Alphatoxin Nanopore Detection of Aflatoxin, Ochratoxin and Fumonisin in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
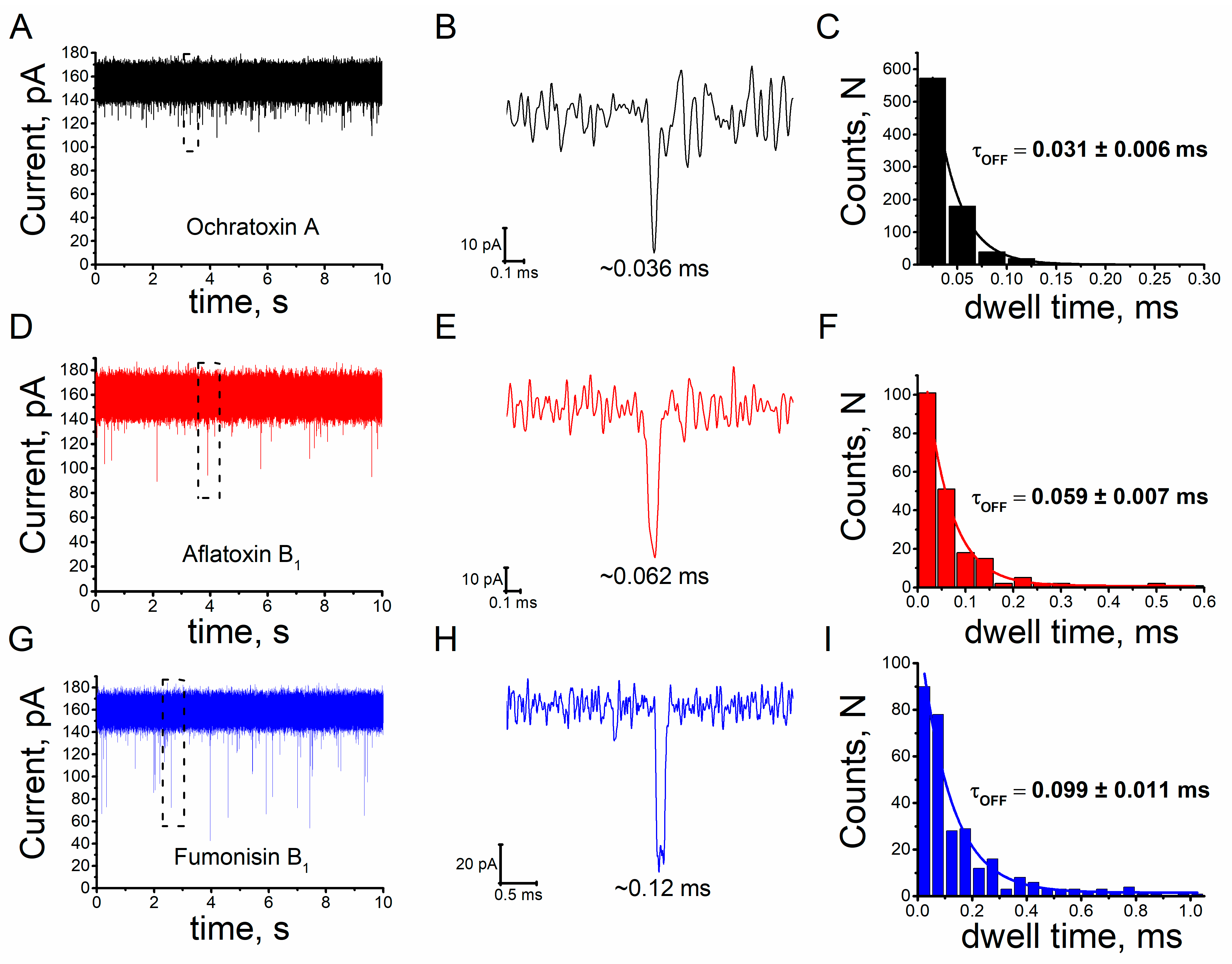
2.1. Mycotoxins Induce Reversible and Characteristic Blockage of Ionic Current in Single Alphatoxin Nanopore
2.2. Augmentation of the Salt Concentration Increases the Frequency of Ionic Current Blockage in the Unitary Alphatoxin Nanopore
2.3. Sensitivity of the Unitary Alphatoxin Nanopore to Mycotoxins
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Planar Lipid Bilayer Formation, Insertion of Single Alphatoxin Nanopore and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W. Mycotoxins, mycotoxicoses, mycotoxicology and Mycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Carballo, D.; Tolosa, J.; Ferrer, E.; Berrada, H. Dietary exposure assessment to mycotoxins through total diet studies. A review. Food Chem. Toxicol. 2019, 128, 8–20. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenár, F.; Mahakamchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination Across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-occurrence of mycotoxins in maize food and maize-based feed from small-scale farms in Brazil: A pilot study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef]
- Scussel, V.M.; Savi, G.D.; Costas, L.L.; Xavier, J.J.; Manfio, D.; Bittencourt, K.O.; Aguiar, K.; Stein, S.M. Fumonisins in corn (Zea mays L.) from Southern Brazil. Food Addit. Contam. Part B Surveill. 2014, 7, 151–155. [Google Scholar] [CrossRef]
- De Boevr, M.; Di Mavungu, J.D.; Landschoo, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef]
- Barac, A. Mycotoxins and human disease. In Clinically Relevant Mycoses; Springer: Cham, Switzerland, 2019; pp. 213–225. [Google Scholar] [CrossRef]
- Lombard, M.J. Mycotoxin exposure and infant and young child growth in Africa: What do we know? Ann. Nutr. Metab. 2014, 64, 42–52. [Google Scholar] [CrossRef]
- Pavlović, N.M. Balkan endemic nephropathy-current status and future perspectives. Clin. Kidney J. 2013, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ediage, E.N.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, M.; Magro, S.L.; Nardiello, D.; Palermo, C.; Centonze, D. Determination of fumonisins B₁ and B₂ in maize food products by a new analytical method based on high-performance liquid chromatography and fluorimetric detection with post-column derivatization. Methods Mol. Biol. 2011, 739, 187–194. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef]
- Huang, P.; Liu, Q.; Wang, J.; Ma, Z.; Lu, J.; Kong, W. Development of an economic ultrafast liquid chromatography with tandem mass spectrometry method for trace analysis of multiclass mycotoxins in Polygonum multiflorum. J. Sep. Sci. 2019, 42, 491–500. [Google Scholar] [CrossRef]
- Blechová, P.; Havlová, P.; Gajdosová, D.; Havel, J. New possibilities of matrix-assisted laser desorption ionization time of flight mass spectrometry to analyze barley malt quality. Highly sensitive detection of mycotoxins. Environ. Toxicol. 2006, 21, 403–408. [Google Scholar] [CrossRef]
- Piermarini, S.; Micheli, L.; Ammida, N.H.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Ning, B.; Liu, M.; Lv, Z.; Sun, Z.; Peng, Y.; Chen, C.; Li, J.; Gao, Z. Simultaneous and rapid detection of six different mycotoxins using an immunochip. Biosens. Bioelectron. 2012, 34, 44–50. [Google Scholar] [CrossRef]
- Azri, F.A.; Sukor, R.; Selamat, J.; Abu Bakar, F.; Yusof, N.A.; Hajian, R. Electrochemical Immunosensor for Detection of Aflatoxin B₁ Based on Indirect Competitive ELISA. Toxins 2018, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, F.; Zheng, N.; Luo, Q.; Wang, H.; Wang, H.; Li, S.; Wang, J. Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron. 2014, 56, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Spiegel, H.; Krause, H.J.; Schillberg, S.; Schröper, F. Sensitive Aflatoxin B1 Detection Using Nanoparticle-Based Competitive Magnetic Immunodetection. Toxins 2020, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Cremer, P.S. Stochastic sensors inspired by biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.P.; Júnior, J.J.S.; Machado, D.C.; Melo, M.C.A.; Rodrigues, C.G. Stochastic biosensing by a single protein nanopore in the development of analytical tools. Quim. Nova 2015, 38, 817–827. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Han, Y.; Zhou, S.; Guan, X. Nanopore stochastic detection: Diversity, sensitivity, and beyond. Acc. Chem. Res. 2013, 46, 2867–2877. [Google Scholar] [CrossRef]
- Gurnev, P.A.; Nestorovich, E.M. Channel-Forming Bacterial Toxins in Biosensing and Macromolecule Delivery. Toxins 2014, 6, 2483–2540. [Google Scholar] [CrossRef]
- Ying, Y.L.; Li, D.W.; Liu, Y.; Dey, S.K.; Kraatz, H.B.; Long, Y.T. Recognizing the translocation signals of individual peptide–oligonucleotide conjugates using an α-hemolysin nanopore. Chem. Commun. 2012, 48, 8784–8786. [Google Scholar] [CrossRef]
- Movileanu, L.; Schmittschmitt, J.P.; Scholtz, J.M.; Bayley, H. Interactions of peptides with a protein pore. Biophys. J. 2005, 89, 1030–1045. [Google Scholar] [CrossRef]
- Mereuta, L.; Asandei, A.; Schiopu, I.; Park, Y.; Luchian, T. Nanopore-Assisted, Sequence-Specific Detection, and Single-Molecule Hybridization Analysis of Short, Single-Stranded DNAs. Anal. Chem. 2019, 91, 8630–8637. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Machado, D.C.; Chevtchenko, S.F.; Krasilnikov, O.V. Mechanism of KCl enhancement in detection of nonionic polymers by nanopore sensors. Biophys. J. 2008, 95, 5186–5192. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, Q.; Guan, X. Stochastic nanopore sensors for the detection of terrorist agents: Current status and challenges. Anal. Chim. Acta 2010, 675, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Osaki, T.; Sasaki, H.; Takinoue, M.; Yoshizawa, S.; Takeuchi, S. Rapid detection of a cocaine-binding aptamer using biological nanopores on a chip. J. Am. Chem. Soc. 2011, 133, 8474–8477. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Gu, L.Q.; Cheley, S.; Braha, O.; Bayley, H. Stochastic sensing of TNT with a genetically engineered pore. Chembiochem 2005, 6, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Ying, Y.L.; Gu, Z.; Cao, C.; Yan, B.Y.; Wang, H.F.; Long, Y.T. Single molecule study of initial structural features on the amyloidosis process. Chem. Commun. 2016, 52, 5542–5545. [Google Scholar] [CrossRef]
- Gupta, J.; Zhao, Q.; Wang, G.; Kang, X.; Guan, X. Simultaneous detection of CMPA and PMPA, hydrolytes of soman and cyclosarin nerve agents, by nanopore analysis. Sens. Actuators B Chem. 2013, 176, 625–631. [Google Scholar] [CrossRef]
- Júnior, J.J.S.; Soares, T.A.; Pol-Fachin, L.; Machado, D.C.; Rusu, V.H.; Aguiar, J.P.; Rodrigues, C.G. Alpha-hemolysin nanopore allows discrimination of the microcystins variants. RSC Adv. 2019, 9, 14683–14691. [Google Scholar] [CrossRef]
- Li, T.; Su, Z.; Li, Y.; Xi, L.; Li, G. An aptamer-assisted biological nanopore biosensor for ultra-sensitive detection of ochratoxin A with a portable single-molecule measuring instrument. Talanta 2022, 248, 123619. [Google Scholar] [CrossRef]
- Meni Wanunu, M.; Morrison, W.; Rabin, Y.; Grosberg, A.Y.; Meller, A. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nat. Nanotechnol. 2010, 5, 160–165. [Google Scholar] [CrossRef]
- Mereuta, L.; Asandei, A.; Ho Seo, C.; Park, Y.; Luchian, T. Quantitative Understanding of pH- and Salt-Mediated Conformational Folding of Histidine-Containing, β-Hairpin-like Peptides, through Single-Molecule Probing with Protein Nanopores. ACS Appl. Mater. Interfaces 2014, 6, 13242–13256. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Machado, D.C.; da Silva, A.M.; Júnior, J.J.; Krasilnikov, O.V. Hofmeister effect in confined spaces: Halogen ions and single molecule detection. Biophys. J. 2011, 100, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.C.; Júnior, J.J.S.; Melo, M.C.A.; Silva, A.M.B.; Fontes, A.; Rodrigues, C.G. Effects of alkali and ammonium ions in the detection of poly(ethyleneglycol) by alpha-hemolysin nanopore sensor. RSC Adv. 2016, 6, 56647–56655. [Google Scholar] [CrossRef]
- Wei, K.; Yao, F.; Kang, X.F. Single-molecule porphyrin-metal ion interaction and sensing application. Biosens. Bioelectron. 2018, 109, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Yan, S.; Du, X.; Zhang, P.; Chen, H.Y.; Huang, S. Retarded Translocation of Nucleic Acids through α-Hemolysin Nanopore in the Presence of a Calcium Flux. ACS Appl. Mater. Interfaces 2020, 12, 26926–26935. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef]
- Robertson, J.W.; Rodrigues, C.G.; Stanford, V.M.; Rubinson, K.A.; Krasilnikov, O.V.; Kasianowicz, J.J. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl. Acad. Sci. USA 2007, 104, 8207–8211. [Google Scholar] [CrossRef]
- Krasilnikov, O.V.; Rodrigues, C.G.; Bezrukov, S.M. Single polymer molecules in a protein nanopore in the limit of a strong polymer-pore attraction. Phys. Rev. Lett. 2006, 97, 018301. [Google Scholar] [CrossRef]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control. 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Jiang, F.; Li, P.; Zong, C.; Yang, H. Surface-plasmon-coupled chemiluminescence amplification of silver nanoparticles modified immunosensor for high-throughput ultrasensitive detection of multiple mycotoxins. Anal. Chim. Acta 2020, 1114, 58–65. [Google Scholar] [CrossRef]
- Bayley, H.; Braha, O.; Gu, L.Q. Stochastic sensing with protein pores. Adv. Mater. 2000, 12, 139–142. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.W.; van IJzendoorn, L.J.; Prins, M.W. Particle Motion Analysis Reveals Nanoscale Bond Characteristics and Enhances Dynamic Range for Biosensing. ACS Nano 2016, 10, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.A.R.d.; Silva Júnior, J.J.d.; Cavalcanti, M.I.d.S.; Machado, D.C.; Medeiros, P.L.; Rodrigues, C.G. Alphatoxin Nanopore Detection of Aflatoxin, Ochratoxin and Fumonisin in Aqueous Solution. Toxins 2023, 15, 183. https://doi.org/10.3390/toxins15030183
Silva AARd, Silva Júnior JJd, Cavalcanti MIdS, Machado DC, Medeiros PL, Rodrigues CG. Alphatoxin Nanopore Detection of Aflatoxin, Ochratoxin and Fumonisin in Aqueous Solution. Toxins. 2023; 15(3):183. https://doi.org/10.3390/toxins15030183
Chicago/Turabian StyleSilva, Artur Alves Rodrigues da, Janilson José da Silva Júnior, Maria Isabel dos Santos Cavalcanti, Dijanah Cota Machado, Paloma Lys Medeiros, and Claudio Gabriel Rodrigues. 2023. "Alphatoxin Nanopore Detection of Aflatoxin, Ochratoxin and Fumonisin in Aqueous Solution" Toxins 15, no. 3: 183. https://doi.org/10.3390/toxins15030183
APA StyleSilva, A. A. R. d., Silva Júnior, J. J. d., Cavalcanti, M. I. d. S., Machado, D. C., Medeiros, P. L., & Rodrigues, C. G. (2023). Alphatoxin Nanopore Detection of Aflatoxin, Ochratoxin and Fumonisin in Aqueous Solution. Toxins, 15(3), 183. https://doi.org/10.3390/toxins15030183




