Microcystins Exposure Associated with Blood Lipid Profiles and Dyslipidemia: A Cross-Sectional Study in Hunan Province, China
Abstract
1. Introduction
2. Results
2.1. General Characteristics of the Study Population
2.2. The Distribution of Lipid Profiles and the Prevalence of Dyslipidemia According to Serum MCs Concentration
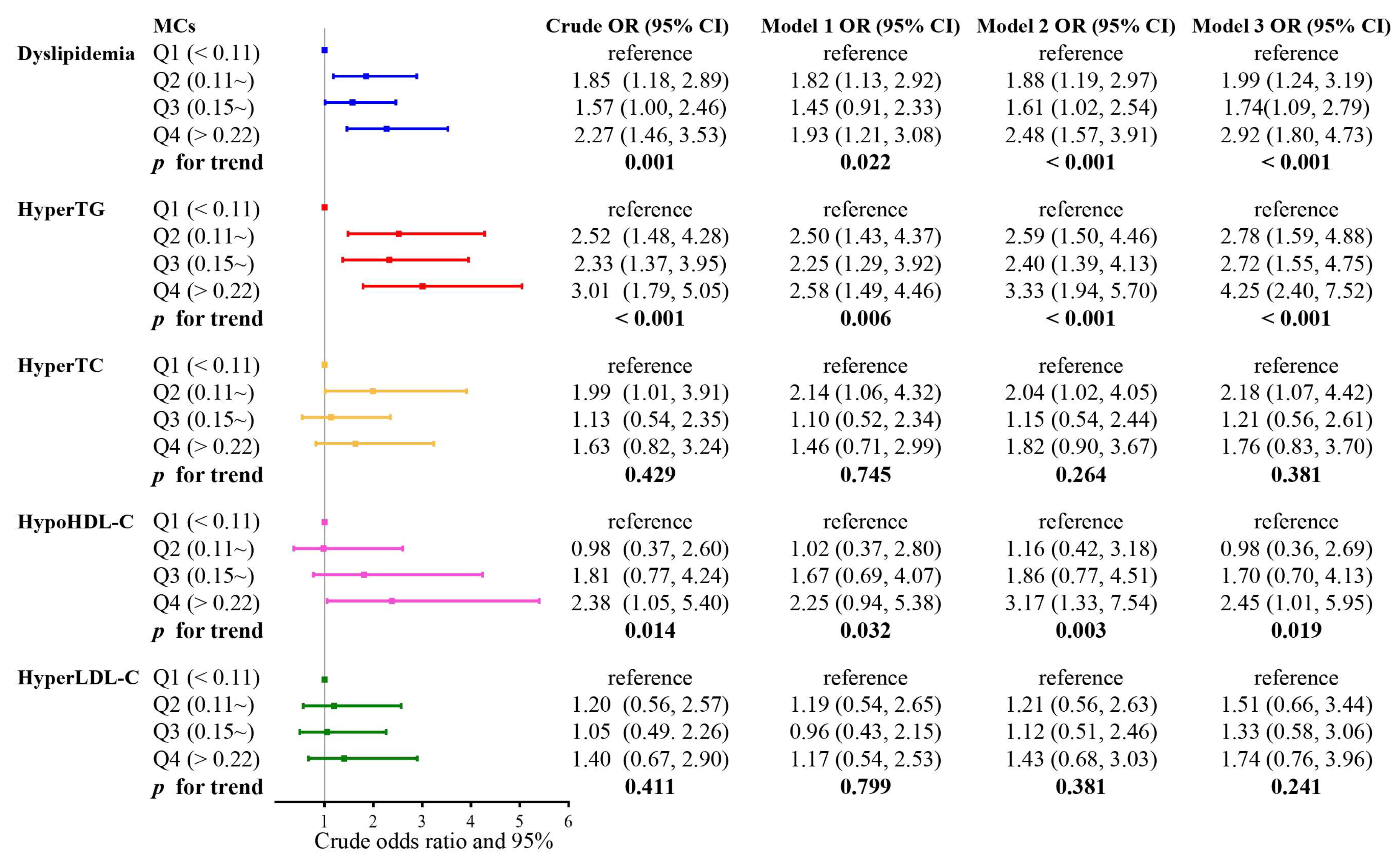
2.3. The Associations of Serum MCs Quartiles with the Risk of Dyslipidemia
2.4. Association of Serum MCs with Blood Lipids Levels
2.5. Stratified Analysis
2.6. Interaction of MCs with Metal Exposure
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Study Design and Population
5.2. Data Collection
5.3. Serum MCs Concentration Detection
5.4. Measurement of Lipid Markers and Diagnosis of Abnormal Blood Lipids
5.5. Covariates and Models
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Xiao, J.; Yang, Z.; Ji, L.; Jia, W.; Weng, J.; Lu, J.; Shan, Z.; Liu, J.; Tian, H.; et al. Serum Lipids and Lipoproteins in Chinese Men and Women. Circulation 2012, 125, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey, M.C.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- GBD. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Anderson, K.M.; Castelli, W.P.; Levy, D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA 1987, 257, 2176–2180. [Google Scholar] [CrossRef]
- Tirschwell, D.L.; Smith, N.L.; Heckbert, S.R.; Lemaitre, R.N.; Longstreth, W.J.; Psaty, B.M. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 2004, 63, 1868–1875. [Google Scholar] [CrossRef]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. S1), 81–84. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Langsted, A.; Nordestgaard, B.G. Nonfasting Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis. JAMA Intern. Med. 2016, 176, 1834–1842. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.J.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Wang, Y.; Gong, J.; Wang, G.; Ge, W.; Chen, R.; Meng, X.; Zhao, Y.; Kan, H. Associations of long-term exposure to ambient nitrogen dioxide with indicators of diabetes and dyslipidemia in China: A nationwide analysis. Chemosphere 2021, 269, 128724. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.T.; Ramet, M.E.; Patel, A.R.; Pandian, N.G.; Mendelsohn, M.E.; Karas, R.H. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002, 144, 165–172. [Google Scholar] [CrossRef]
- de Jesus, M.; Mohammed, T.; Singh, M.; Tiu, J.G.; Kim, A.S. Etiology and Management of Dyslipidemia in Patients With Cancer. Front. Cardiovasc. Med. 2022, 9, 892335. [Google Scholar] [CrossRef]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar]
- Li, Z.; Xu, Y.; Huang, Z.; Wei, Y.; Hou, J.; Long, T.; Wang, F.; Cheng, X.; Duan, Y.; Chen, X.; et al. Association of multiple metals with lipid markers against different exposure profiles: A population-based cross-sectional study in China. Chemosphere 2021, 264, 128505. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef]
- Lyon-Colbert, A.; Su, S.; Cude, C. A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins 2018, 10, 254. [Google Scholar] [CrossRef]
- WHO. Cyanobacterial Toxins: Microcystin-LR in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Cai, D.; Wei, J.; Huang, F.; Feng, H.; Peng, T.; Luo, J.; Yang, F. The detoxification activities and mechanisms of microcystinase towards MC-LR. Ecotoxicol. Environ. Saf. 2022, 236, 113436. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020, 174, 115638. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kang, L.; Sun, X.; Jia, R.; Zhang, Y.; Ma, J.; Li, H.; Ai, H. Spatiotemporal distribution and potential risk assessment of microcystins in the Yulin River, a tributary of the Three Gorges Reservoir, China. J. Hazard. Mater. 2018, 347, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Steinman, A.D.; Gu, Y.; Zhu, G.; Xie, L. Occurrence and risk assessment of microcystin and its relationship with environmental factors in lakes of the eastern plain ecoregion, China. Environ. Sci. Pollut. Res. 2020, 27, 45095–45107. [Google Scholar] [CrossRef]
- Cordeiro-Araujo, M.K.; Chia, M.A.; Arruda-Neto, J.; Tornisielo, V.L.; Vilca, F.Z.; Bittencourt-Oliveira, M. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566–567, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Bouaicha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Parental Transfer of Microcystin-LR-Induced Innate Immune Dysfunction of Zebrafish: A Cross-Generational Study. Environ. Sci. Technol. 2020, 54, 1014–1023. [Google Scholar] [CrossRef]
- Pan, C.; Qin, H.; Jin, H.; Chen, W.; Guo, H.; Han, X. Environmental exposure to microcystin-LR increases the risks of urinary bladder proliferation and carcinogenesis: Evidence from case control, animal, and in vitro studies. Toxicology 2022, 480, 153326. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Svircev, Z.; Chen, L.; Santha, K.; Drobac, B.D.; Susak, S.; Vulin, A.; Palanacki, M.T.; Codd, G.A.; Meriluoto, J. A review and assessment of cyanobacterial toxins as cardiovascular health hazards. Arch. Toxicol. 2022, 96, 2829–2863. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ma, Y.; Chen, X.; Losiewicz, M.D.; Du, X.; Tian, Z.; Zhang, S.; Shi, L.; Zhang, H.; et al. Long-term exposure to low concentrations of MC-LR induces blood-testis barrier damage through the RhoA/ROCK pathway. Ecotoxicol. Environ. Saf. 2022, 236, 113454. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Steinman, A.D. Microcystin-LR induced thyroid dysfunction and metabolic disorders in mice. Toxicology 2015, 328, 135–141. [Google Scholar] [CrossRef]
- Chi, C.; Giri, S.S.; Yu, X.W.; Liu, Y.; Chen, K.K.; Liu, W.B.; Zhang, D.D.; Jiang, G.Z.; Li, X.F.; Gao, X.; et al. Lipid metabolism, immune and apoptosis transcriptomic responses of the hepatopancreas of Chinese mitten crab to the exposure to microcystin-LR. Ecotoxicol. Environ. Saf. 2022, 236, 113439. [Google Scholar] [CrossRef] [PubMed]
- Al-Badrani, M.; Saha, P.; Mondal, A.; Seth, R.K.; Sarkar, S.; Kimono, D.; Bose, D.; Porter, D.E.; Scott, G.I.; Brooks, B.; et al. Early microcystin-LR exposure-linked inflammasome activation in mice causes development of fatty liver disease and insulin resistance. Environ. Toxicol. Pharmacol. 2020, 80, 103457. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, G.; Chen, J.; Lin, J.; Zeng, C.; Chen, J.; Deng, J.; Xie, P. Prolonged exposure to low-dose microcystin induces nonalcoholic steatohepatitis in mice: A systems toxicology study. Arch. Toxicol. 2017, 91, 465–480. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; He, J.; Chen, J.; Giesy, J.P.; Xie, P. Responses of the Proteome and Metabolome in Livers of Zebrafish Exposed Chronically to Environmentally Relevant Concentrations of Microcystin-LR. Environ. Sci. Technol. 2017, 51, 596–607. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.X.; Wu, B.; Yin, J.; Yu, Y.; Yang, L. Comprehensive insights into microcystin-LR effects on hepatic lipid metabolism using cross-omics technologies. J. Hazard. Mater. 2016, 315, 126–134. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, X.; Yang, L.; Xu, L.; Zhang, Z.; Wu, J.; Wang, Y. Microcystin-LR altered mRNA and protein expression of endoplasmic reticulum stress signaling molecules related to hepatic lipid metabolism abnormalities in mice. Environ. Toxicol. Pharmacol. 2015, 40, 114–121. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shu, Y.; Dai, Y.; Gao, Y.; Liu, S.; Wang, W.; Jiang, H.; Zhang, H.; Hong, P.; Wu, H. Microcystin-leucine arginine exposure induced intestinal lipid accumulation and MC-LR efflux disorder in Lithobates catesbeianus tadpoles. Toxicology 2022, 465, 153058. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Clara, T.; Huang, F.; Wei, J.; Yang, F. Identification and characterization of the dominant Microcystis sp. cyanobacteria detected in Lake Dong Ting, China. J. Toxicol. Environ. Health A 2019, 82, 1143–1150. [Google Scholar] [CrossRef]
- Zheng, C.; Zeng, H.; Lin, H.; Wang, J.; Feng, X.; Qiu, Z.; Chen, J.A.; Luo, J.; Luo, Y.; Huang, Y.; et al. Serum microcystin levels positively linked with risk of hepatocellular carcinoma: A case-control study in southwest China. Hepatology 2017, 66, 1519–1528. [Google Scholar] [CrossRef]
- Zeng, H.; Tan, Y.; Wang, L.; Xiang, M.; Zhou, Z.; Chen, J.A.; Wang, J.; Zhang, R.; Tian, Y.; Luo, J.; et al. Association of serum microcystin levels with neurobehavior of school-age children in rural area of Southwest China: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 212, 111990. [Google Scholar] [CrossRef]
- Lin, Y.; An, R.; Wu, C.; Liu, H.; Deng, J.; Tan, H.; Chen, L.; Chen, M.; Ma, S. Serum microcystin-LR levels and risk of gestational diabetes mellitus: A Chinese nested case-control study. Front. Endocrinol. 2022, 13, 1047866. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.A.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef]
- Birbeck, J.A.; Westrick, J.A.; O’Neill, G.M.; Spies, B.; Szlag, D.C. Comparative Analysis of Microcystin Prevalence in Michigan Lakes by Online Concentration LC/MS/MS and ELISA. Toxins 2019, 11, 13. [Google Scholar] [CrossRef]
- Du, J.; Chen, Y.; Zhou, N.; Song, Y.; Wang, W.; Hong, X. Associations between self-reported sleep duration and abnormal serum lipids in eastern China: A population-based cross-sectional survey. Sleep Med. 2022, 95, 1–8. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, F.; Luo, H.; Liu, F. Occupational noise exposure and the prevalence of dyslipidemia in a cross-sectional study. BMC Public Health 2021, 21, 1258. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Li, S.; Wang, C.; Liu, Y.; Li, N.; Liu, F.; Huang, S.; Liu, S.; Lu, Y.; Mao, Z.; et al. Is long-term PM1 exposure associated with blood lipids and dyslipidemias in a Chinese rural population? Environ. Int. 2020, 138, 105637. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Tian, Q.J.; Liang, S.C.; Zhou, Y.Y.; Zou, H.C. Bioaccumulation of heavy metals by the dominant plants growing in Huayuan manganese and lead/zinc mineland, Xiangxi. Huan Jing Ke Xue 2012, 33, 2038–2045. [Google Scholar] [PubMed]
- Du, Y.; Chen, L.; Ding, P.; Liu, L.; He, Q.; Chen, B.; Duan, Y. Different exposure profile of heavy metal and health risk between residents near a Pb-Zn mine and a Mn mine in Huayuan county, South China. Chemosphere 2019, 216, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, W.; Zuo, Y.; Lin, L.; Song, L. Heavy metal migration and risk transference associated with cyanobacterial blooms in eutrophic freshwater. Sci. Total Environ. 2018, 613–614, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Pichardo, S.; Jos, A.; Gomez-Amores, L.; Mate, A.; Vazquez, C.M.; Camean, A.M. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 2005, 45, 395–402. [Google Scholar] [CrossRef]
- Lin, W.; Hou, J.; Guo, H.; Li, L.; Wang, L.; Zhang, D.; Li, D.; Tang, R. 1The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish. Environ. Pollut. 2018, 235, 197–206. [Google Scholar] [CrossRef]
- Bouaicha, N.; Maatouk, I. Microcystin-LR and nodularin induce intracellular glutathione alteration, reactive oxygen species production and lipid peroxidation in primary cultured rat hepatocytes. Toxicol. Lett. 2004, 148, 53–63. [Google Scholar] [CrossRef]
- Goel, A.; Dani, V.; Dhawan, D.K. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Interact. 2005, 156, 131–140. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Z.; Liu, L.; Luo, C.; Lu, G.; Li, Q.; Gao, X. Zinc Regulates Lipid Metabolism and MMPs Expression in Lipid Disturbance Rabbits. Biol. Trace Elem. Res. 2015, 168, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Faghihi, A.; Motaghinejad, M.; Shiasi, M.; Imanparast, F.; Amiri, H.L.; Shidfar, F. Zinc and Selenium Co-supplementation Reduces Some Lipid Peroxidation and Angiogenesis Markers in a Rat Model of NAFLD-Fed High Fat Diet. Biol. Trace Elem. Res. 2018, 181, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Du, C.; Yang, Y.; Feng, X.; Zhu, L.; Chen, J.; Yang, F. MC-LR Aggravates Liver Lipid Metabolism Disorders in Obese Mice Fed a High-Fat Diet via PI3K/AKT/mTOR/SREBP1 Signaling Pathway. Toxins 2022, 14, 833. [Google Scholar] [CrossRef]
- Andrinolo, D.; Sedan, D.; Telese, L.; Aura, C.; Masera, S.; Giannuzzi, L.; Marra, C.A.; de Alaniz, M.J. Hepatic recovery after damage produced by sub-chronic intoxication with the cyanotoxin microcystin LR. Toxicon 2008, 51, 457–467. [Google Scholar] [CrossRef]
- Sedan, D.; Laguens, M.; Copparoni, G.; Aranda, J.O.; Giannuzzi, L.; Marra, C.A.; Andrinolo, D. Hepatic and intestine alterations in mice after prolonged exposure to low oral doses of Microcystin-LR. Toxicon 2015, 104, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Babour, A.; Bicknell, A.A.; Tourtellotte, J.; Niwa, M. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 2010, 142, 256–269. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can. J. Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef]
- Pan, C.; Yan, M.; Jin, H.; Guo, H.; Han, X. Chronic exposure to MC-LR increases the risks of microcytic anemia: Evidence from human and mice. Environ. Pollut. 2021, 288, 117966. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, X.L.; Leong, P.M.; Zhang, H.M.; Yang, X.G.; Kong, L.Z.; Zhai, F.Y.; Cheng, Y.Y.; Guo, J.S.; Su, Y.X. New Chinese dietary guidelines: Healthy eating patterns and food-based dietary recommendations. Asia Pac. J. Clin. Nutr. 2018, 27, 908–913. [Google Scholar]
- Scinicariello, F.; Buser, M.C. Urinary antimony and leukocyte telomere length: An analysis of NHANES 1999–2002. Environ. Res. 2016, 150, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; He, W.; Shao, Y.; Liu, B.; Huang, H.; Liang, J.; Liao, Q.; Tang, Y.; Mo, M.; Zhou, Y.; et al. Associations between prenatal multiple plasma metal exposure and newborn telomere length: Effect modification by maternal age and infant sex. Environ. Pollut. 2022, 315, 120451. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 720) | Dyslipidemia | p Value a | |
|---|---|---|---|---|
| Yes (n = 255) | No (n = 465) | |||
| Age, years | 52.69 ± 14.93 | 54.18 ± 15.40 | 49.98 ± 13.63 | <0.001 b |
| Gender, n (%) | 0.266 | |||
| male | 501(69.6) | 184 (72.2) | 317 (68.2) | |
| female | 219 (30.8) | 71 (27.8) | 148 (30.4) | |
| Ethnicity, n (%) | 0.443 | |||
| Han | 582 (80.8) | 210 (82.4) | 372 (80.0) | |
| others | 138 (19.2) | 45 (17.6) | 93 (20.0) | |
| Educational level, n (%) | 0.039 | |||
| primary school or below | 271 (37.6) | 78 (30.6) | 193 (41.5) | |
| junior high school | 161 (22.4) | 63 (24.7) | 98 (21.1) | |
| senior high school | 191 (24.7) | 76 (29.8) | 115 (24.7) | |
| university degree or above | 97 (13.5) | 38 (14.9) | 59 (12.7) | |
| Annual family income, CNY, n (%) | 0.001 | |||
| ≤30,000 | 440 (61.1) | 134 52.5) | 306 (65.8) | |
| 30,000–100,000 | 257 (35.7) | 108 (42.4) | 149 (32.0) | |
| >100,000 | 23 (3.2) | 13 (5.1) | 10 (2.2) | |
| Occupation, n (%) | 0.510 | |||
| physical labor | 642 (89.2) | 230 (90.2) | 412 (88.6) | |
| mental labor | 78 (10.8) | 25 (9.8) | 53 (11.4) | |
| Body Mass Index, kg/m2, n (%) | <0.001 | |||
| <18.5 | 25 (3.5) | 3 (1.2) | 22 (4.7) | |
| 18.5~23.9 | 299 (41.5) | 66 (25.9) | 233 (50.1) | |
| 24.0~27.9 | 287 (39.9) | 126 (49.4) | 161 (34.6) | |
| ≥28.0 | 109 (15.1) | 60 (23.5) | 49 (10.5) | |
| Smoking, n (%) | 0.355 | |||
| never | 378 (52.5) | 126 (49.4) | 252 (54.2) | |
| former | 68 (9.4) | 23 (9.0) | 45(9.7) | |
| current | 274 (38.1) | 106 (41.6) | 168 (36.1) | |
| Alcohol drinking, n (%) | 0.304 | |||
| never | 531 (73.8) | 182 (71.4) | 349 (75.1) | |
| former | 47 (6.5) | 15 (5.9) | 32 (6.9) | |
| current | 142 (19.7) | 58 (22.7) | 84 (18.1) | |
| Physical exercise, n (%) | 0.371 | |||
| yes | 304 (42.2) | 102 (40.0) | 202 (43.4) | |
| no | 416 (57.8) | 153(60.0) | 263 (56.6) | |
| Low intake of vegetables and fruits, n (%) | 575(79.9) | 206 (80.0) | 369 (79.4) | 0.647 |
| SSBs Frequency (d/w), n (%) | 0.015 | |||
| <1 | 640 (88.9) | 231 (90.6) | 409 (88.0) | |
| 1~2 | 61 (8.5) | 17 (6.7) | 44 (9.5) | |
| 3~6 | 11 (1.5) | 1 (0.4) | 10 (2.2) | |
| >7 | 8 (1.1) | 6 (2.4) | 2 (0.4) | |
| Family history of dyslipidemia, n (%) | 0.021 | |||
| yes | 18 (2.5) | 11 (4.3) | 7 (1.5) | |
| no | 702 (97.5) | 244 (95.7) | 458 (98.5) | |
| Hypertension, n (%) | 0.328 | |||
| yes | 196 (27.2) | 75 (29.4) | 122 (26.0) | |
| no | 524 (72.8) | 180 (70.6) | 344 (74.0) | |
| History of chronic hepatitis, n (%) | 0.277 | |||
| yes | 34 (4.7) | 15 (5.9) | 19 (4.1) | |
| no | 686 (95.3) | 240 (94.1) | 446 (95.9) | |
| Diabetes, n (%) | 0.741 | |||
| yes | 26 (3.6) | 10 (3.9) | 16 (3.4) | |
| no | 694 (96.4) | 245 (96.1) | 449 (96.6) | |
| Serum MCs, μg/L | 0.15 (0.11, 0.22) | 0.17 (0.12, 0.24) | 0.15 (0.11, 0.21) | 0.002 b |
| Dyslipidemia, n (%) | 255 (35.4) | − | − | − |
| Plasma Zn, μg/L | 891.24 (761.44, 1048.95) | 928.13 (800.41, 1081.30) | 866.91 (735.19, 1016.27) | 0.001 b |
| Plasma Se, μg/L | 87.51 (67.05, 107.88) | 90.51 (70.77, 106.88) | 84.91 (62.31, 107.95) | 0.119 b |
| plasma Fe, μg/L | 1052.63 (775.81, 1387.48) | 1110.91 (872.72, 1373.08) | 1023.71 (739.06, 1395.20) | 0.051 b |
| Urinary Ti, μg/L | 137.28 (77.44, 193.13) | 128.64 (71.62, 184.42) | 142.40 (80.06, 201.88) | 0.124 b |
| Urinary Mo, μg/L | 103.16 (59.00, 154.90) | 84.94 (55.21, 165.37) | 107.35 (61.72, 165.37) | 0.020 b |
| Urinary Cd, μg/L | 4.72 (2.08, 8.44) | 5.34 (2.00, 11.03) | 4.58 (2.15, 7.37) | 0.039 b |
| MCs (μg/L) | n | TG (mM) | TC (mM) | HDL-C (mM) | LDL-C (mM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | a p Value | Mean ± SD | a p Value | Mean ± SD | a p Value | Mean ± SD | a p Value | ||
| Q1 | 189 | 1.66 ± 1.23 | ref | 4.88 ± 0.89 | ref | 1.42 ± 0.33 | ref | 2.86 ± 0.83 | ref |
| Q2 | 171 | 2.22 ± 2.85 | 0.003 | 5.54 ± 5.83 | 0.253 | 1.37 ± 0.28 | 0.130 | 2.90 ± 0.94 | 0.958 |
| Q3 | 181 | 2.14 ± 2.10 | 0.002 | 4.92 ± 0.98 | 0.807 | 1.34 ± 0.30 | 0.002 | 2.79 ± 0.89 | 0.259 |
| Q4 | 179 | 2.13 ± 1.54 | <0.001 | 5.01 ± 0.94 | 0.259 | 1.30 ± 0.28 | <0.001 | 2.83 ± 0.92 | 0.155 |
| Variables | Adjusted Odds Ratios (95% CI) by Quartile of Serum MCs Concentration (μg/L) | p for Trend | |||
|---|---|---|---|---|---|
| Q1 (n = 189) | Q2 (n = 171) | Q3 (n = 181) | Q4 (n = 179) | ||
| Age | |||||
| <53 | reference | 3.75 (1.84, 7.64) *** | 3.41 (1.66, 7.00) ** | 4.12 (2.01, 8.44) *** | 0.001 |
| ≥53 | reference | 1.23 (0.48, 3.12) | 0.67 (0.26, 1.77) | 2.31 (0.91, 5.90) | 0.104 |
| Gender | |||||
| male | reference | 3.75 (1.96, 7.17) *** | 2.36 (1.24, 4.48) ** | 3.30 (1.68, 6.48) ** | 0.009 |
| female | reference | 0.46 (0.15, 1.46) | 0.56 (0.18, 1.69) | 1.78 (0.60, 5.27) | 0.109 |
| Annual family income, CNY | |||||
| ≤30,000 | reference | 1.48 (0.73, 2.99) | 1.28 (0.64, 2.58) | 2.14 (1.07, 4.29) * | 0.043 |
| >30,000 | reference | 3.86 (1.59, 9.37) ** | 1.99 (0.82, 4.81) | 4.38 (1.70, 11.26) ** | 0.011 |
| Body Mass Index, kg/m2 | |||||
| <24 | reference | 2.43 (0.98, 6.03) | 2.25 (0.87, 5.83) | 3.01 (1.16, 7.83) * | 0.043 |
| 24.0~27.9 | reference | 1.37 (0.63, 3.01) | 0.78 (0.35, 1.72) | 1.69 (0.75, 3.81) | 0.297 |
| ≥28.0 | reference | 1.55 (0.08, 31.75) | 11.29 (1.02, 124.77) * | 7.13 (0.49, 104.30) | 0.107 |
| Smoking status | |||||
| current or former | reference | 5.60 (2.43, 12.92) *** | 2.28 (1.00, 5.18) | 4.06 (1.67, 9.92) ** | 0.031 |
| never | reference | 1.09 (0.53, 2.25) | 1.41 (0.70, 2.84) | 2.55 (1.25, 5.18) * | 0.005 |
| Alcohol consumption | |||||
| current or former | reference | 5.04 (1.45, 17.61) * | 1.60 (0.50, 5.18) | 3.65 (1.02, 13.06) * | 0.181 |
| never | reference | 1.67 (0.91, 3.09) | 1.61 (0.87, 2.99) | 2.81 (1.52, 5.24) ** | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Cao, M.; Tang, P.; Deng, S.; Chen, L.; Tang, Y.; Zhu, L.; Chen, X.; Huang, Z.; Shen, M.; et al. Microcystins Exposure Associated with Blood Lipid Profiles and Dyslipidemia: A Cross-Sectional Study in Hunan Province, China. Toxins 2023, 15, 293. https://doi.org/10.3390/toxins15040293
Feng S, Cao M, Tang P, Deng S, Chen L, Tang Y, Zhu L, Chen X, Huang Z, Shen M, et al. Microcystins Exposure Associated with Blood Lipid Profiles and Dyslipidemia: A Cross-Sectional Study in Hunan Province, China. Toxins. 2023; 15(4):293. https://doi.org/10.3390/toxins15040293
Chicago/Turabian StyleFeng, Shuidong, Mengyue Cao, Peng Tang, Shuxiang Deng, Limou Chen, Yan Tang, Lemei Zhu, Xiang Chen, Zhijun Huang, Minxue Shen, and et al. 2023. "Microcystins Exposure Associated with Blood Lipid Profiles and Dyslipidemia: A Cross-Sectional Study in Hunan Province, China" Toxins 15, no. 4: 293. https://doi.org/10.3390/toxins15040293
APA StyleFeng, S., Cao, M., Tang, P., Deng, S., Chen, L., Tang, Y., Zhu, L., Chen, X., Huang, Z., Shen, M., & Yang, F. (2023). Microcystins Exposure Associated with Blood Lipid Profiles and Dyslipidemia: A Cross-Sectional Study in Hunan Province, China. Toxins, 15(4), 293. https://doi.org/10.3390/toxins15040293






