Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice
Abstract
1. Introduction
2. Results
2.1. Impact of MC-LR Treatment on the General Condition and Body Weight of Obese Mice
2.2. Impact of MC-LR Treatment on the Histopathological Changes in the Colorectal Tissue of Obese Mice
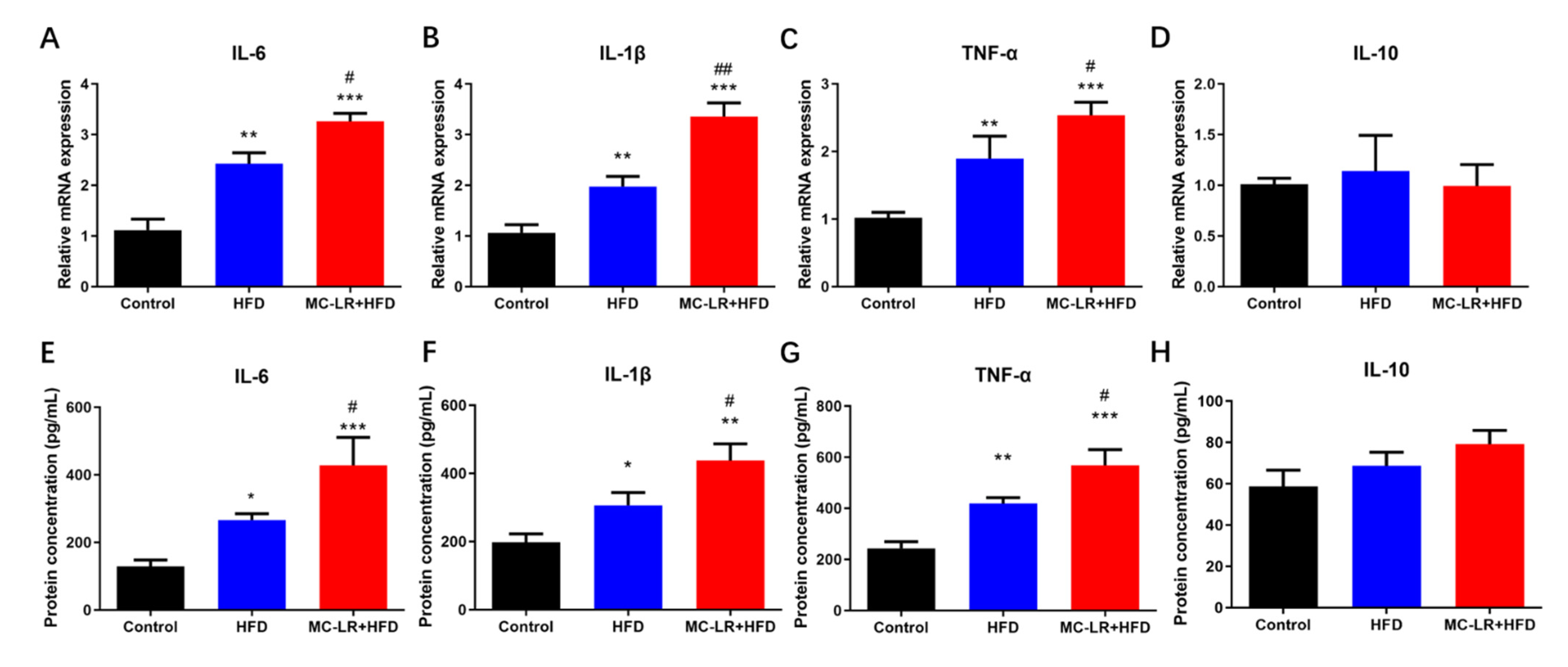
2.3. Impact of MC-LR Treatment on the Expression of Inflammatory Mediators Factors in the Colorectal Tissue of Obese Mice
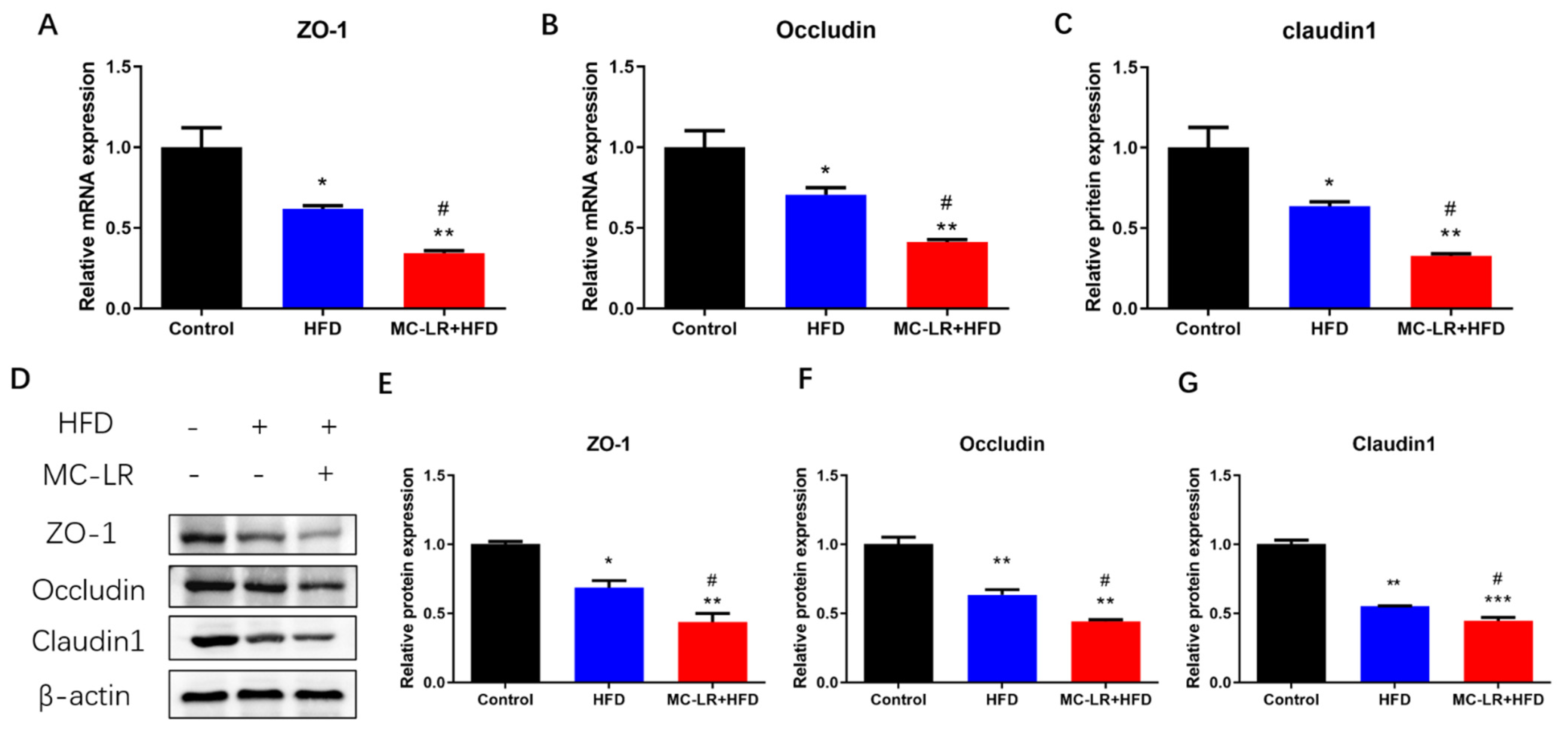
2.4. Impact of MC-LR Treatment on the Expression of Tight Junction-Related Factors in the Colorectal Tissue of Obese Mice
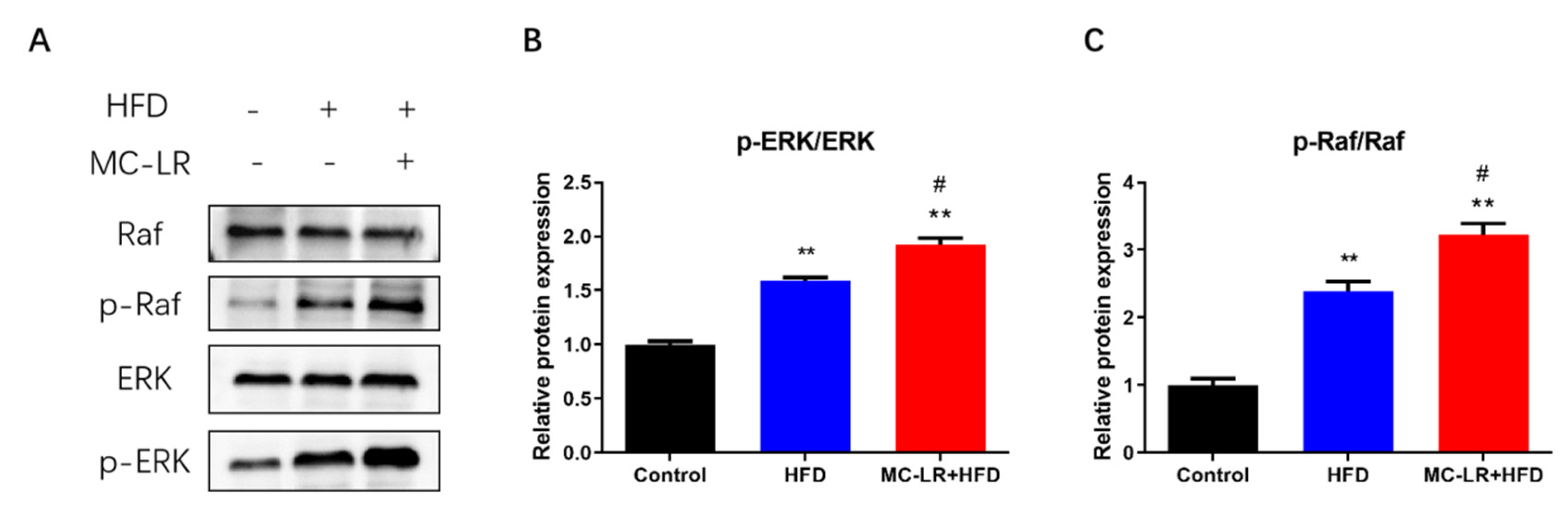
2.5. Impact of MC-LR Treatment on the Expression of Raf/ERK Signaling Pathway-Related Proteins in the Colorectal of Obese Mice
3. Discussion
3.1. MC-LR Promoted Colorectal Damage in Obese Mice
3.2. MC-LR Promoted Colorectal Inflammatory Response and Barrier Disruption in Obese Mice
3.3. MC-LR Promoted Colorectal Inflammatory Response and Barrier Disruption by Activating Raf/ERK Signaling Pathway in Obese Mice
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Animals and Diet
5.3. Histological Analysis
5.4. WB
5.5. qRT-PCR
5.6. ELISA
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020, 174, 115638. [Google Scholar] [CrossRef]
- Pan, C.; Yan, M.; Jin, H.; Guo, H.; Han, X. Chronic exposure to MC-LR increases the risks of microcytic anemia: Evidence from human and mice. Environ. Pollut. 2021, 288, 117966. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.S.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.; Antunes, M.B.; de Melo, F.D.; Lyra, T.M.; Barreto, V.S.; et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.R.; Wharton, R.E.; Lee, C.; Mojica, M.A.; Krajewski, L.C.; Gordon, S.C.; Schaefer, A.M.; Johnson, R.C.; Hamelin, E.I. Measurement of Microcystin Activity in Human Plasma Using Immunocapture and Protein Phosphatase Inhibition Assay. Toxins 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef]
- Gu, S.; Jiang, M.; Zhang, B. Microcystin-LR in Primary Liver Cancers: An Overview. Toxins 2022, 14, 715. [Google Scholar] [CrossRef]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res 2023, 229, 119397. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Zuo, J.; Huo, T.; Du, X.; Yang, Q.; Wu, Q.; Shen, J.; Liu, C.; Hung, T.C.; Yan, W.; Li, G. The joint effect of parental exposure to microcystin-LR and polystyrene nanoplastics on the growth of zebrafish offspring. J. Hazard. Mater. 2021, 410, 124677. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, T.; Wang, Y.T.; He, J.; Zhao, X.; Wang, Y.K.; Giesy, J.P.; Chen, F.; Chen, Y.; Tuo, X.; et al. Effects of acute exposure to microcystins on hypothalamic-pituitary-adrenal (HPA), -gonad (HPG) and -thyroid (HPT) axes of female rats. Sci. Total Environ. 2021, 778, 145196. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Liu, Y.; Jeppesen, E.; Svenning, J.C.; Wu, J.; Zhang, W.; Zhou, T.; Wang, P.; Nangombe, S.; et al. From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize megafauna. Innovation 2021, 2, 100092. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the Microstructure and Inflammation-Related Factors of Jejunum in Mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, H.; Chen, K. Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar] [PubMed]
- Zhou, R.; Llorente, C.; Cao, J.; Zaramela, L.S.; Zeng, S.; Gao, B.; Li, S.Z.; Welch, R.D.; Huang, F.Q.; Qi, L.W.; et al. Intestinal alpha1-2-Fucosylation Contributes to Obesity and Steatohepatitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 293–320. [Google Scholar] [CrossRef]
- Monsted, M.O.; Falck, N.D.; Pedersen, K.; Buschard, K.; Holm, L.J.; Haupt-Jorgensen, M. Intestinal permeability in type 1 diabetes: An updated comprehensive overview. J. Autoimmun. 2021, 122, 102674. [Google Scholar] [CrossRef]
- Arman, T.; Lynch, K.D.; Montonye, M.L.; Goedken, M.; Clarke, J.D. Sub-Chronic Microcystin-LR Liver Toxicity in Preexisting Diet-Induced Nonalcoholic Steatohepatitis in Rats. Toxins 2019, 11, 398. [Google Scholar] [CrossRef]
- Arman, T.; Lynch, K.D.; Goedken, M.; Clarke, J.D. Sub-chronic microcystin-LR renal toxicity in rats fed a high fat/high cholesterol diet. Chemosphere 2021, 269, 128773. [Google Scholar] [CrossRef]
- Fawell, J.K.; Mitchell, R.E.; Everett, D.J.; Hill, R.E. The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Fink, G.; Sundram, S. Clozapine-induced ERK1 and ERK2 signaling in prefrontal cortex is mediated by the EGF receptor. J. Mol. Neurosci. 2009, 39, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Liu, F.; Chen, Y.M.; Liu, Y.J.; Wang, X.D.; Du, S.Y. CTGF-mediated ERK signaling pathway influences the inflammatory factors and intestinal flora in ulcerative colitis. Biomed. Pharmacother. 2019, 111, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Gao, N.; Chen, J.; Fan, L.; Zeng, Z.; Gao, G.; Li, L.; Fang, G.; Hu, K.; Pang, X.; et al. Erk and MAPK signaling is essential for intestinal development through Wnt pathway modulation. Development 2020, 147, dev185678. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Qiu, Y.; Yu, M.; Yin, J.; Yang, H.; Mei, J. KGF inhibits hypoxia-induced intestinal epithelial cell apoptosis by upregulating AKT/ERK pathway-dependent E-cadherin expression. Biomed. Pharmacother. 2018, 105, 1318–1324. [Google Scholar] [CrossRef]
- Chu, H.; Du, C.; Yang, Y.; Feng, X.; Zhu, L.; Chen, J.; Yang, F. MC-LR Aggravates Liver Lipid Metabolism Disorders in Obese Mice Fed a High-Fat Diet via PI3K/AKT/mTOR/SREBP1 Signaling Pathway. Toxins 2022, 14, 833. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Oliveira, V.M.; Oliveira, R.; Da, C.P.; Grisolia, C.K.; Pires, O.J. Histopathological effects of [D-Leu(1)]Microcystin-LR variants on liver, skeletal muscle and intestinal tract of Hypophthalmichthys molitrix (Valenciennes, 1844). Toxicon 2010, 55, 1255–1262. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, L.; Li, J.; Chen, Y.; Jin, J.; Kawan, A.; Zhang, X. Pathological damage and immunomodulatory effects of zebrafish exposed to microcystin-LR. Toxicon 2016, 118, 13–20. [Google Scholar] [CrossRef]
- Su, R.C.; Blomquist, T.M.; Kleinhenz, A.L.; Khalaf, F.K.; Dube, P.; Lad, A.; Breidenbach, J.D.; Mohammed, C.J.; Zhang, S.; Baum, C.E.; et al. Exposure to the Harmful Algal Bloom (HAB) Toxin Microcystin-LR (MC-LR) Prolongs and Increases Severity of Dextran Sulfate Sodium (DSS)-Induced Colitis. Toxins 2019, 11, 371. [Google Scholar] [CrossRef]
- Rocha, M.F.; Sidrim, J.J.; Soares, A.M.; Jimenez, G.C.; Guerrant, R.L.; Ribeiro, R.A.; Lima, A.A. Supernatants from macrophages stimulated with microcystin-LR induce electrogenic intestinal response in rabbit ileum. Pharmacol. Toxicol. 2000, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Su, R.C.; Warner, E.A.; Breidenbach, J.D.; Lad, A.; Blomquist, T.M.; Kleinhenz, A.L.; Modyanov, N.; Malhotra, D.; Kennedy, D.J.; Haller, S.T. CD40 Receptor Knockout Protects against Microcystin-LR (MC-LR) Prolongation and Exacerbation of Dextran Sulfate Sodium (DSS)-Induced Colitis. Biomedicines 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pina-Canseco, M.S.; Paez-Arenas, A.; Masso, F.; Perez-Campos, E.; Martinez-Cruz, R.; Hernandez-Cruz, P.; Majluf-Cruz, A.; Martinez-Cruz, M.; Perez-Campos, M.L.; Perez-Santiago, A.D.; et al. Protein C activation peptide inhibits the expression of ICAM-1, VCAM-1, and interleukin-8 induced by TNF-alpha in human dermal microvascular endothelial cells. Folia Histochem. Cytobiol. 2012, 50, 407–413. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.; Yu, B.; Yu, G. Characterization of in vitro effects of microcystin-LR on intestinal epithelial cells. Environ. Toxicol. 2017, 32, 1539–1547. [Google Scholar] [CrossRef]
- Zhuang, L.; Jin, Z.; Li, H.; Wu, S.; Tong, X.; Wang, H.; Li, M. Effects of Chronic Exposure to Microcystin-LR on the Gut Microbiota of Male Mice. Int. J. Toxicol. 2021, 40, 171–177. [Google Scholar] [CrossRef]
- Ding, W.; Shangguan, Y.; Zhu, Y.; Sultan, Y.; Feng, Y.; Zhang, B.; Liu, Y.; Ma, J.; Li, X. Negative impacts of microcystin-LR and glyphosate on zebrafish intestine: Linked with gut microbiota and microRNAs? Environ. Pollut. 2021, 286, 117685. [Google Scholar] [CrossRef]
- Forster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Mehta, S.; Nijhuis, A.; Kumagai, T.; Lindsay, J.; Silver, A. Defects in the adherens junction complex (E-cadherin/ beta-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015, 360, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. Ebiomedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Sarkar, S.; Kimono, D.; Albadrani, M.; Seth, R.K.; Busbee, P.; Alghetaa, H.; Porter, D.E.; Scott, G.I.; Brooks, B.; Nagarkatti, M.; et al. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 8742. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanz, J.; Fernandez-Rojo, M.A.; Potriquet, J.; Mukhopadhyay, P.; Brust, A.; Wilhelm, P.; Smallwood, T.B.; Clark, R.J.; Fry, B.G.; Alewood, P.F.; et al. ERK and mTORC1 Inhibitors Enhance the Anti-Cancer Capacity of the Octpep-1 Venom-Derived Peptide in Melanoma BRAF(V600E) Mutations. Toxins 2021, 13, 146. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bian, R.; Li, J.; Qiu, L.; Lu, B.; Ouyang, X. Chronic exposure to microcystin-LR reduces thyroid hormone levels by activating p38/MAPK and MEK/ERK signal pathway. Ecotoxicol. Environ. Saf. 2019, 173, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, X.; Li, M.; Liu, J. P44/42 MAPK signal pathway-mediated hyperphosphorylation of paxillin and redistribution of E-cadherin was involved in microcystin-LR-reduced cellular adhesion in a human liver cell line. Chemosphere 2018, 200, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tang, Y. Ntrk1 promotes mesangial cell proliferation and inflammation in rat glomerulonephritis model by activating the STAT3 and p38/ERK MAPK signaling pathways. BMC Nephrol. 2022, 23, 413. [Google Scholar] [CrossRef]

| Group | Body Weight (g) |
|---|---|
| Control | 33.96 ± 1.374 |
| HFD | 50.58 ± 2.312 *** |
| HFD + MC-LR | 51.52 ± 2.782 *** |
| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| IL-6 | CCACGGCCTTCCCTACTTC | TTGGGAGTGGTATCCTCTGTGA |
| TNF-α | CCCACGTCGTAGCAAACCA | ACAAGGTACAACCCATCGGC |
| IL-1β | GCACTACAGGCTCCGAGATGAA | GTCGTTGCTTGGTTCTCCTTGT |
| IL-10 | AGAGCTGCGGACTGCCTTCA | ACCTGCTCCACTGCCTTGCT |
| ZO-1 | GCGATTCAGCAGCAACAGAACC | AGGACCGTGTAATGGCAGACTC |
| Occludin | GCGGCTATGGAGGCTATGGCTA | AGGAAGCGATGAAGCAGAAGGC |
| Claudin1 | GGACAACATCGTGACCGCTCAG | TCCAGGCACCTCATGCACTTCA |
| β-Actin | TCAAGATCATTGCTCCTCCTGAG | ACATCTGCTGGAAGGTGGACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zheng, S.; Chu, H.; Du, C.; Chen, M.; Emran, M.Y.; Chen, J.; Yang, F.; Tian, L. Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice. Toxins 2023, 15, 262. https://doi.org/10.3390/toxins15040262
Yang Y, Zheng S, Chu H, Du C, Chen M, Emran MY, Chen J, Yang F, Tian L. Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice. Toxins. 2023; 15(4):262. https://doi.org/10.3390/toxins15040262
Chicago/Turabian StyleYang, Yue, Shuilin Zheng, Hanyu Chu, Can Du, Mengshi Chen, Mohammed Y. Emran, Jihua Chen, Fei Yang, and Li Tian. 2023. "Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice" Toxins 15, no. 4: 262. https://doi.org/10.3390/toxins15040262
APA StyleYang, Y., Zheng, S., Chu, H., Du, C., Chen, M., Emran, M. Y., Chen, J., Yang, F., & Tian, L. (2023). Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice. Toxins, 15(4), 262. https://doi.org/10.3390/toxins15040262






