Exploration of Synergistic Pesticidal Activities, Control Effects and Toxicology Study of a Monoterpene Essential Oil with Two Natural Alkaloids
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Chemicals
2.2. Biological Assay
2.2.1. Insecticidal Activity of Matrine (MT), Oxymatrine (OMT) and Their Binary Complexes against P. xylostella by the Leaf-Loading Poison Method
2.2.2. Insecticidal Activity of MT, OMT, MT/OMT (8/2), 1,8-Cineole (CN) and MT/OMT (8/2)/CN against P. xylostella by the Leaf-Dipping Method
2.2.3. Control Efficiency of MT/OMT (8/2), and MT/OMT (8/2)/CN against P. xylostella in the Greenhouse
2.2.4. Acaricidal Activity of MT, OMT, CN, and Their Mixtures against T. urticae by the Slide-Dipping Method
2.2.5. LC50 of MT, OMT, MT/OMT (3/7), and MT/OMT (3/7)/CN against T. urticae
2.2.6. Control Efficiency of MT/OMT (3/7), and MT/OMT (3/7)/CN against T. urticae in the Greenhouse
2.3. Enzyme Activity Assay against P. xylostella
2.3.1. Sample Preparation Using Leaf-Dipping Method
2.3.2. Preparation of Homogenous Liquid
2.3.3. CarE and GST Activity Assay According to Bradford’s Method
2.4. Analysis of Morphology of Cuticles
2.4.1. Pretreatment of Mites
2.4.2. Observation of Morphology and Structural Changes of Cuticles in Spider Mites
2.5. Statistic Analysis
3. Results and Discussion
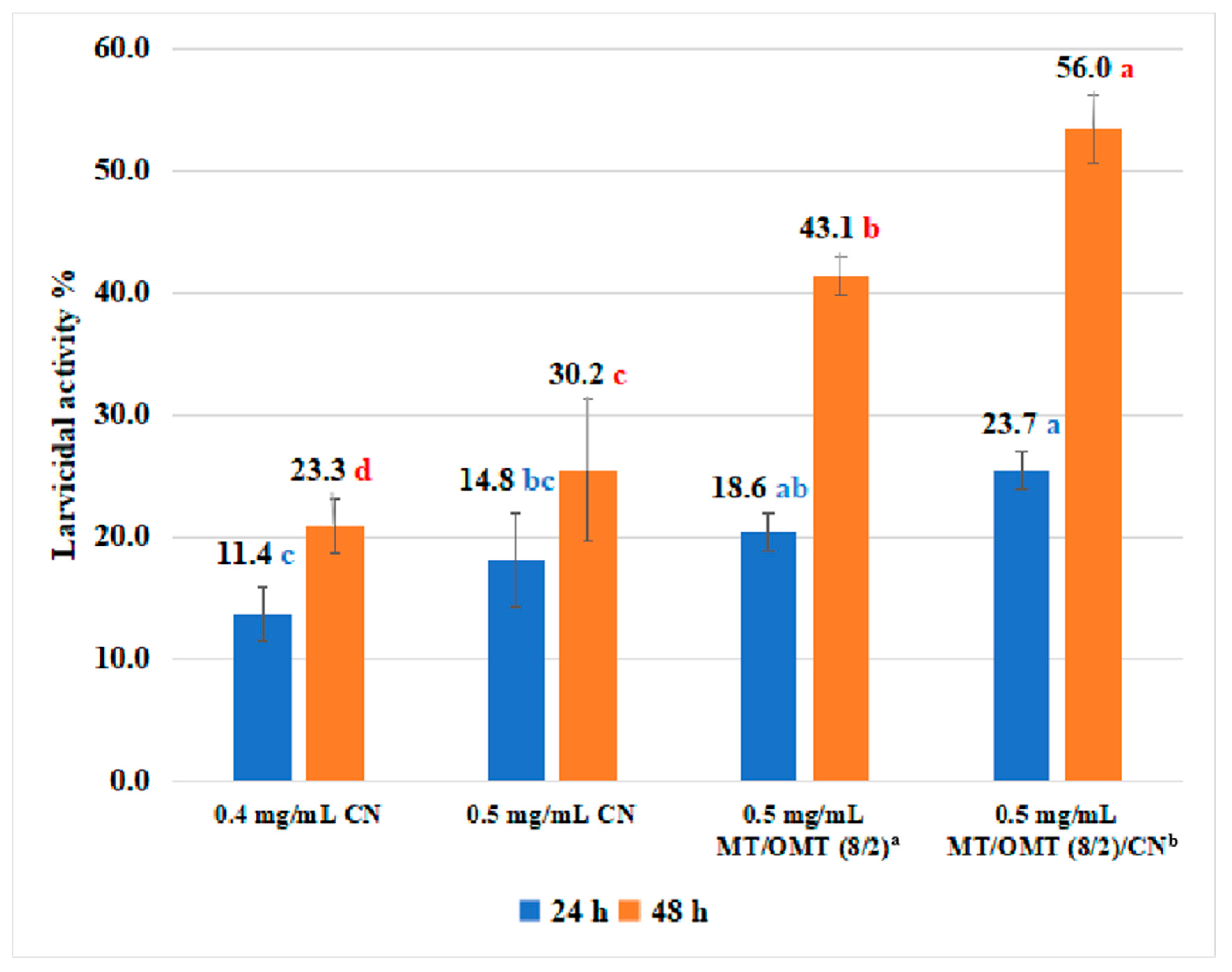
3.1. Insecticidal Activity
3.2. Acaricidal Activity
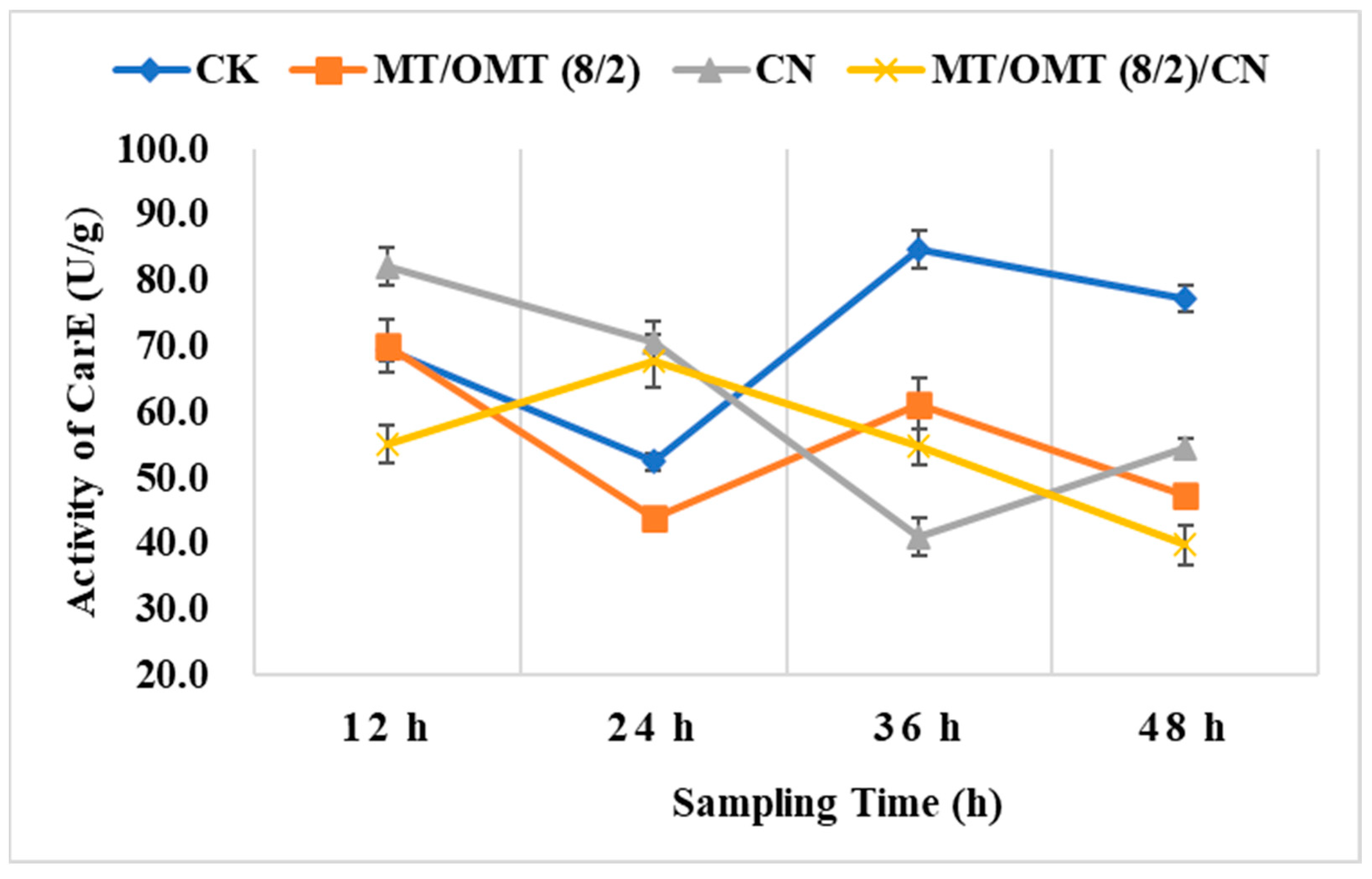
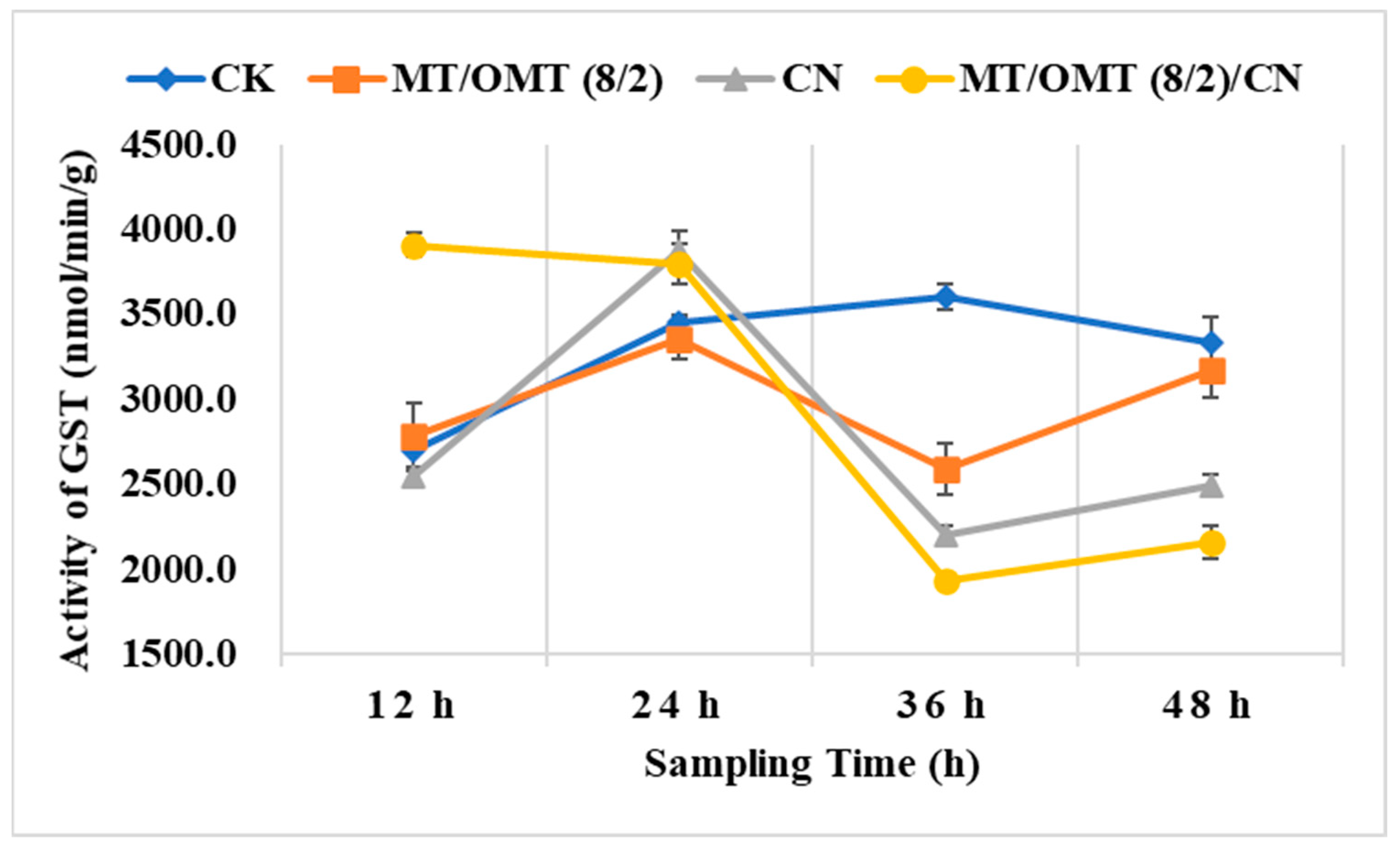
3.3. Changes of Detoxification Enzymes Activities
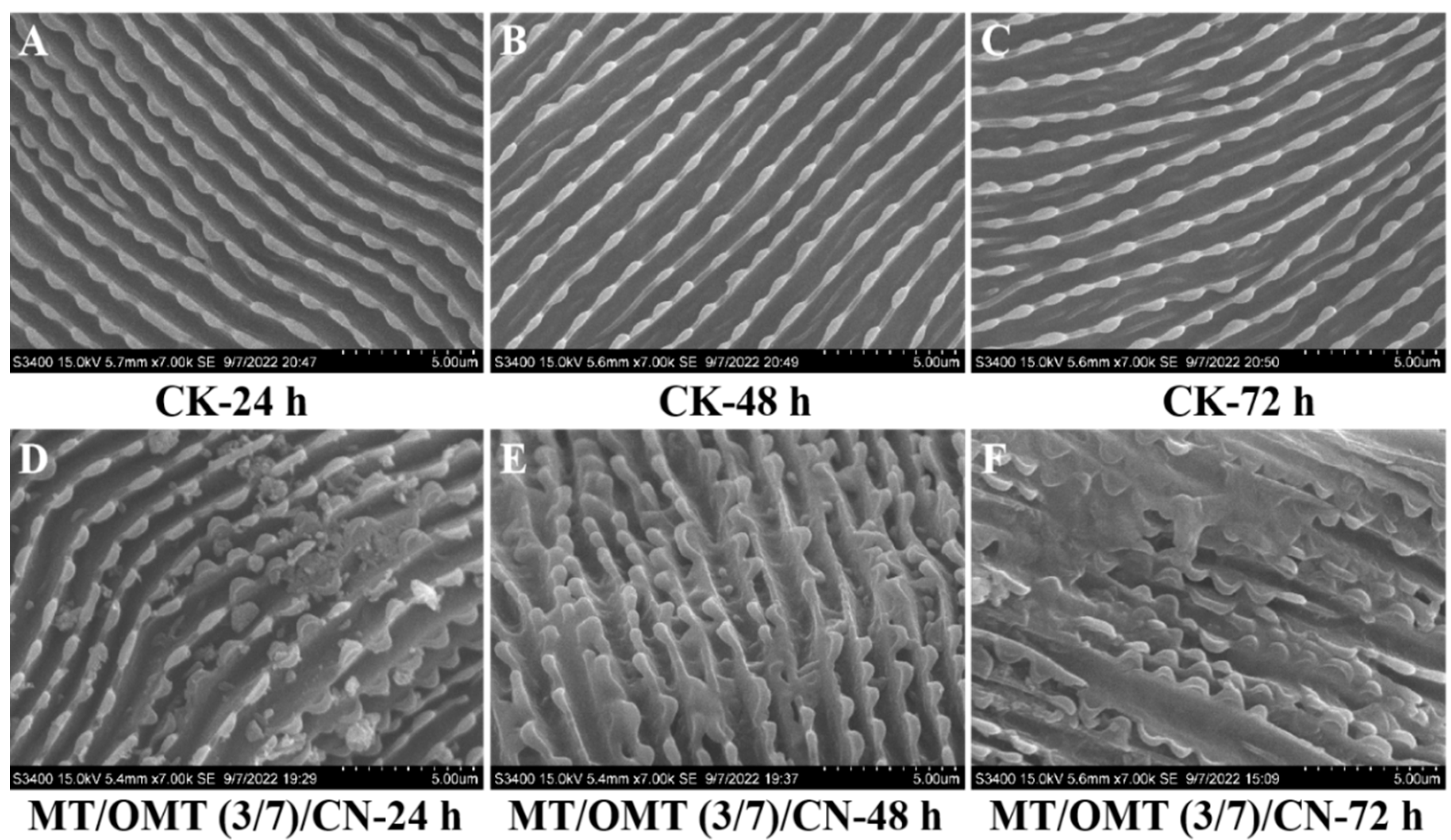
3.4. Toxicological Study of Structural Changes of Cuticles by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, S.; Liu, Z.; Chen, J.; Sun, G.; Jiang, Y.; Li, M.; Xiong, L.; Chen, S.; Zhou, Y.; Asad, M.; et al. Silencing arginine kinase/integrin β1 subunit by transgenic plant expressing dsRNA inhibits the development and survival of Plutella xylostella. Pest Manag. Sci. 2020, 76, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, L.M.; Beccacece, H.M. The emerging importance of noncoding RNAs in the insecticide tolerance, with special emphasis on Plutella xylostella (Lepidoptera: Plutellidae). Wiley Interdiscip. Rev. RNA 2019, 10, e1539. [Google Scholar] [CrossRef] [PubMed]
- Banazeer, A.; Afzal, M.B.S.; Hassan, S.; Ijaz, M.; Shad, S.A.; Serrão, J.E. Status of insecticide resistance in Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) from 1997 to 2019: Cross-resistance, genetics, biological costs, underlying mechanisms, and implications for management. Phytoparasitica 2021, 50, 465–485. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Badawy, M.E.; Mahmoud, N.F.; Marei, A.E.-S.M. Acaricidal activity, biochemical effects and molecular docking of some monoterpenes against two-spotted spider mite (Tetranychus urticae Koch). Pestic. Biochem. Physiol. 2019, 156, 105–115. [Google Scholar] [CrossRef]
- Ndiaye, S.G.; Welty, C. Augmentation and conservation biological control of Tetranychus urticae on hops in Ohio. Biol. Control. 2022, 173, 104980. [Google Scholar] [CrossRef]
- Mavridis, K.; Papapostolou, K.M.; Ilias, A.; Michaelidou, K.; Stavrakaki, M.; Roditakis, E.; Tsagkarakou, A.; Bass, C.; Vontas, J. Next-generation molecular diagnostics (TaqMan qPCR and ddPCR) for monitoring insecticide resistance in Bemisia tabaci. Pest Manag. Sci. 2022, 78, 4994–5001. [Google Scholar] [CrossRef]
- Shan, J.; Sun, X.; Li, R.; Zhu, B.; Liang, P.; Gao, X. Identification of ABCG transporter genes associated with chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2021, 77, 3491–3499. [Google Scholar] [CrossRef]
- Yamanaka, T.; Kitabayashi, S.; Jouraku, A.; Kanamori, H.; Kuwazaki, S.; Sudo, M. A feasibility trial of genomics-based diagnosis detecting insecticide resistance of the diamondback moth. Pest Manag. Sci. 2022, 78, 1573–1581. [Google Scholar] [CrossRef]
- Poonsri, W.; Pluempanupat, W.; Chitchirachan, P.; Bullangpoti, V.; Koul, O. Insecticidal alkanes from Bauhinia scandens var. horsfieldii against Plutella xylostella L. (Lepidoptera: Plutellidae). Ind. Crop. Prod. 2015, 65, 170–174. [Google Scholar] [CrossRef]
- Venzon, M.; Togni, P.H.B.; Perez, A.L.; Oliveira, J.M. Control of two-spotted spider mites with neem-based products on a leafy vegetable. Crop Prot. 2020, 128, 105006. [Google Scholar] [CrossRef]
- Fan, Q.; Li, X.; Wei, C.; Wang, P.; Sun, H.; Zheng, S.; Li, Y.; Tian, Z.; Liu, J.; Zhang, Y. Extraction, structure characterization and biological activity determination of (S)-(-)-palasonin from Butea monosperma (Lam.) Kuntze seeds. Ind. Crop. Prod. 2022, 187, 115393. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Wang, Y.; Thapa, S.; Zhang, S.; Xu, H. Synthesis of piperine-based ester derivatives with diverse aromatic rings and their agricultural bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann. Insects 2023, 14, 40. [Google Scholar] [CrossRef]
- Hao, M.; Xu, J.; Wen, H.; Du, J.; Zhang, S.; Lv, M.; Xu, H. Recent advances on biological activities and structural modifications of dehydroabietic acid. Toxins 2022, 14, 632. [Google Scholar] [CrossRef]
- Yang, R.; Lv, M.; Xu, H. Synthesis of piperine analogs containing isoxazoline/pyrazoline scaffold and their pesticidal bioactivities. J. Agric. Food Chem. 2018, 66, 11254–11264. [Google Scholar] [CrossRef]
- Qu, H.; Lv, M.; Yu, X.; Lian, X.; Xu, H. Discovery of some piperine-based phenylsulfonylhydrazone derivatives as potent botanically narcotic agents. Sci. Rep. 2015, 5, 13077. [Google Scholar] [CrossRef]
- Koul, O.; Singh, R.; Kaur, B.; Kanda, D. Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind. Crop Prod. 2013, 49, 428–436. [Google Scholar] [CrossRef]
- Huang, J.-L.; Lv, M.; Xu, H. Semisynthesis of some matrine ether derivatives as insecticidal agents. RSC Adv. 2017, 7, 15997–16004. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Zhang, B.; Lv, M.; Xu, H. Non-food bioactive products: Semisynthesis, biological activities, and mechanisms of action of oximinoether derivatives of matrine from Sophora flavescens. Ind. Crop. Prod. 2019, 131, 134–141. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Z.; Hao, M.; Lv, M.; Xu, H. Evaluation of biological activities, and exploration on mechanism of action of matrine-cholesterol derivatives. Bioorg. Chem. 2020, 94, 103439. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, H. Research progress of agricultural bioactivities and structural modifications of matrine and its analogues. Chin. J. Pestic. Sci. 2019, 21, 609–626. [Google Scholar]
- Lv, M.; Ma, Q.; Zhang, S.; Xu, H. Agrochemical properties evaluation of some imines alkaloids of matrine/oxymatrine. Bioorganic Med. Chem. Lett. 2021, 48, 128246. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, M.; Sun, Z.; Li, S. Preparation of matrinic/oxymatrinic amide derivatives as insecticidal/acaricidal agents and study on the mechanisms of action against Tetranychus cinnabarinus. J. Agric. Food Chem. 2019, 67, 12182–12190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, N.; Sun, J.; Zhang, L.; Guo, J.; Hao, X.; Sun, Y. Oxymatrine inhibits bocavirus MVC replication, reduces viral gene expression and decreases apoptosis induced by viral infection. Virol. Sin. 2019, 34, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Zhao, J.; Zhang, Y.; Chen, Y.; Liu, Y.; Xu, F. Oxymatrine exerts organ- and tissue-protective effects by regulating in-flammation, oxidative stress, apoptosis, and fibrosis: From bench to bedside. Pharmacol. Res. 2020, 151, 104541. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, P.; Ye, J.; Liao, Y.; Du, Z.; Chen, F.; Juanjuan, H.; Zhang, S.; Zhai, W. Oxymatrine inhibits the migration and invasion of hepatocellular carcinoma cells by reducing the activity of MMP-2/-9 via regulating p38 signaling pathway. J. Cancer 2019, 10, 5397–5403. [Google Scholar] [CrossRef]
- Arena, J.S.; Merlo, C.; Defagó, M.T.; Zygadlo, J.A. Insecticidal and antibacterial effects of some essential oils against the poultry pest Alphitobius diaperinus and its associated microorganisms. J. Pest Sci. 2019, 93, 403–414. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, X.; Cao, J.; Wu, Z.; Pan, D. Effects of 1,8-cineole on carbohydrate metabolism related cell structure changes of salmonella. Front. Microbiol. 2018, 9, 1078. [Google Scholar] [CrossRef]
- Meng, C.; Zeng, W.; Lv, J.; Wang, Y.; Gao, M.; Chang, R.; Li, Q.; Wang, X. 1,8-cineole ameliorates ischaemic brain damage via TRPC6/CREB pathways in rats. J. Pharm. Pharmacol. 2021, 73, 979–985. [Google Scholar] [CrossRef]
- de Albuquerque Lima, T.; de Queiroz Baptista, N.M.; de Oliveira, A.P.; da Silva, P.A.; de Gusmão, N.B.; dos Santos Correia, M.T.; Napoleão, T.H.; da Silva, M.V.; Paiva, P.M. Insecticidal activity of a chemotype VI essential oil from Lippia alba leaves collected at Caatinga and the major compound (1,8-cineole) against Nasutitermes corniger and Sitophilus zeamais. Pestic. Biochem. Physiol. 2021, 177, 104901. [Google Scholar] [CrossRef]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Liao, M.; Li, S.; Wu, H.; Gao, Q.; Shi, S.; Huang, Y.; Cao, H. Transcriptomic analysis of Sitophilus zeamais in response to limonene fumigation. Pest. Manag. Sci. 2022, 78, 4774–4782. [Google Scholar] [CrossRef]
- Born, F.D.; da Camara, C.A.; de Melo, J.P.; de Moraes, M.M. Acaricidal property of the essential oil from Lippia gracilis against Tetranychus urticae and a natural enemy, Neoseiulus californicus, under greenhouse conditions. Exp. Appl. Acarol 2018, 75, 491–502. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Boukouvala, M.C.; Ntalaka, C.T.; Maggi, F.; Spinozzi, E.; Petrelli, R.; Perinelli, D.R.; Benelli, G.; et al. Carlina acaulis essential oil nanoemulsion as a new grain protectant against different developmental stages of three stored-product beetles. Pest Manag. Sci. 2022, 78, 2434–2442. [Google Scholar] [CrossRef]
- Ebadollahi, A. Chemical composition, acaricidal and insecticidal effects of essential oil from Achillea filipendulina against two arthropod pests; Oryzaephilus surinamensis and Tetranychus urticae. Toxin Rev. 2016, 36, 132–137. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Sendi, J.J.; Aliakbar, A. Efficacy of Nanoencapsulated Thymus eriocalyx and Thymus kotschyanus Essential Oils by a Mesoporous Material MCM-41 Against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Èntomol. 2017, 110, 2413–2420. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Sendi, J.J. Acaricidal potentials of the terpene-rich essential oils of two Iranian Eucalyptus species against Tetranychus urticae Koch. J. Oleo Sci. 2017, 66, 307–314. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Yang, C.; Liu, T.; Liu, X.; Yuan, H. Isolation and insecticidal activity of essential oil from Artemisia la-vandulaefolia DC. against Plutella xylostella. Toxins 2021, 13, 842. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crop. Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Sun, Z.; Lv, M.; Huang, W.; Li, T.; Xu, H. Development of botanical pesticides: Exploration on the phenotype of vestigial wings of insect pests induced by plant natural products or their derivatives by blocking tyrosine phosphorylation of insulin receptor 1. J. Agric. Food Chem. 2022, 70, 2117–2126. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, H.; He, W.; Deng, Y.; Sun, R.; Zhong, G. Synergistic effects of botanical curcumin-induced programmed cell death on the management of Spodoptera litura Fabricius with avermectin. Ecotoxicol. Environ. Saf. 2021, 229, 113097. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Shi, Y.; Liao, M.; Xiao, J.; Li, X.; Zhou, L.; Liu, C.; Liu, P.; Cao, H. Laboratory and field evaluation of the aphidicidal activity of moso bamboo (Phyllostachys pubescens) leaf extract and identification of the active components. Pest Manag. Sci. 2019, 75, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Weng, H.; Ma, Z.; Zhang, X. Efficacy of binary combinations between methyl salicylate and carvacrol against thrips Anaphothrips obscurus: Laboratory and field trials. Pest Manag. Sci. 2019, 76, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Nong, Q.-Y.; Liu, Y.-A.; Qin, L.-T.; Liu, M.; Mo, L.-Y.; Liang, Y.-P.; Zeng, H.-H. Toxic mechanism of three azole fungicides and their mixture to green alga Chlorella pyrenoidosa. Chemosphere 2020, 262, 127793. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2015, 72, 474–480. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crop. Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, X.; Yu, X.; Wu, Y.; Jiang, D. Resistance to insecticides and synergistic and antagonistic effects of essential oils on dimefluthrin toxicity in a field population of Culex quinquefasciatus Say. Ecotoxicol. Environ. Saf. 2018, 169, 928–936. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.-Y.; Jin, D.-C. Protective and detoxifying enzyme activity and ABCG subfamily gene expression in Sogatella furcifera under insecticide stress. Front. Physiol. 2019, 9, 1890. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Tian, Z.; Li, Y.; Ye, X.; Li, R.; Li, X.; Zheng, S.; Liu, J.; Zhang, Y. Identification of key residues of carboxylesterase PxEst-6 involved in pyrethroid metabolism in Plutella xylostella (L.). J. Hazard. Mater. 2021, 407, 124612. [Google Scholar] [CrossRef]
- Zhu, W.; Duan, Y.; Chen, J.; Merzendorfer, H.; Zou, X.; Yang, Q. SERCA interacts with chitin synthase and participates in cu-ticular chitin biogenesis in Drosophila. Insect. Biochem. Mol. Biol. 2022, 145, 103783. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Lv, M.; Hao, M. Construction of cholesterol oxime ether derivatives containing isoxazoline/isoxazole fragments and their agricultural bioactive properties/control efficiency. J. Agric. Food Chem. 2021, 69, 8098–8109. [Google Scholar] [CrossRef]
- Wu, C.; Yu, X.; Wang, B.; Liu, J.; Meng, F.; Zhao, Y.; Xiong, L.; Yang, N.; Li, Y.; Li, Z. Synthesis, insecticidal evaluation, and 3D-QASR of novel anthranilic diamide derivatives containing N-arylpyrrole as potential ryanodine receptor activators. J. Agric. Food Chem. 2020, 68, 9319–9328. [Google Scholar] [CrossRef]
- Sun, Y.P.; Johnson, E.R. Analysis of joint action of insecticides against house flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Nian, X.; He, Y.; Lu, L.; Zhao, R. Evaluation of alternative Plutella xylostella control by two Isaria fumosorosea conidial formu-lations-oil-based formulation and wettable powder-combined with Bacillus thuringiensis. Pest Manag. Sci. 2015, 71, 1675–1684. [Google Scholar] [CrossRef]
- Hao, M.; Sun, Z.; Xu, J.; Lv, M.; Xu, H. Semisynthesis and pesticidal activities of derivatives of the diterpenoid andrographolide and investigation on the stress response of Aphis citricola Van der Goot (Homoptera: Aphididae). J. Agric. Food Chem. 2020, 68, 4131–4143. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Wang, J.; Wang, Z.; Xu, J.; Fang, S.; Xu, H. High value-added application of natural plant products in crop protection: Construction and pesticidal activities of piperine-type ester derivatives and their toxicology study. J. Agric. Food Chem. 2022, 70, 16126–16134. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Wan, F.; Jian, Y.; Guo, F.; Chen, J.; Ning, Y.; Ding, W. Graphene oxide–acaricide nanocomposites advance acaricidal activity of acaricides against Tetranychus cinnabarinus by directly inhibiting the transcription of a cuticle protein gene. Environ. Sci. Nano 2021, 8, 3122–3137. [Google Scholar] [CrossRef]
- Togawa, T.; Dunn, A.; Emmons, A.C.; Willis, J.H. CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem. Mol. Biol. 2007, 37, 675–688. [Google Scholar] [CrossRef]
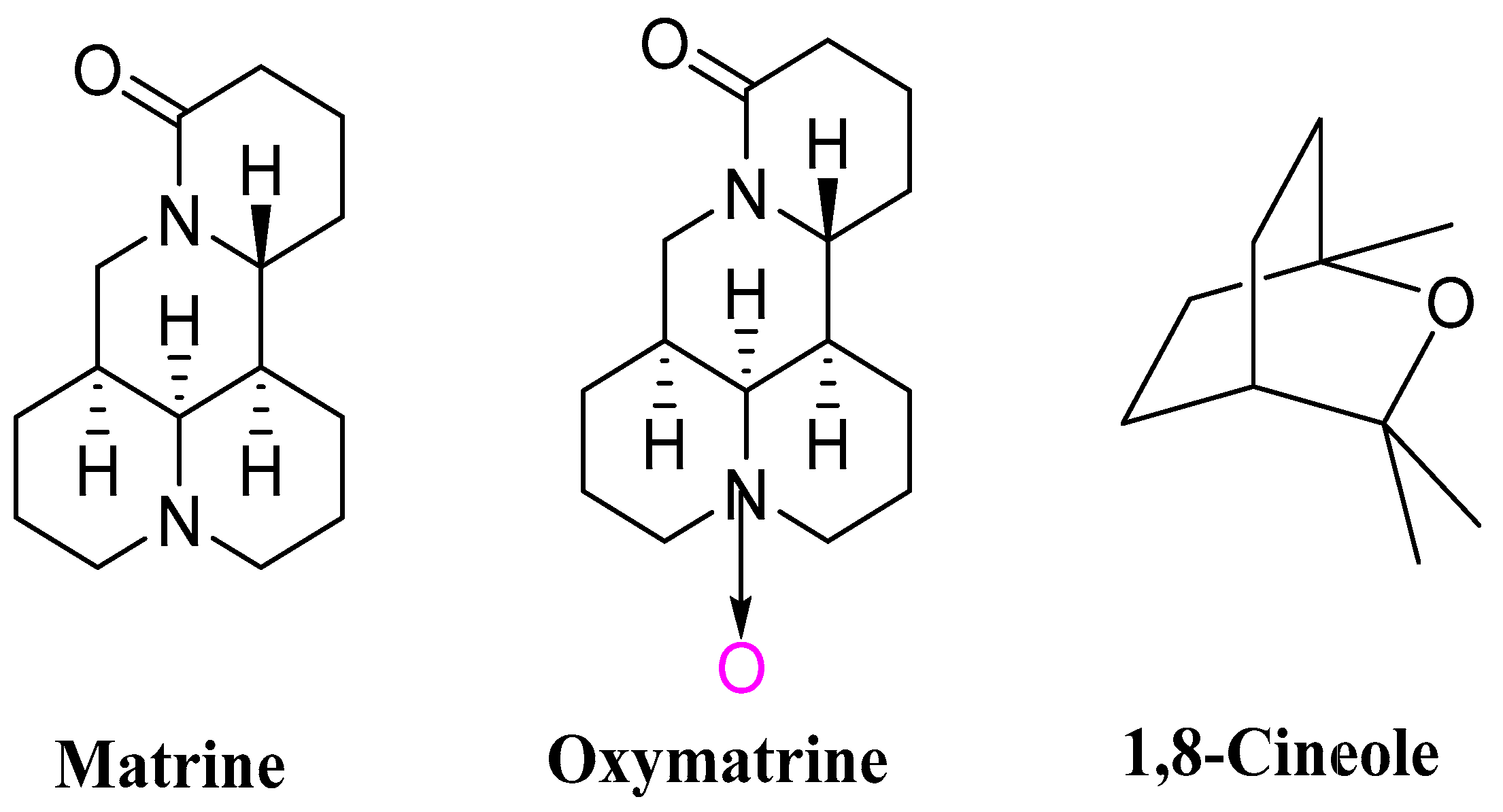


| Compound | Corrected Mortality Rate (Mean ± SE, %) | |
|---|---|---|
| 24 h | 48 h | |
| MT | 9.1 ± 2.2 | 20.9 ± 2.2 b b |
| MT/OMT (9/1) a | 11.3 ± 0 | 16.2 ± 0 bc |
| MT/OMT (8/2) | 13.6 ± 2.2 | 27.9 ± 2.2 a |
| MT/OMT (7/3) | 11.3 ± 3.8 | 20.9 ± 2.2 b |
| MT/OMT (6/4) | 2.3 ± 2.2 | 18.6 ± 2.2 bc |
| MT/OMT (5/5) | 4.5 ± 0 | 20.9 ± 2.2 b |
| MT/OMT (4/6) | 9.1 ± 2.2 | 18.6 ± 2.2 bc |
| MT/OMT (3/7) | 4.5 ± 0 | 13.9 ± 2.2 c |
| MT/OMT (2/8) | 2.3 ± 2.2 | 18.6 ± 2.2 bc |
| MT/OMT (1/9) | 4.5 ± 0 | 16.2 ± 0 bc |
| OMT | 2.3 ± 2.2 | 16.2 ± 0 bc |
| toosendanin | 11.3 ± 0 | 32.5 ± 2.2 a |
| Compound | Corrected Mortality Rate (%) at Concentration (mg/mL) a | ||||
|---|---|---|---|---|---|
| 2 (0.5) b | 1 (0.25) b | 0.5 (0.125) b | 0.25 (0.0625) b | 0.125 (0.03125) b | |
| MT | 65.1 ± 3.8 | 53.4 ± 2.2 | 39.5 ± 2.2 | 23.2 ± 0 | 13.9 ± 2.2 |
| OMT | 60.4 ± 4.4 | 48.8 ± 2.2 | 32.5 ± 4.4 | 18.6 ± 2.2 | 11.6 ± 2.2 |
| MT/OMT (8/2)c | 72.4 ± 3.3 | 58.6 ± 0 | 41.3 ± 1.6 | 27.5 ± 2.8 | 15.5 ± 1.6 |
| MT/OMT (8/2)/CNd | 81.0 ± 1.6 | 65.5 ± 1.6 | 53.4 ± 2.8 | 37.9 ± 0 | 24.1 ± 1.6 |
| β-cypermethrin | 83.7 ± 2.2 | 55.8 ± 5.8 | 37.2 ± 3.8 | 25.5 ± 2.2 | 13.9 ± 2.2 |
| Compound | Linear Regression Equationa | LC50 (mg/mL) | Confidence Interval 95% (mg/mL) | r | χ2 | df | P | Co-Toxicity Coefficient |
|---|---|---|---|---|---|---|---|---|
| MT | Y = 0.057 + 1.240X | 0.899 | 0.636~1.455 | 0.997 | 0.171 | 3 | 0.982 | / |
| OMT | Y = −0.08 + 1.258X | 1.158 | 0.806~2.045 | 0.993 | 0.155 | 3 | 0.984 | / |
| MT/OMT (8/2) b | Y = −0.201 + 1.338X | 0.708 | 0.539~0.970 | 0.998 | 0.025 | 3 | 0.999 | 133 |
| MT/OMT (8/2)/ CN c | Y = 0.456 + 1.280X | 0.441 | 0.324~0.585 | 0.999 | 0.161 | 3 | 0.984 | 213 |
| β-cypermethrin | Y = 1.281 + 1.652X | 0.168 | 0.128~0.226 | 0.980 | 1.845 | 3 | 0.605 | / |
| Compound | Control Efficiency (%) c | ||
|---|---|---|---|
| 1st Day | 3rd Day | 5th Day | |
| MT/OMT (8/2) a | 10.1 ± 1.6 b d | 25.8 ± 1.6 b | 36.8 ± 0 c |
| MT/OMT (8/2)/CN b | 15.5 ± 4.4 b | 32.1 ± 3.3 b | 49.0 ± 3.3 b |
| β-cypermethrin | 40.6 ± 4.4 a | 58.6 ± 2.8 a | 71.9 ± 1.6 a |
| Compound | Corrected Mortality Rate (Mean ± SE, %) | |
|---|---|---|
| 48 h | 72 h | |
| MT | 7.0 ± 2.5 | 23.5 ± 1.7 cde d |
| MT/OMT (9/1) a | 10.5 ± 1.6 | 20.4 ± 3.2 cdef |
| MT/OMT (8/2) | 6.9 ± 0.7 | 24.3 ± 2.3 cde |
| MT/OMT (7/3) | 4.5 ± 1.0 | 21.9 ± 5.8 cdef |
| MT/OMT (6/4) | 10.3 ± 0.5 | 16.0 ± 3.4 ef |
| MT/OMT (5/5) | 10.9 ± 1.6 | 24.6 ± 4.4 cde |
| MT/OMT (4/6) | 8.7 ± 2.0 | 27.1 ± 3.4 bcde |
| MT/OMT (3/7) | 9.4 ± 3.2 | 32.2 ± 0.9 bc |
| MT/OMT (2/8) | 9.8 ± 2.2 | 28.9 ± 1.6 bcd |
| MT/OMT (1/9) | 4.0 ± 1.2 | 25.2 ± 6.3 cde |
| OMT | 6.0 ± 1.3 | 18.2 ± 2.9 def |
| 1,8-cineole (CN) b | 6.5 ± 0.2 | 11.2 ± 0.4 f |
| MT/OMT (3/7)/CN c | 17.8 ± 0.3 | 38.0 ± 3.2 b |
| spirodiclofen | 47.7 ± 4.7 | 88.6 ± 2.3 a |
| Compound | Corrected Mortality Rate (%) at Concentration (mg/mL) a | |||||
|---|---|---|---|---|---|---|
| 6 | 3 | 1.5 | 0.75 | 0.375 | / | |
| MT | 80.3 ± 0.9 | 56.6 ± 1.6 | 40.5 ± 2.4 | 24.1 ± 2.7 | 15.6 ± 3.9 | / |
| OMT | 75.1 ± 2.4 | 53.9 ± 2.2 | 38.5 ± 2.1 | 20.5 ± 3.5 | 10.3 ± 1.0 | / |
| Compound | Corrected mortality rate (%) at concentration (mg/mL) a | |||||
| 4 (0.5) b | 2 (0.25) b | 1 (0.125) b | 0.5 (0.0625) b | 0.25 (0.03125) b | 0.125 | |
| MT/OMT (3/7) c | 72.6 ± 0.4 | 53.2 ± 1.3 | 40.4 ± 2.7 | 32.2 ± 0.9 | 21.7 ± 0.6 | 13.4 ± 1.5 |
| MT/OMT (3/7)/CN d | 80.1 ± 0.8 | 61.0 ± 0.5 | 49.6 ± 2.2 | 38.0 ± 3.2 | 29.6 ± 3.5 | 17.4 ± 1.6 |
| spirodiclofen | 90.1 ± 0.5 | 72.5 ± 0.2 | 54.5 ± 0.8 | 36.8 ± 2.6 | 26.0 ± 1.2 | / |
| Compound | Linear Regression Equation a | LC50 (mg/mL) | Confidence Interval 95% (mg/mL) | r | χ2 | df | P | Co-Toxicity Coefficient |
|---|---|---|---|---|---|---|---|---|
| MT | Y = −0.471 + 1.549X | 2.014 | 1.667~2.451 | 0.987 | 2.004 | 3 | 0.572 | / |
| OMT | Y = −0.604 + 1.606X | 2.377 | 1.970~2.924 | 0.994 | 0.398 | 3 | 0.941 | / |
| MT/OMT (3/7) b | Y = −0.160 + 1.083X | 1.405 | 1.108~1.858 | 0.986 | 1.681 | 4 | 0.794 | 160 |
| MT/OMT (3/7)/CN c | Y = 0.054 + 1.103X | 0.894 | 0.713~1.134 | 0.992 | 2.166 | 4 | 0.705 | 252 |
| spirodiclofen | Y = 1.597 + 1.558X | 0.094 | 0.077~0.113 | 0.996 | 2.198 | 3 | 0.532 | / |
| Compound | Control Efficiency (%) c | ||
|---|---|---|---|
| 1st Day | 3rd Day | 5th Day | |
| MT/OMT (3/7) a | 7.7 ± 0.8 b d | 19.6 ± 1.9 c | 31.3 ± 1.1 c |
| MT/OMT (3/7)/CN b | 13.8 ± 1.8 b | 31.9 ± 1.0 b | 42.3 ± 1.2 b |
| spirodiclofen | 25.0 ± 2.4 a | 58.3 ± 1.0 a | 73.9 ± 1.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Lv, M.; Fang, S.; Wang, Y.; Wen, H.; Zhang, S.; Xu, H. Exploration of Synergistic Pesticidal Activities, Control Effects and Toxicology Study of a Monoterpene Essential Oil with Two Natural Alkaloids. Toxins 2023, 15, 240. https://doi.org/10.3390/toxins15040240
Xu J, Lv M, Fang S, Wang Y, Wen H, Zhang S, Xu H. Exploration of Synergistic Pesticidal Activities, Control Effects and Toxicology Study of a Monoterpene Essential Oil with Two Natural Alkaloids. Toxins. 2023; 15(4):240. https://doi.org/10.3390/toxins15040240
Chicago/Turabian StyleXu, Jianwei, Min Lv, Shanshan Fang, Yanyan Wang, Houpeng Wen, Shaoyong Zhang, and Hui Xu. 2023. "Exploration of Synergistic Pesticidal Activities, Control Effects and Toxicology Study of a Monoterpene Essential Oil with Two Natural Alkaloids" Toxins 15, no. 4: 240. https://doi.org/10.3390/toxins15040240
APA StyleXu, J., Lv, M., Fang, S., Wang, Y., Wen, H., Zhang, S., & Xu, H. (2023). Exploration of Synergistic Pesticidal Activities, Control Effects and Toxicology Study of a Monoterpene Essential Oil with Two Natural Alkaloids. Toxins, 15(4), 240. https://doi.org/10.3390/toxins15040240




