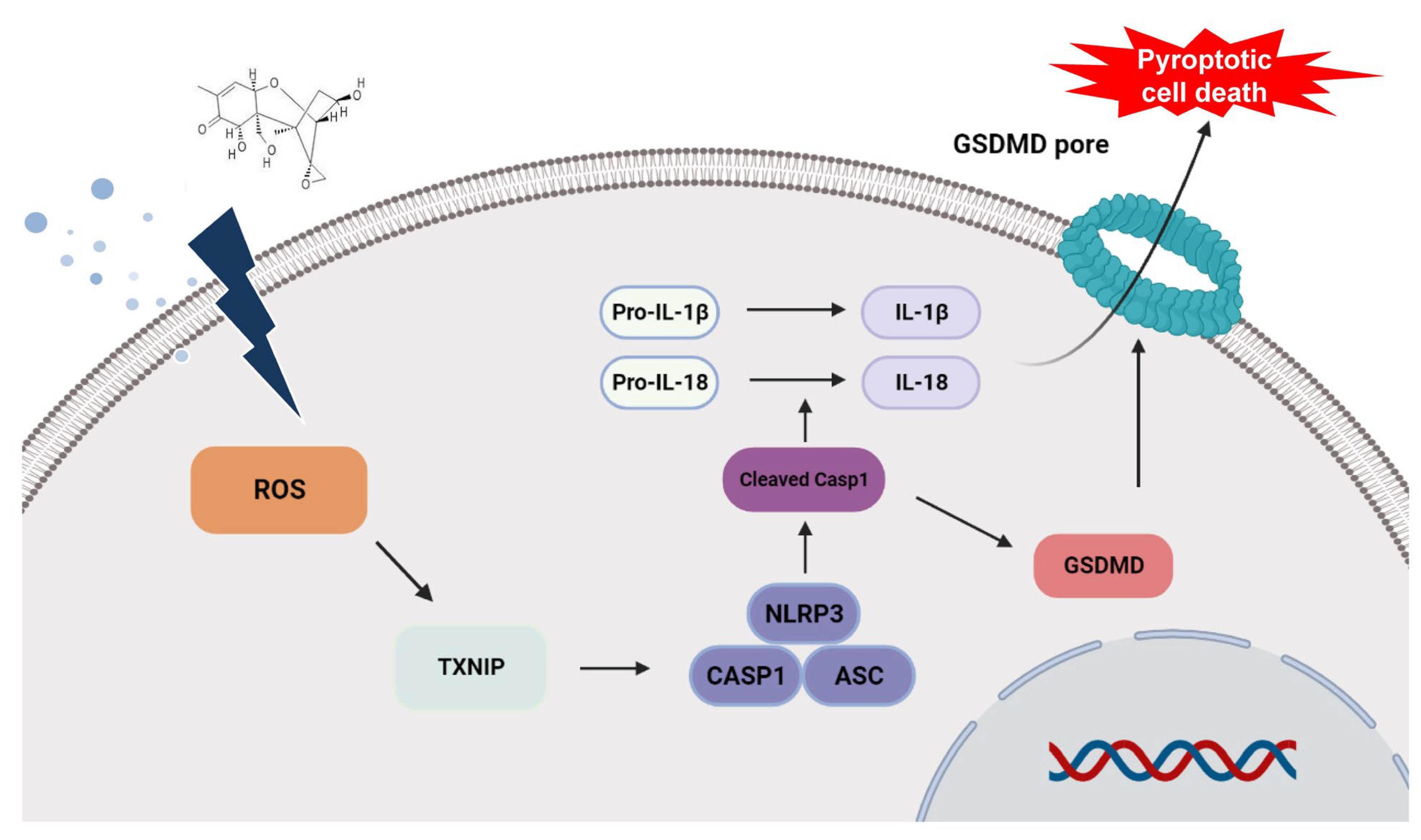
1. Introduction
2. Results
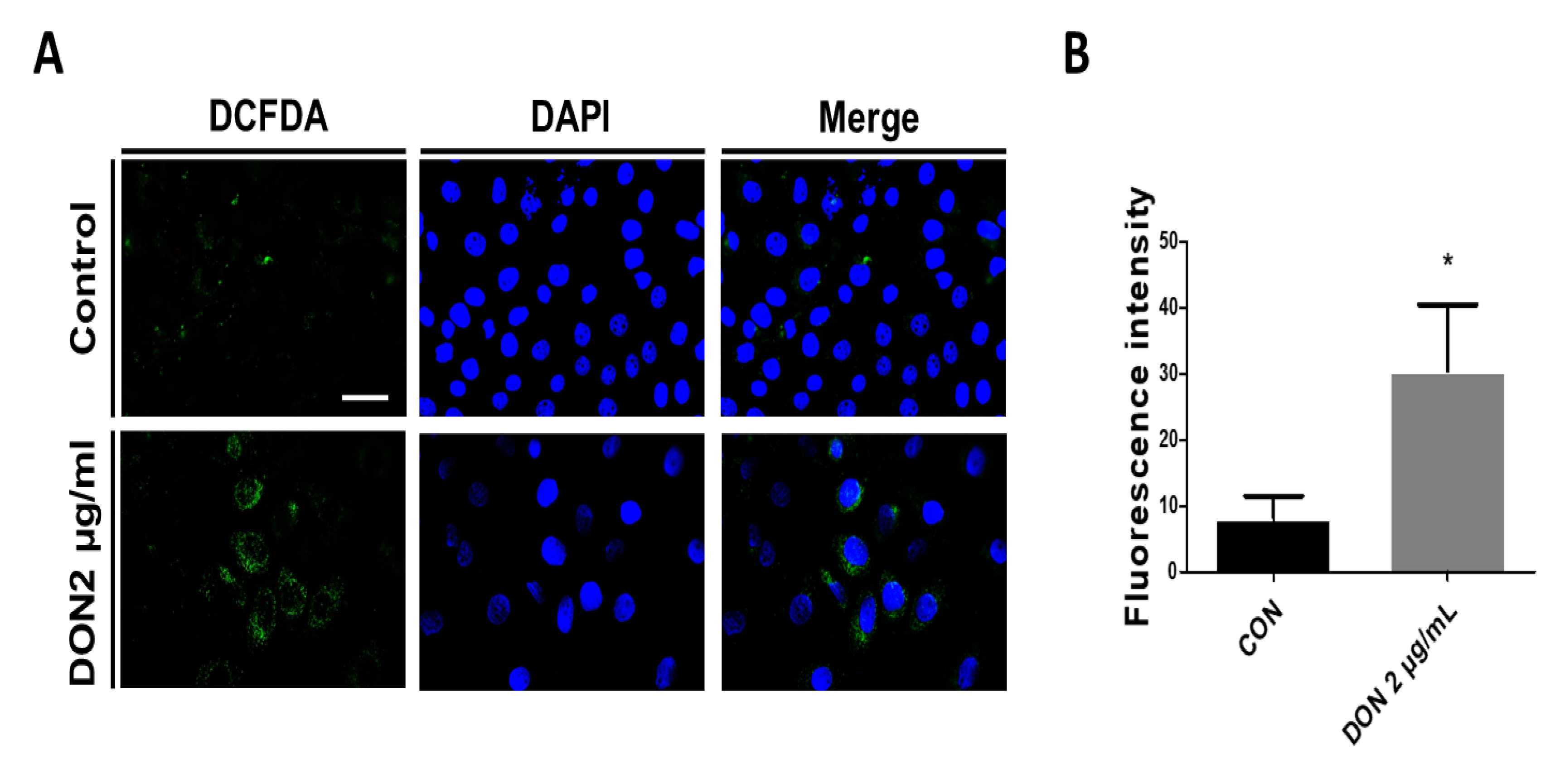
2.1. DON Induced ROS Production in Porcine Intestinal Epithelial Cells
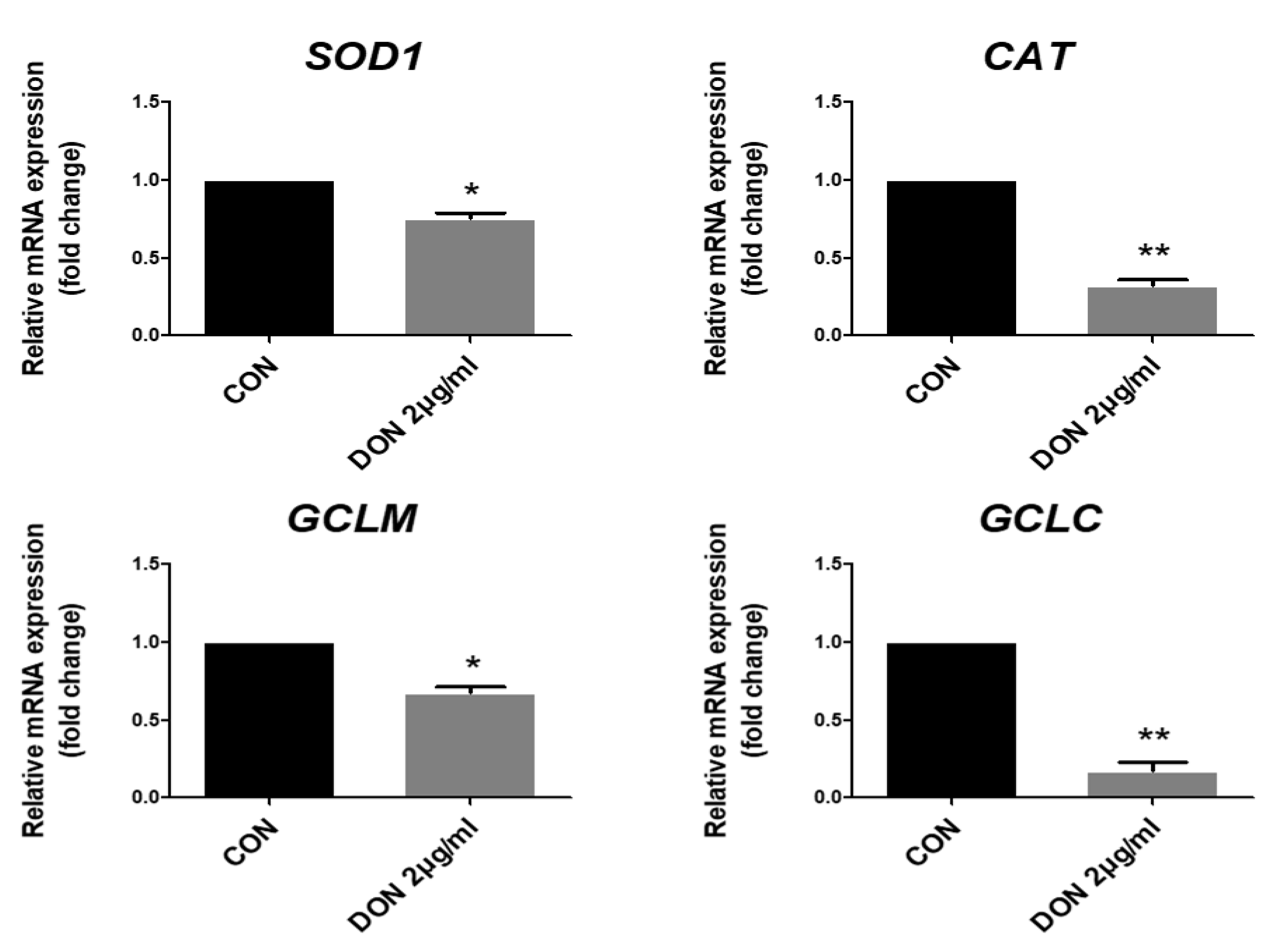
2.2. DON Decreased the Expression of Antioxidant-Related Genes in Porcine Intestinal Epithelial Cells
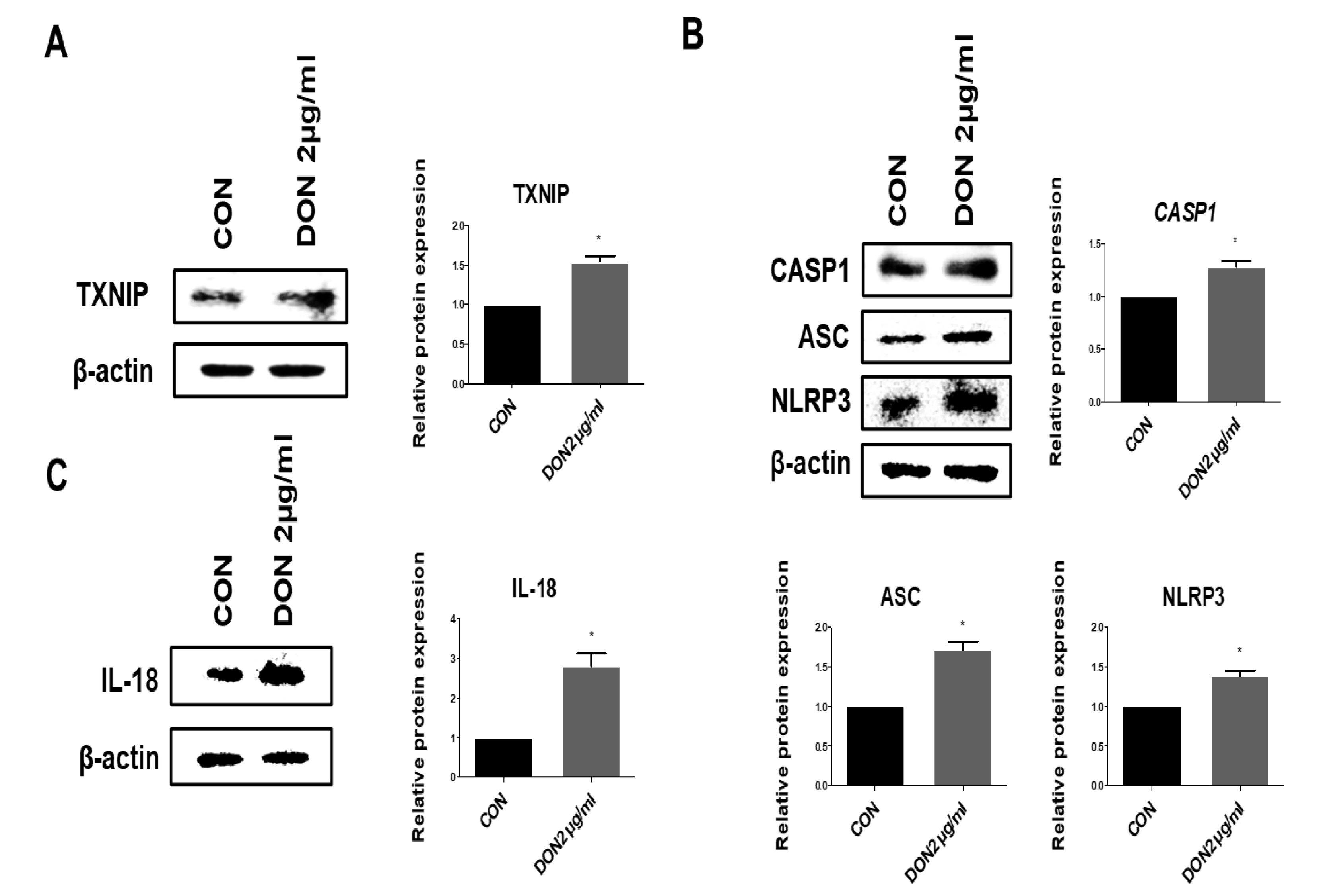
2.3. Effect of Thioredoxin Interacting Protein, Inflammasome, and Pro-Inflammatory Cytokines in the mRNA Levels after DON Treatment
2.4. Effect of TXNIP, Inflammasome, and Pro-Inflammatory Cytokines in the Protein Levels after DON Treatment
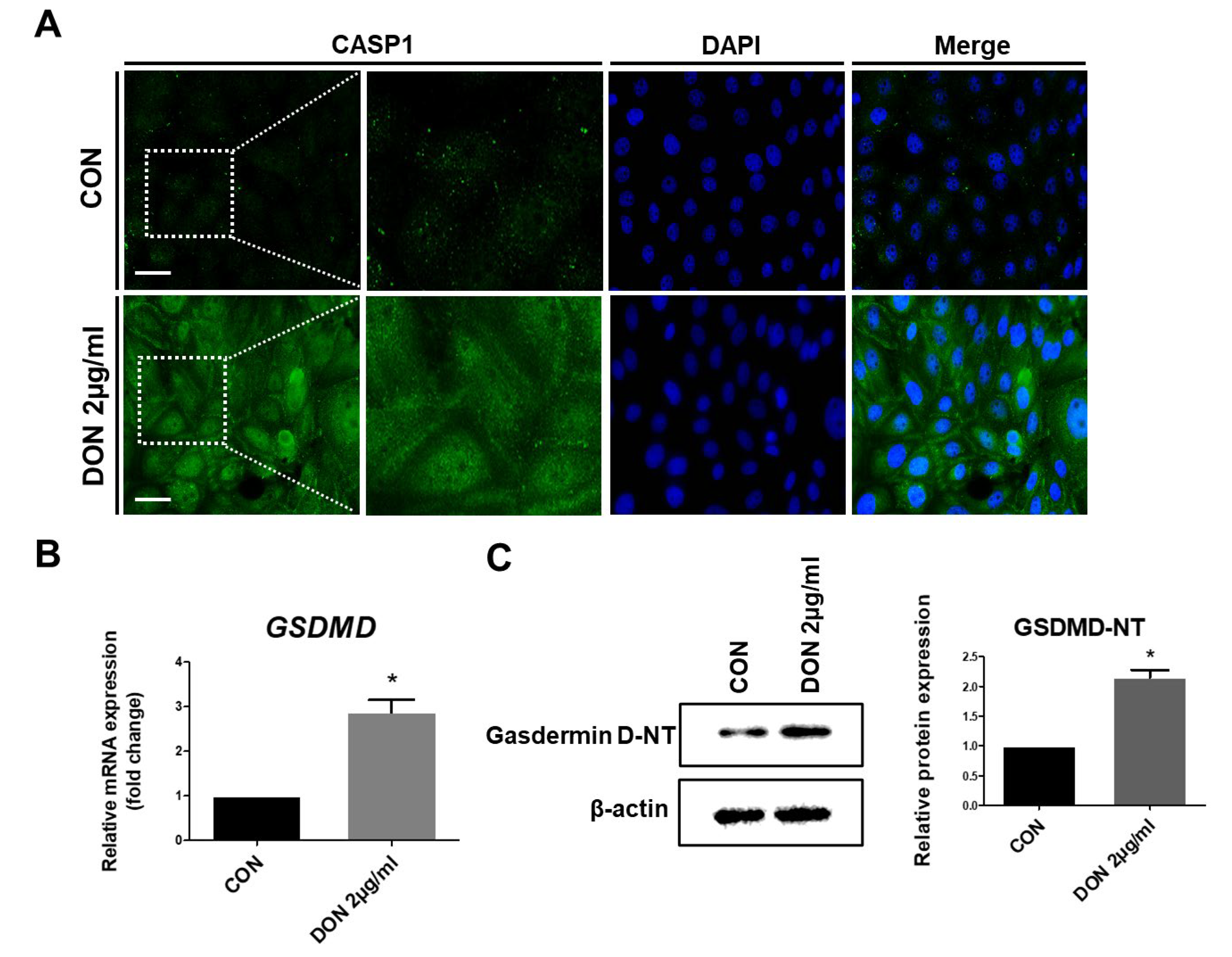
2.5. DON Induced Pyroptotic Cell Death in IPEC-J2 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Treatment
5.2. Cell Passage and Cryopreservation
5.3. Intracellular ROS Detection
5.4. Quantitative Real-Time PCR and Western Blotting
5.5. Immunofluorescence Staining of Porcine Intestinal Epithelial Cells
5.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastasiadis, V.; Raptis, I.; Economou, A.; Kakabakos, S.E.; Petrou, P.S. Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor. Biosensors 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Fan, Y.; Chen, C.; Zuo, Z.; Deng, J. Research Progress on the Toxic Antagonism of Selenium Against Mycotoxins. Biol. Trace Element Res. 2018, 190, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (Vomitoxin)-Induced Anorexia Is Induced by the Release of Intestinal Hormones in Mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Vatzia, E.; Pierron, A.; Saalmüller, A.; Mayer, E.; Gerner, W. Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins 2019, 11, 644. [Google Scholar] [CrossRef]
- Tiemann, U.; Dänicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef]
- Kowalska, K.; Kozieł, M.J.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Piastowska-Ciesielska, A.W. Deoxynivalenol induces apoptosis and autophagy in human prostate epithelial cells via PI3K/Akt signaling pathway. Arch. Toxicol. 2021, 96, 231–241. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, H.; Yang, Z.; Hu, L.; Liu, Q.; Wang, Y.; Wei, H.-K.; Peng, J. Gly-Pro-Ala peptide and FGSHF3 exert protective effects in DON-induced toxicity and intestinal damage via decreasing oxidative stress. Food Res. Int. 2020, 139, 109840. [Google Scholar] [CrossRef]
- Hanyu, H.; Yokoi, Y.; Nakamura, K.; Ayabe, T.; Tanaka, K.; Uno, K.; Miyajima, K.; Saito, Y.; Iwatsuki, K.; Shimizu, M.; et al. Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure. Toxins 2020, 12, 610. [Google Scholar] [CrossRef]
- Monaco, A.; Ovryn, B.; Axis, J.; Amsler, K. The Epithelial Cell Leak Pathway. Int. J. Mol. Sci. 2021, 22, 7677. [Google Scholar] [CrossRef]
- Itoh, M.; Bissell, M.J. The Organization of Tight Junctions in Epithelia: Implications for Mammary Gland Biology and Breast Tumorigenesis. J. Mammary Gland. Biol. Neoplasia 2003, 8, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Metz, L.; Meddings, J.B.; Sharkey, K.A.; Yong, V.W. The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. Intestinal Permeability Defects: Is It Time to Treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, Y.; He, C.; Lin, X.; Li, C.; Xu, W.; Duan, N.; Wu, S.; Wang, Z. Application of PEG-CdSe@ZnS quantum dots for ROS imaging and evaluation of deoxynivalenol-mediated oxidative stress in living cells. Food Chem. Toxicol. 2020, 146, 111834. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2018, 34, 975–991. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free. Radic. Biol. Med. 2019, 147, 8–22. [Google Scholar] [CrossRef]
- Hebbar, S.; Knust, E. Reactive oxygen species (ROS) constitute an additional player in regulating epithelial development. Bioessays 2021, 43, e2100096. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Liu, S.; Zhu, Q.; Lu, F. Astilbin ameliorates deoxynivalenol-induced oxidative stress and apoptosis in intestinal porcine epithelial cells (IPEC-J2). J. Appl. Toxicol. 2020, 40, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xiao, H.; Li, X.; Zhu, J.; Gao, J.; Wang, L.; Hu, C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants 2021, 10, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.-H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, G. Mechanisms and Therapeutic Regulation of Pyroptosis in Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2020, 21, 1456. [Google Scholar] [CrossRef]
- Seoane, P.I.; Lee, B.; Hoyle, C.; Yu, S.; Lopez-Castejon, G.; Lowe, M.; Brough, D. The NLRP3–inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 2020, 219, e202006194. [Google Scholar] [CrossRef]
- Liao, J.; Hu, Z.; Li, Q.; Li, H.; Chen, W.; Huo, H.; Han, Q.; Zhang, H.; Guo, J.; Hu, L.; et al. Endoplasmic Reticulum Stress Contributes to Copper-Induced Pyroptosis via Regulating the IRE1α-XBP1 Pathway in Pig Jejunal Epithelial Cells. J. Agric. Food Chem. 2022, 70, 1293–1303. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.-Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxidative Med. Cell. Longev. 2017, 2017, 9743280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-L.; Zhang, G.-L. Deoxynivalenol and Zearalenone: Different Mycotoxins with Different Toxic Effects in the Sertoli Cells of Equus asinus. Cells 2021, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Guo, W.; Zhao, Y.; Liu, G.; Wu, J.; Chang, C. Deoxynivalenol-Induced Cytotoxicity and Apoptosis in IPEC-J2 Cells Through the Activation of Autophagy by Inhibiting PI3K-AKT-mTOR Signaling Pathway. ACS Omega 2019, 4, 18478–18486. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and Zearalenone—Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Deng, C.; Ji, C.; Qin, W.; Cao, X.; Zhong, J.; Li, Y.; Srinivas, S.; Feng, Y.; Deng, X. Deoxynivalenol inhibits proliferation and induces apoptosis in human umbilical vein endothelial cells. Environ. Toxicol. Pharmacol. 2016, 43, 232–241. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.; Kim, D.; Moon, Y. Mycotoxin detoxifiers attenuate deoxynivalenol-induced pro-inflammatory barrier insult in porcine enterocytes as an in vitro evaluation model of feed mycotoxin reduction. Toxicol. Vitr. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Rohweder, D.; Kersten, S.; Valenta, H.; Sondermann, S.; Schollenberger, M.; Drochner, W.; Dänicke, S. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from wheat straw and chaff in pigs. Arch. Anim. Nutr. 2013, 67, 37–47. [Google Scholar] [CrossRef]
- Nagl, V.; Schatzmayr, G. Deoxynivalenol and its masked forms in food and feed. Curr. Opin. Food Sci. 2015, 5, 43–49. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Yu, L.; Wang, Y.; Wang, H.; Wu, Z.; Wu, S.; Bao, W. SLC4A11 and MFSD3 Gene Expression Changes in Deoxynivalenol Treated IPEC-J2 Cells. Front. Genet. 2021, 12, 697883. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Q.; He, W.; Ge, L.; Huang, K. Deoxynivalenol aggravates the immunosuppression in piglets and PAMs under the condition of PEDV infection through inhibiting TLR4/NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 231, 113209. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.-J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2019, 467, 1–12. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free. Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef]
- Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Shen, Y.; Wu, S. Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins 2022, 14, 275. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Lin, Z.-F.; Xu, H.-B.; Wang, J.-Y.; Lin, Q.; Ruan, Z.; Liu, F.-B.; Jin, W.; Huang, H.-H.; Chen, X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem. Biophys. Res. Commun. 2013, 441, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, B.; Yao, J.; Duan, D.; Fang, J. Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food Funct. 2015, 6, 2091–2100. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Rajput, S.A.; Shaukat, A.; Rajput, I.R.; Kamboh, A.A.; Iqbal, Z.; Saeed, M.; Akhtar, R.W.; Shah, S.A.H.; Raza, M.A.; El Askary, A.; et al. Ginsenoside Rb1 prevents deoxynivalenol-induced immune injury via alleviating oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112333. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Jia, Y.; Cui, R.; Wang, C.; Feng, Y.; Li, Z.; Tong, Y.; Qu, K.; Liu, C.; Zhang, J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020, 32, 101534. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.-Z.; Wang, S.-L.; Pan, P.; Yao, J.; Wu, K.; Li, Z.-S.; Bai, Y.; Linghu, E.-Q. Targeting NLRP3 Inflammasome in Inflammatory Bowel Disease: Putting out the Fire of Inflammation. Inflammation 2019, 42, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.-F.; Yu, T.; Chu, X.-M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ma, Q.; Liu, Y.-N.; Peng, N.; Yuan, F.-F.; Li, X.-G.; Li, M.; Wu, Y.-S.; Li, B.-L.; Song, W.-B.; et al. Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. J. Hepatol. 2015, 63, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, S.; He, W.; Liu, S.; Mao, X.; Yin, L.; Yue, D.; Zhang, P.; Huang, K.; Chen, X. Crucial Function of Caveolin-1 in Deoxynivalenol-Induced Enterotoxicity by Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis. J. Agric. Food Chem. 2022, 70, 12968–12981. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Lee, S.; Jayasooriya, R.G.P.T.; Jin, C.-Y.; Choi, Y.H.; Kim, G.-Y. Deoxynivalenol enhances IL-1ß expression in BV2 microglial cells through activation of the NF-?B pathway and the ASC/NLRP3 inflammasome. EXCLI J. 2019, 18, 356–369. [Google Scholar] [CrossRef]
- Song, J.-L.; Sun, Y.-J.; Liu, G.-Q.; Zhang, G.-L. Deoxynivalenol and zearalenone: Different mycotoxins with different toxic effects in donkey (Equus asinus) endometrial epithelial cells. Theriogenology 2021, 179, 162–176. [Google Scholar] [CrossRef]
- Kang, T.H.; Kang, K.S.; Lee, S.I. Deoxynivalenol Induces Apoptosis via FOXO3a-Signaling Pathway in Small-Intestinal Cells in Pig. Toxics 2022, 10, 535. [Google Scholar] [CrossRef]
- Rao, X.; Lai, D.; Huang, X. A New Method for Quantitative Real-Time Polymerase Chain Reaction Data Analysis. J. Comput. Biol. 2013, 20, 703–711. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.H.; Shin, S.; Park, J.; Lee, B.R.; Lee, S.I. Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells. Toxins 2023, 15, 300. https://doi.org/10.3390/toxins15040300
Kang TH, Shin S, Park J, Lee BR, Lee SI. Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells. Toxins. 2023; 15(4):300. https://doi.org/10.3390/toxins15040300
Chicago/Turabian StyleKang, Tae Hong, Sangsu Shin, JeongWoong Park, Bo Ram Lee, and Sang In Lee. 2023. "Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells" Toxins 15, no. 4: 300. https://doi.org/10.3390/toxins15040300
APA StyleKang, T. H., Shin, S., Park, J., Lee, B. R., & Lee, S. I. (2023). Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells. Toxins, 15(4), 300. https://doi.org/10.3390/toxins15040300




