High-Throughput IgG Epitope Mapping of Tetanus Neurotoxin: Implications for Immunotherapy and Vaccine Design
Abstract
1. Introduction
2. Results
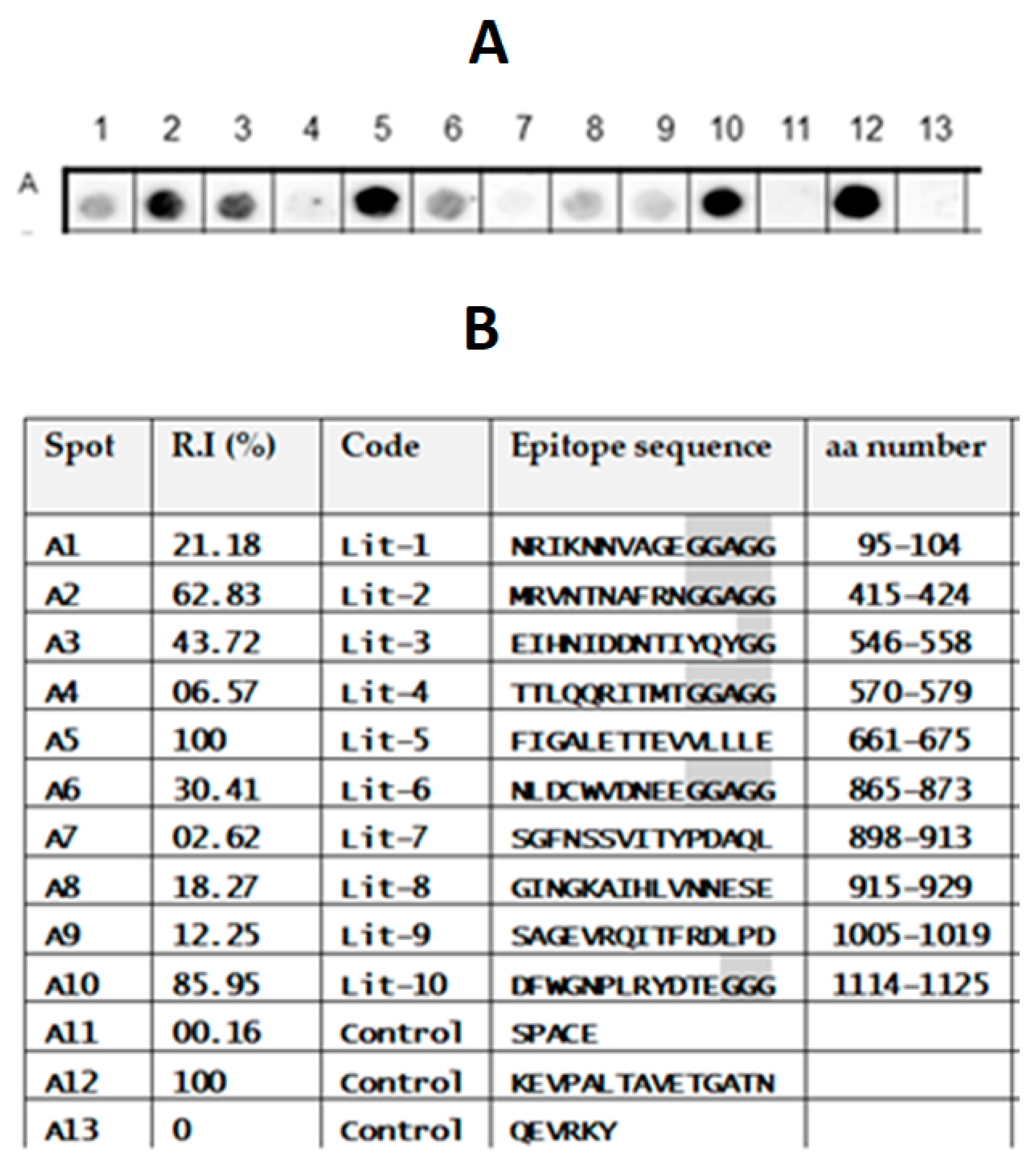
2.1. Identification of the Immunodominant IgG Epitopes in TeNT
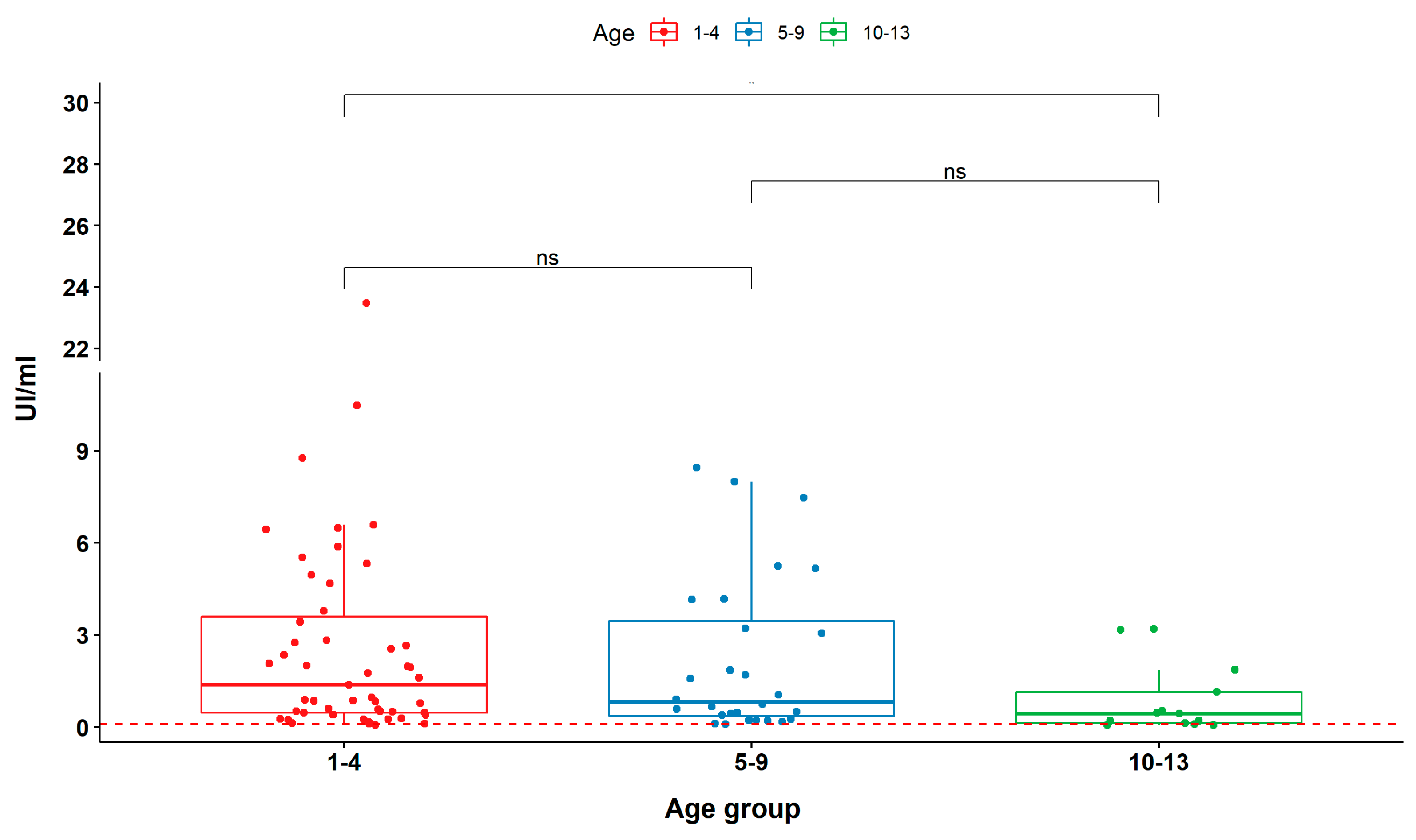
2.2. Epitope Reactivity by ELISA-MAP4
2.3. The Spatial Location of TeNT Reactive Epitopes
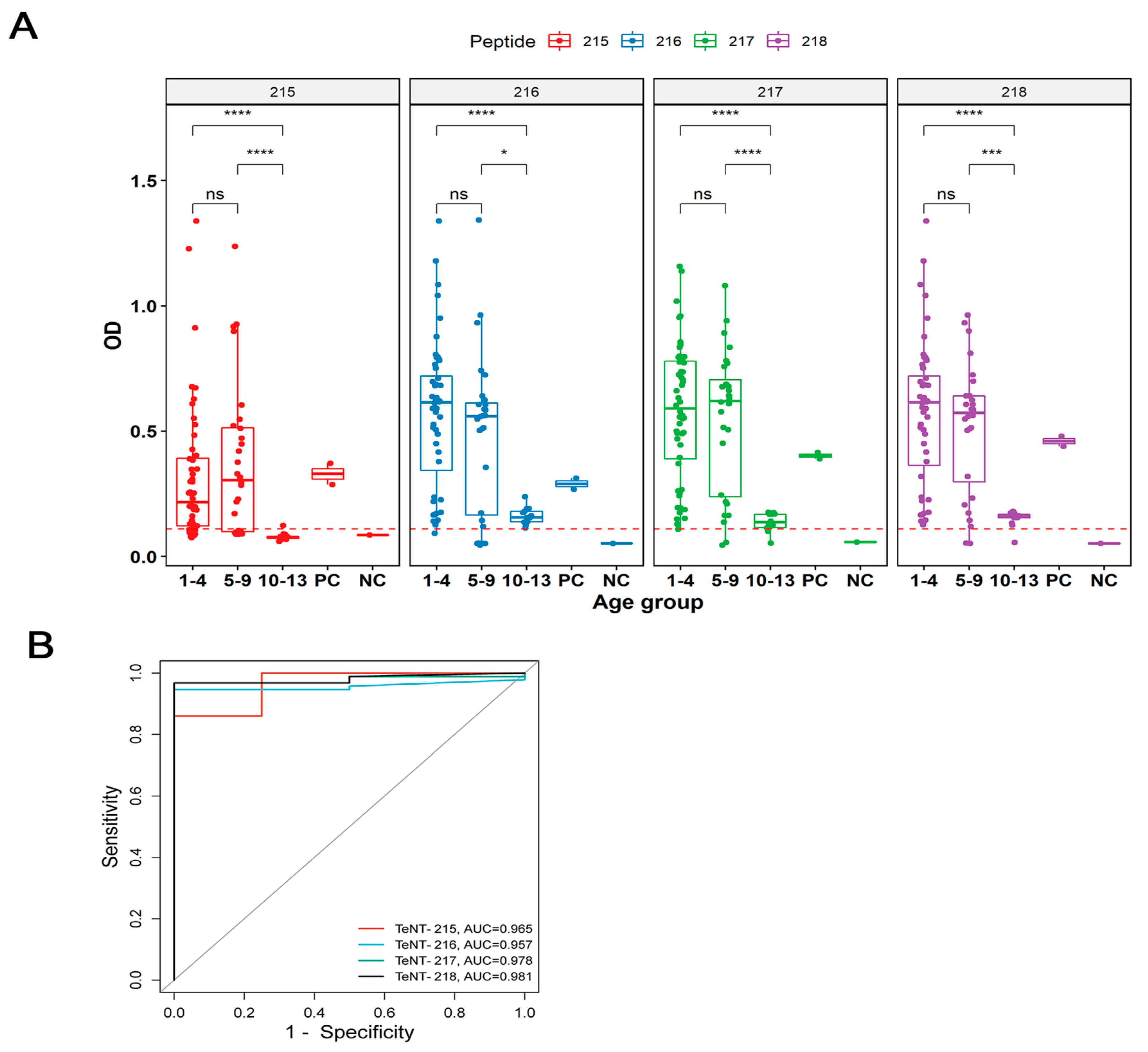
2.4. Validation of TeNT IgG Epitopes Deposited in the IEDB Database
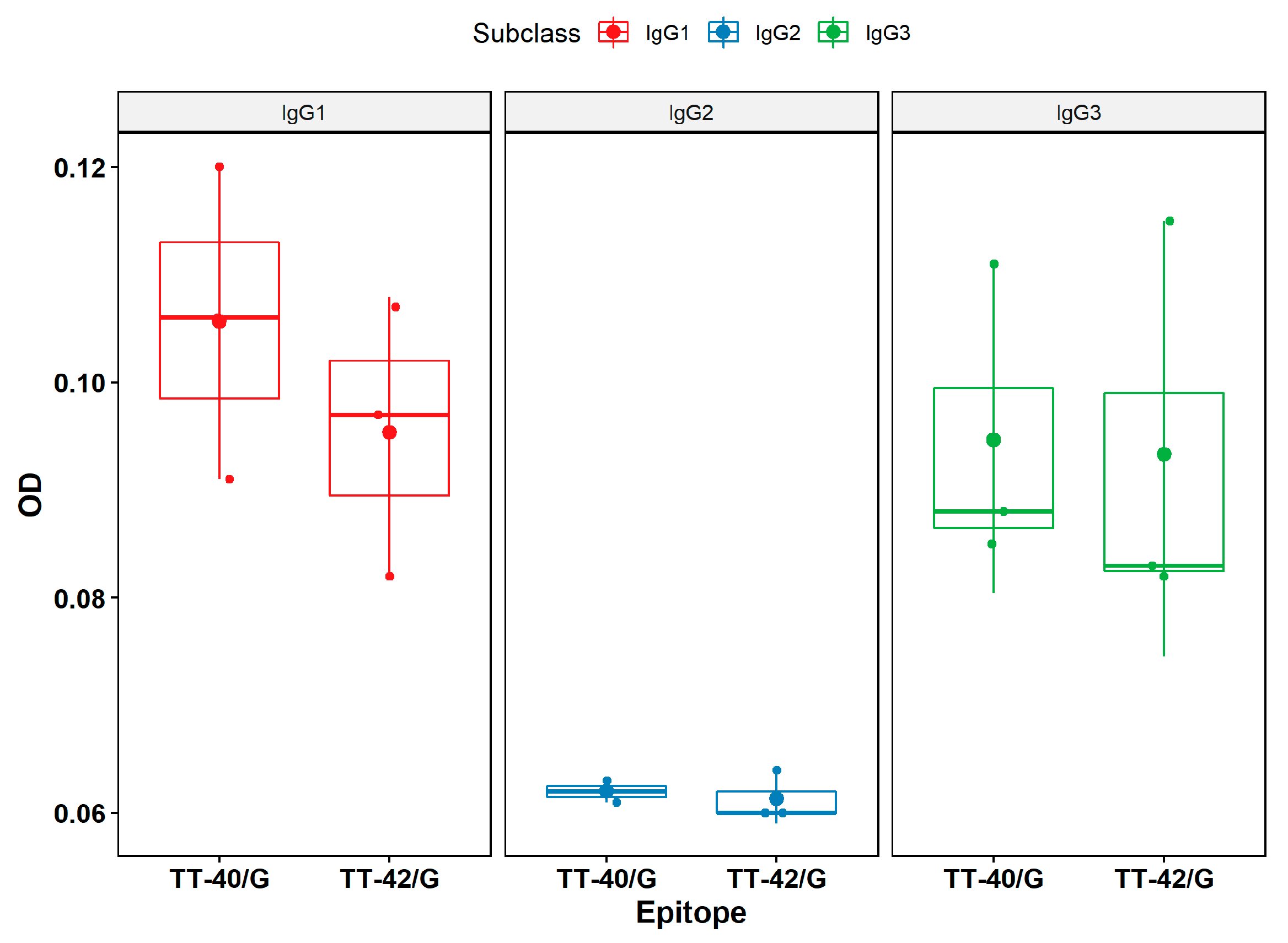
2.5. Identification of Subclass of Immunoglobulins through ELISA
2.6. Cross-Immune IgG Epitopes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Human Sera and Ethics Statement
5.2. Mapping of Specific B-Cell Epitopes of the TeNT Protein
5.3. Screening of SPOT membranes
5.4. Scanning and Measurement of Spot Signal Intensities
5.5. Peptides and MAP4 Synthesis
5.6. Peptide-ELISA Serodiagnosis
5.7. ELISA for Specific IgG1, IgG2, and IgG3 Isotypes
5.8. Structural Localization of the IgG Epitopes
5.9. Bioinformatics Tools
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and tetanus neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Lista, F.; Montecucco, C. The role of the single interchains disulfide bond in tetanus and botulinum neurotoxins and the development of antitetanus and antibotulism drugs. Cell. Microbiol. 2019, 21, e13037. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; World Bank. State of the World’s Vaccines and Immunization; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Clarke, K.E.N.; MacNeil, A.; Hadler, S.; Scott, C.; Tiwari, T.S.P.; Cherian, T. Global epidemiology of diphtheria, 2000–2017. Emerg. Infect. Dis. 2019, 2, 1834–1842. [Google Scholar] [CrossRef]
- Demicheli, V.; Barale, A.; Rivetti, A. Vaccines for women for preventing neonatal tetanus. Cochrane Database Syst Rev. 2015, 2015, CD002959. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W.; Andemeskel, A.T.; Kyei, K.; Woldeamanuel, M.W.; Woldeamanuel, W. Case fatality of adult tetanus in Africa: Systematic review and meta-analysis. J. Neurol. Sci. 2016, 368, 292–299. [Google Scholar] [CrossRef]
- World Health Organization. Tetanus vaccines: WHO position paper, February 2017—Recommendations. Vaccine 2018, 36, 3573–3575. [Google Scholar] [CrossRef]
- Nigussie, J.; Girma, B.; Molla, A.; Mareg, M. Tetanus toxoid vaccination coverage and associated factors among childbearing women in Ethiopia: A systematic review and meta-analysis. BioMed Res. Int. 2021, 2021, 5529315. [Google Scholar] [CrossRef]
- Rossetto, O.; Caccin, P.; Rigoni, M.; Tonello, F.; Bortoletto, N.; Stevens, R.C.; Montecucco, C. Active-site mutagenesis of tetanus neurotoxin implicates TYR-375 and GLU-271 in metalloproteolytic activity. Toxicon 2001, 39, 1151–1159. [Google Scholar] [CrossRef]
- Rossetto, O.; Scorzeto, M.; Megighian, A.; Montecucco, C. Tetanus neurotoxin. Toxicon 2013, 66, 59–63. [Google Scholar] [CrossRef]
- Rao, K.N.; Kumaran, D.; Binz, T.; Swaminathan, S. Structural analysis of the catalytic domain of tetanus neurotoxin. Toxicon 2005, 45, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Blum, F.C.; Tepp, W.H.; Johnson, E.A.; Barbieri, J.T. Multiple domains of tetanus toxin direct entry into primary neurons. Traffic 2014, 15, 1057–1065. [Google Scholar] [CrossRef]
- Calvo, A.C.; Oliván, S.; Manzano, R.; Zaragoza, P.; Aguilera, J.; Osta, R. Fragment C of tetanus toxin: New insights into its neuronal signaling pathway. Int. J. Mol. Sci. 2012, 13, 6883–6901. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, Z.; Kim, J.-J.P.; Barbieri, J.T.; Baldwin, M.R. Gangliosides as high-affinity receptors for tetanus neurotoxin. J. Biol. Chem. 2009, 284, 26569–26577. [Google Scholar] [CrossRef] [PubMed]
- Zuverink, M.; Bluma, M.; Barbieri, J.T. Tetanus toxin cis-loop contributes to light-chain translocation. mSphere 2020, 5, e00244-20. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Khosravi-Eghbal, R.; Reza Mahmoudi, A.; Jeddi-Tehrani, M.; Rabbani, H.; Shokri, F. Comparative in vitro and in vivo assessment of toxin neutralization by antitetanus toxin monoclonal antibodies. Hum. Vaccin. Immunother. 2014, 10, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Fotinou, C.; Emsley, P.; Black, I.; Ando, H.; Ishida, H.; Kiso, M.; Sinha, K.A.; Fairweather, N.F.; Isaacs, N.W. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests crosslinking between ganglioside receptors and the toxin. J. Biol. Chem. 2001, 276, 32274–32281. [Google Scholar] [CrossRef] [PubMed]
- Von Kossel, K.; Ferreira, R.; Marinho, L.; Castro, L.; Nigro, A.; Montero, E. Gangliosideos-estudo do colágeno e da resposta inflamatória no processo cicatricial. Acta Cir. Bras. 2000, 15, 16–19. [Google Scholar] [CrossRef]
- Schnaar, R.L. Gangliosides of the vertebrate nervous system. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef]
- Kyu, H.H.; Mumford, J.E.; Stanaway, J.D.; Barber, R.M.; Hancock, J.R.; Vos, T.; Murray, C.J.; Naghavi, M. Mortality from Tetanus between 1990 and 2015: Findings from the global burden of disease study 2015. BMC Public Health 2017, 179, 179. [Google Scholar] [CrossRef]
- Thaysen-Andersen, M.; Jørgensen, S.B.; Wilhelmsen, E.S.; Petersen, J.W.; Højrup, P. Investigation of the detoxification mechanism of formaldehyde-treated tetanus toxin. Vaccine 2007, 25, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Behrensdorf-Nicol, H.A.; Kegel, B.; Bonifas, U.; Silberbach, K.; Klimek, J.; Weißer, K.; Krämer, B. Residual enzymatic activity of the tetanus toxin light chain present in tetanus toxoid batches used for vaccine production. Vaccine 2008, 26, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Disease Control and Prevention (CDC). Prevention of pertussis, Tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2008, 57, 1–51. [Google Scholar]
- Sekaran, N.K.; Edwards, K.M. Extensive swelling reaction associated with diphtheria and tetanus toxoids and acellular pertussis vaccine. Pediatr. Infect. Dis. J. 2006, 25, 374–375. [Google Scholar] [CrossRef]
- Moller, J.; Kraner, M.E.; Burkovski, A. More than a toxin: Protein inventory of Clostridium tetani toxoid vaccines. Proteomes 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghotloo, S.; Golsaz-Shirazi, F.; Amiri, M.M.; Jeddi-Tehrani, M.; Shokri, F. Epitope mapping of tetanus toxin by monoclonal antibodies: Implication for immunotherapy and vaccine design. Neurotox. Res. 2020, 37, 239–249. [Google Scholar] [CrossRef]
- Kitamura, N.; Bahkali, K.; Chem, E.D.; Quilty, B.J.; Edwards, T.; Toizumi, M.; Yoshida, L.M. Waning rate of immunity and duration of protective immunity against diphtheria toxoid as a function of age and number of doses: Systematic review and quantitative data analysis. Hum. Vaccin. Immunother. 2022, 18, 2099700. [Google Scholar] [CrossRef]
- Swart, E.M.; van Gageldonk, P.G.; de Melker, H.E.; van der Klis, F.R.; Berbers, G.A.; Mollema, L. Long-Term Protection against Diphtheria in the Netherlands after 50 Years of Vaccination: Results from a seroepidemiological study. PLoS ONE 2016, 11, e0148605. [Google Scholar] [CrossRef]
- Nabel, G.J. Designing tomorrow’s vaccines. N. Engl. J. Med. 2013, 368, 551–560. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Yang, L.; Zhou, Y.; Li, M.; Xu, W.; Qin, Y.; Su, J.; Zhao, W.; Gu, C.; et al. Immunoglobin G sero-dynamics aided host specific linear epitope identification and differentiation of infected from vaccinated hosts. J. Virol. 2022, 96, e0014322. [Google Scholar] [CrossRef]
- Liu, F.J.; Shi, D.Y.; Li, Z.Y.; Lu, J.S.; Wang, R.; Pang, X.B.; Yang, Z.X.; Yu, Y.Z. Evaluation of a recombinant tetanus toxin subunit vaccine. Toxicon 2020, 187, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Przedpelski, A.; Tepp, W.H.; Pellett, S.; Johnson, E.A.; Barbieri, J.T. A novel high-potency tetanus vaccine. mBio 2020, 11, e01668-20. [Google Scholar] [CrossRef] [PubMed]
- Ahnert-Hilger, G.; Bizzini, B.; Goretzki, K.; Müller, H.; Völckers, C.; Habermann, E. Monoclonal antibodies against tetanus toxin and toxoid. Med. Microbiol. Immunol. 1983, 172, 123–135. [Google Scholar] [CrossRef]
- Andersen-Beckh, B.; Binz, T.; Kurazono, H.; Mayer, T.; Eisel, U.; Niemann, H. Expression of tetanus toxin subfragments in vitro and characterization of epitopes. Infect. Immun. 1989, 57, 3498–34505. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.B.; Cryz, S.J., Jr.; Schürch, U.; Ganss, M.T.; Bruderer, U. Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J. Immunol. 1993, 151, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Tahmasebi, F.; Younesi, V.; Razavi, A.; Khoshnoodi, J.; Bayat, A.A.; Abbasi, E.; Rabbani, H.; Jeddi-Tehrani, M.; Shokri, F.J. Characterization of neutralizing monoclonal antibodies directed against tetanus toxin fragment C. J. Immunotoxicol. 2014, 11, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, R.; Fang, T.; Yu, T.; Chi, X.; Zhang, X.; Liu, S.; Fu, L.; Yu, C.; Chen, W. Tetanus neurotoxin neutralizing antibodies screened from a human immune SCFV antibody phage display library. Toxins 2016, 8, 266. [Google Scholar] [CrossRef]
- Palermo, A.; Weber, L.K.; Rentschler, S.; Isse, A.; Sedlmayr, M.; Herbster, K.; List, V.; Hubbuch, J.; Löffler, F.F.; Nesterov-Müller, A.; et al. Identification of a tetanus toxin specific epitope in single amino acid resolution. Biotechnol. J. 2017, 12, 1700197. [Google Scholar] [CrossRef]
- Ghaderi, S.; Bozorgmehr, M.R.; Ahmadi, M.; Tarahomjoo, S. Identification of conformational B-cell epitopes in Diphtheria toxin at varying temperatures using molecular dynamics simulations. Arch. Razi. Inst. 2021, 75, 427–437. [Google Scholar] [CrossRef]
- Houy, C.; Ming, M.; Ettorre, L.; Jin, R.; Thangavadivel, N.; Chen, T.; Su, J.; Gajewska, B. Epitope profiling of diphtheria toxoid provides enhanced monitoring for consistency testing during manufacturing process changes. Vaccines 2022, 10, 775. [Google Scholar] [CrossRef]
- Stein, P.E.; Boodhoo, A.; Armstrong, G.D.; Cockle, S.A.; Klein, M.H.; Read, R.J. The crystal structure of pertussis toxin. Structure 1994, 2, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Imoto, Y.; Hikima, T.; Inoue, T. Structural flexibility of the tetanus neurotoxin revealed by crystallographic and solution scattering analyses. J. Struct. Biol. X 2021, 5, 100045. [Google Scholar] [CrossRef] [PubMed]
- Poulain, B. Molecular mechanism of action of tetanus toxin and botulinum neurotoxins. Pathol. Biol. 1994, 42, 173–182. [Google Scholar] [PubMed]
- Rummel, A.; Bade, S.; Alves, J.; Bigalke, H.; Binz, T. Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J. Mol. Biol. 2003, 326, 835–847. [Google Scholar] [CrossRef]
- Masuyer, G.; Conrad, J.; Stenmark, P. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep. 2017, 18, 1306–1317. [Google Scholar] [CrossRef]
- Surana, S.; Tosolini, A.P.; Meyer, I.F.G.; Fellows, A.D.; Novoselov, S.S.; Schiavo, G. The travel diaries of tetanus and botulinum neurotoxins. Toxicon 2018, 147, 58–67. [Google Scholar] [CrossRef]
- Bayart, C.; Mularoni, A.; Hemmani, N.; Kerachni, S.; Jose, J.; Gouet, P.; Paladino, J.; Le Borgne, M. Tetanus toxin fragment C: Structure, drug discovery research, and production. Pharmaceuticals 2022, 15, 756. [Google Scholar] [CrossRef]
- Louch, H.A.; Buczko, E.S.; Woody, M.A.; Venable, R.M.; Vann, W.F. Identification of a binding site for ganglioside on the receptor binding domain of tetanus toxin. Biochemistry 2002, 41, 13644–13652. [Google Scholar] [CrossRef]
- Usuwanthim Glenny, A.T.; Hopkins, B.E. Diphtheria toxoid as an immunizing agent. Br. J. Exp. Pathol. 1923, 4, 283. [Google Scholar]
- Alsarraf, H.; Dedic, E.; Bjerrum, M.J.; Østergaard, O.; Kristensen, M.P.; Petersen, J.W.; Jørgensen, R. Biophysical comparison of diphtheria and tetanus toxins with the formaldehyde-detoxified toxoids, the main components of diphtheria and tetanus vaccines. Virulence 2017, 8, 1880–1889. [Google Scholar] [CrossRef]
- Metz, B.; Michiels, T.; Uittenbogaard, J.; Danial, M.; Tilstra, W.; Meiring, H.D.; Hennink, W.E.; Crommelin, D.J.; Kersten, G.F.; Jiskoot, W. Identification of formaldehyde-induced modifications in diphtheria toxin. J. Pharm Sci 2020, 109, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus, and tetanus neurotoxin: From peripheral uptake to central nervous tissue targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef]
- Geethadevi, A.; Jadhav, K.; Kumar, G.; Parashar, D. scFv6.C4 DNA vaccine with fragment C of tetanus toxin increases protective immunity against CEA-expressing tumor. Gene Ther. 2021, 28, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Zuverink, M.; Barbieri, J.T. Protein toxins that utilize gangliosides as host receptors. Prog. Mol. Biol. Transl. Sci. 2018, 156, 325–354. [Google Scholar] [CrossRef]
- Aboud, S.; Matre, R.; Lyamuya, E.F.; Kristoffersen, E.K. Levels and avidity of antibodies to tetanus toxoid in children aged 1–15 years in Dar es Salaam and Bagamoyo, Tanzania. Ann. Trop. Paediatr. 2000, 20, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Papadiochos, I.; Papadiochou, S.; Petsinis, V.; Goutzanis, L.; Atsali, C.; Papadogeorgaki, N. Trismus as a clinical manifestation of tetanus: A case report. J. Oral Facial Pain Headache 2016, 30, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Wei, C.; Ross, R.; Luo, X.; Ma, X.; Qi, Y.; Chai, R.; Cao, J.; Huang, M.; Bo, T. Effects of detoxification process on toxicity and foreign protein of tetanus toxoid and diphtheria toxoid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1207, 123377. [Google Scholar] [CrossRef]
- Bernardes, M.; Lo Presti, S.; Ratzan, K. A case of cephalic tetanus in an elderly patient with trismus. Case Rep. Infect. Dis. 2018, 2018, 1247256. [Google Scholar] [CrossRef]
- Napoleão-Pêgo, P.; Carneiro, F.R.G.; Durans, A.M.; Gomes, L.R.; Morel, C.M.; Provance, D.W., Jr.; De-Simone, S.G. Performance assessment of multi-epitope chimeric antigen for serodiagnosis of acute phase of Mayaro fever. Sci. Rep. 2021, 11, 15374. [Google Scholar] [CrossRef]
- Silva, F.R.; Napoleão-Pêgo, P.; De-Simone, S.G. Identification of linear B epitopes of pertactin of Bordetella pertussis induced by immunization with whole and acellular vaccine. Vaccine 2014, 32, 6251–6258. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; Pina, J.C.; Silva, F.R. Epitope mapping of the diphtheria toxin and development of an ELISA-specific diagnostic assay. Vaccines 2021, 9, 313. [Google Scholar] [CrossRef]
- De-Simone, S.; Souza, A.L.A.; Melgarejo, A.R.; Aguiar, A.S.; Provance, D.W., Jr. Development of elisa assay to detect specific human IgE anti-therapeutic horse sera. Toxicon 2017, 138, 37–42. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Gonçalves, P.S.; Provance, D.W., Jr.; Morel, C.M.; Silva, F.R.B. Cell epitope mapping of the Vibrio cholerae toxins A, B and P. Int. J. Mol. Sci. 2023, 24, 531. [Google Scholar] [CrossRef] [PubMed]
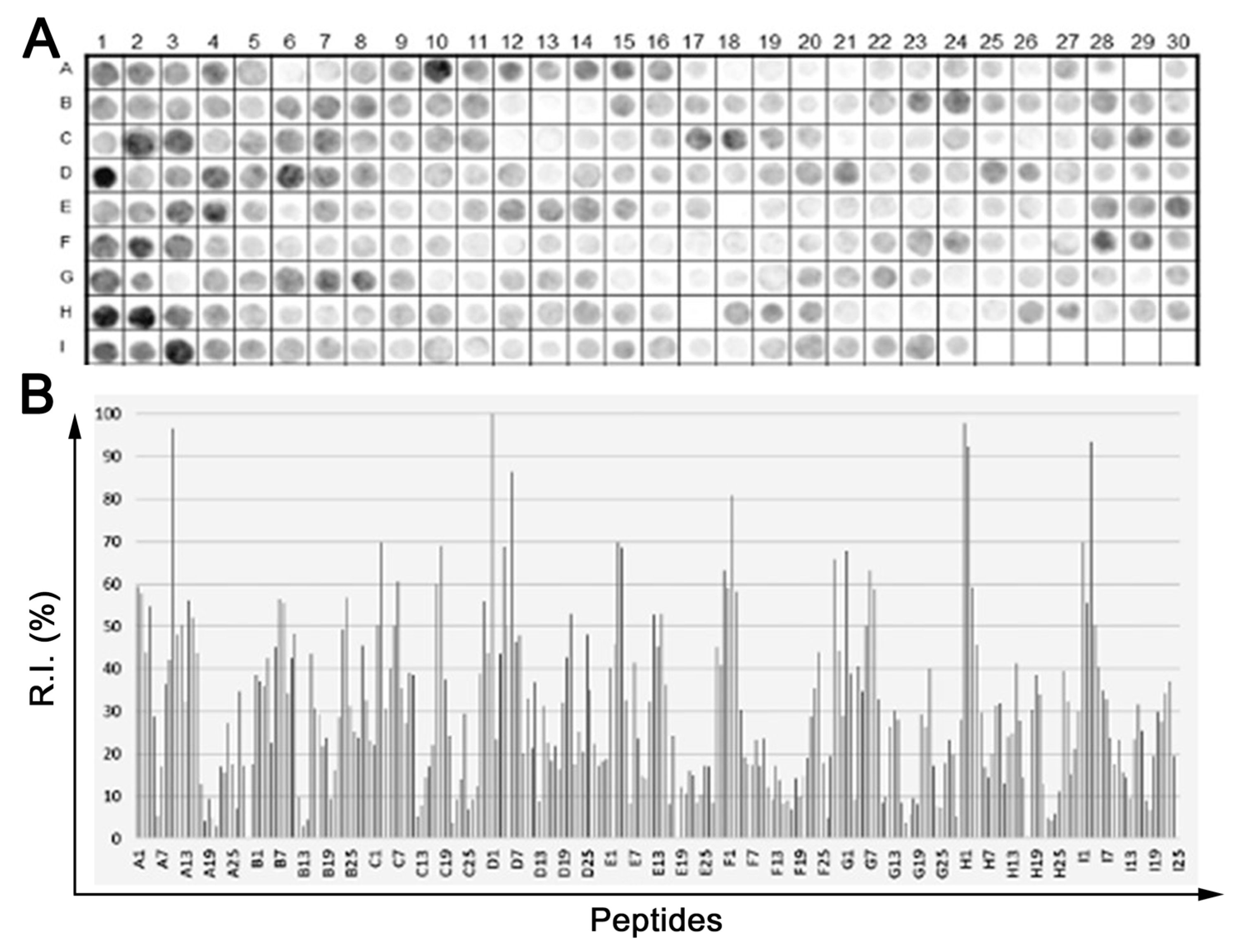
| Code | Sequence of IgG Epitopes | aa Number | 2nd Structure * | Code | Sequence of IgG Epitopes | aa Number | 2nd Structure |
|---|---|---|---|---|---|---|---|
| TT-1/G | DPVNNDTII | 10–15 | C | TT-23/G | IKTIDNFLEKRYEKW | 701–715 | H |
| TT-2/G | VPERYEFGTK | 46–55 | C | TT-24/G | WLGTVNTQFQ | 726–735 | H |
| TT-3/G | IEGASEYYDPNYLRTD | 66–80 | C | TT-25/G | SGPD | 764–767 | C |
| TT-4/G | NRIKNNVAGE | 96–105 | H+C+H | TT-26/G | INKVFSTPIP | 851–860 | C |
| TT-5/G | PYLGSYSLLDKFDTNSNSV | 116–135 | C | TT-27/G | NLDCWVDNEE | 866–875 | H+C+H |
| TT-6/G | EYVPTFDNVIENITS | 201–215 | C | TT-28/G | INND | 889–892 | C |
| TT-7/G | YGMQVSSH | 242–250 | C | TT-29/G | TSGFNSSVITYPDAQLV | 898–914 | C+S+C |
| TT-8/G | AEELFTFGGQD | 269–279 | C | TT-30/G | GINGKAIHLVNNESE | 917–931 | C+S+C |
| TT-9/G | VISCNDPNID | 308–317 | C | TT-31/G | NDMFNN | 944–949 | C |
| TT-10/G | QFDKDSNGQ | 331–339 | C | TT-32/G | VSASHLEQYGTNEYS | 961–975 | C |
| TT-11/G | YNSIMYGFTEIELGK | 351–365 | H | TT-33/G | SAGEVRQITFRDLPD | 1.001–1.021 | C+S+C |
| TT-12/G | LLDDTIYNDTEGFN | 389–401 | C | TT-34/G | DRLSS | 1.039–1.043 | C |
| TT-13/G | MRVNTNAFRN | 416–425 | S+C+H | TT-35/G | TGLGAIREDNN | 1.058–1.068 | C |
| TT-14/G | TNIRENLYNRTASLTDLGGE | 446–465 | C | TT-36/G | DRCNNNNQYV | 1073–1082 | C |
| TT-15/G | EKNSFSEEPFQ | 480–490 | C | TT-37/G | DFWGNPLRYDTE | 1.115–1.126 | C+H+C |
| TT-16/G | YNTKNKPLNFNYSLD | 496–510 | C | TT-38/G | LKNITD | 1.143–1.147 | C |
| TN-17/G | YNLQSKITLPNDRTT | 516–530 | C | TT-39/G | NAPSYTN | 1.153–1.159 | C |
| TT-18/G | EIHNIDDNTIYQY | 551–563 | C+S+C+S | TT-40/G | YTPNNEIDS | 1.180–1.188 | C |
| TT-19/G | TTLQRITMT | 571–579 | C+S | TT-41/G | YPKDGNAFNNLDRIL | 1.211–1.225 | S+C+H |
| TT-20/G | FTNES | 623–627 | C | TT-42/G | GYNAPGIPLYK | 1.228–1.238 | C |
| TT-21/G | FIGALETTGVVLLLE | 661–675 | H+C+H | TT-43/G | HNGQIGNDPNRD | 1.271–1.282 | C |
| TT-22/G | KNLDCWVDNE | 865–874 | H+C | TT-44/G | TDEGWTND | 1.308–1315 | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-Simone, S.G.; Napoleão-Pêgo, P.; Lechuga, G.C.; Carvalho, J.P.R.S.; Gomes, L.R.; Cardozo, S.V.; Morel, C.M.; Provance, D.W., Jr.; Silva, F.R.d. High-Throughput IgG Epitope Mapping of Tetanus Neurotoxin: Implications for Immunotherapy and Vaccine Design. Toxins 2023, 15, 239. https://doi.org/10.3390/toxins15040239
De-Simone SG, Napoleão-Pêgo P, Lechuga GC, Carvalho JPRS, Gomes LR, Cardozo SV, Morel CM, Provance DW Jr., Silva FRd. High-Throughput IgG Epitope Mapping of Tetanus Neurotoxin: Implications for Immunotherapy and Vaccine Design. Toxins. 2023; 15(4):239. https://doi.org/10.3390/toxins15040239
Chicago/Turabian StyleDe-Simone, Salvatore G., Paloma Napoleão-Pêgo, Guilherme C. Lechuga, João P. R. S. Carvalho, Larissa R. Gomes, Sergian V. Cardozo, Carlos M. Morel, David W. Provance, Jr., and Flavio R. da Silva. 2023. "High-Throughput IgG Epitope Mapping of Tetanus Neurotoxin: Implications for Immunotherapy and Vaccine Design" Toxins 15, no. 4: 239. https://doi.org/10.3390/toxins15040239
APA StyleDe-Simone, S. G., Napoleão-Pêgo, P., Lechuga, G. C., Carvalho, J. P. R. S., Gomes, L. R., Cardozo, S. V., Morel, C. M., Provance, D. W., Jr., & Silva, F. R. d. (2023). High-Throughput IgG Epitope Mapping of Tetanus Neurotoxin: Implications for Immunotherapy and Vaccine Design. Toxins, 15(4), 239. https://doi.org/10.3390/toxins15040239








