Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.)
Abstract
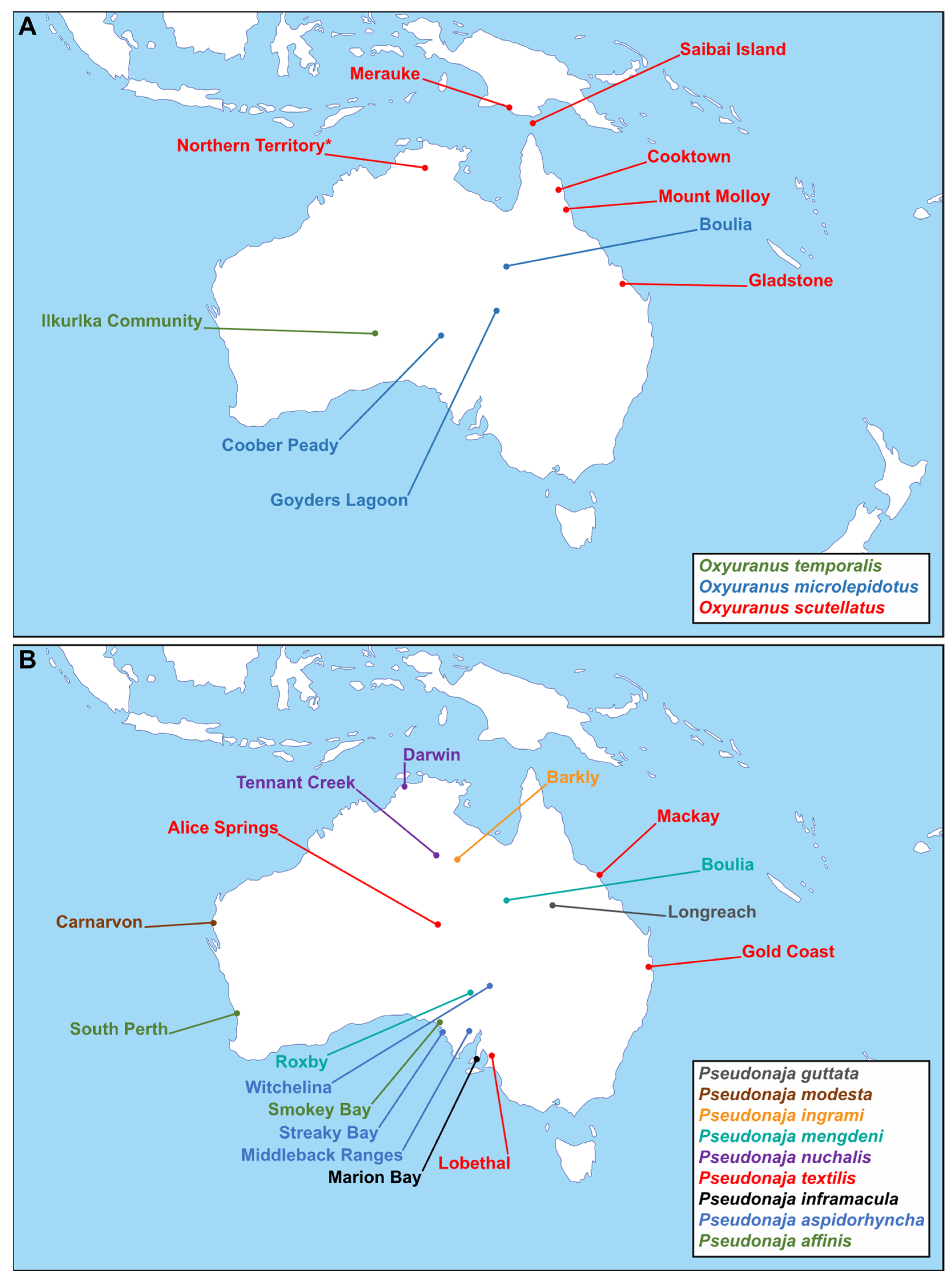
1. Introduction
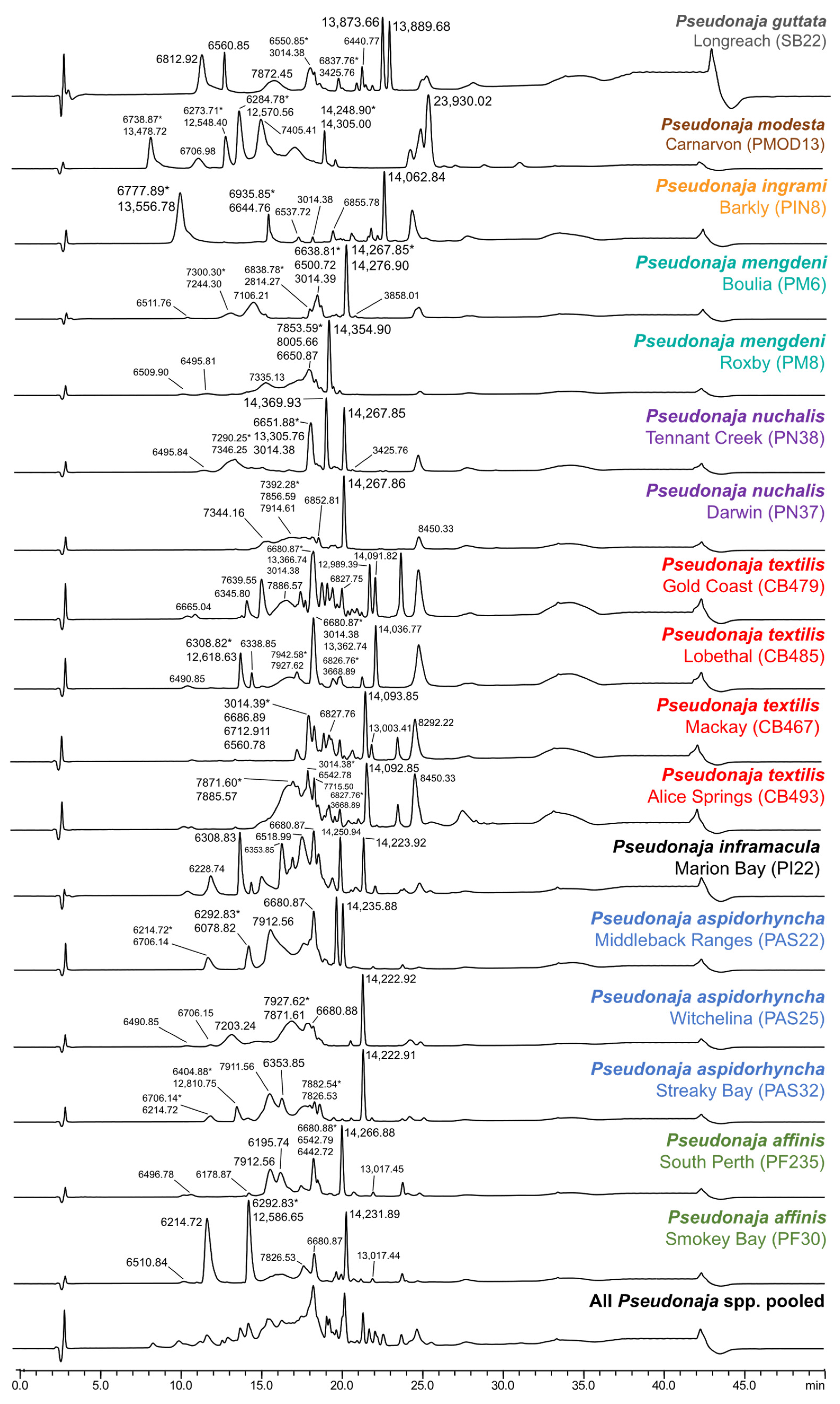
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Venom Preparation
4.2. Liquid Chromatography, At-Line Nanofractionation and Mass Spectrometry
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Thiel, J.; Khan, M.A.; Wouters, R.M.; Harris, R.J.; Casewell, N.R.; Fry, B.G.; Kini, R.M.; Mackessy, S.P.; Vonk, F.J.; Wüster, W.; et al. Convergent evolution of toxin resistance in animals. Biol. Rev. 2022, 97, 1823–1843. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proceedings. Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. Biol. Sci. 2016, 283, 20152841. [Google Scholar] [CrossRef] [PubMed]
- Daltry, J.C.; Wuster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.J., Jr.; Aird, S.D. Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 128, 425–456. [Google Scholar] [CrossRef]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. 2021, 118, e2015579118. [Google Scholar] [CrossRef]
- Jackson, T.N.W.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P.; et al. Rapid Radiations and the Race to Redundancy: An Investigation of the Evolution of Australian Elapid Snake Venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef]
- Zancolli, G.; Casewell, N.R. Venom Systems as Models for Studying the Origin and Regulation of Evolutionary Novelties. Mol. Biol. Evol. 2020, 37, 2777–2790. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharm. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science 2021, 371, 386. [Google Scholar] [CrossRef]
- Durban, J.; Pérez, A.; Sanz, L.; Gómez, A.; Bonilla, F.; Rodríguez, S.; Chacón, D.; Sasa, M.; Angulo, Y.; Gutiérrez, J.M. Integrated “omics” profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genom. 2013, 14, 1–17. [Google Scholar] [CrossRef]
- Mackessy, S.P. Venom ontogeny in the Pacific rattlesnakes Crotalus viridis helleri and C. v. oreganus. Copeia 1988, 1988, 92–101. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; Attarde, S.; Suranse, V.; Iyer, A.; Casewell, N.R.; Whitaker, R.; Martin, G.; Sunagar, K. Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspots. PLOS Negl. Trop. Dis. 2021, 15, e0009247. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLOS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef]
- Jackson, T.N.; Jouanne, H.; Vidal, N. Snake venom in context: Neglected clades and concepts. Front. Ecol. Evol. 2019, 7, 332. [Google Scholar] [CrossRef]
- Smiley-Walters, S.A.; Farrell, T.M.; Gibbs, H.L. High levels of functional divergence in toxicity towards prey among the venoms of individual pigmy rattlesnakes. Biol. Lett. 2019, 15, 20180876. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, U.; Khochare, S.; Attarde, S.; Laxme, R.R.S.; Suranse, V.; Martin, G.; Sunagar, K. Remarkable intrapopulation venom variability in the monocellate cobra (Naja kaouthia) unveils neglected aspects of India’s snakebite problem. J. Proteom. 2021, 242, 104256. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Harrison, R.A.; Renjifo, C.; Wüster, W. Domain Loss Facilitates Accelerated Evolution and Neofunctionalization of Duplicate Snake Venom Metalloproteinase Toxin Genes. Mol. Biol. Evol. 2011, 28, 2637–2649. [Google Scholar] [CrossRef]
- Shibata, H.; Chijiwa, T.; Oda-Ueda, N.; Nakamura, H.; Yamaguchi, K.; Hattori, S.; Matsubara, K.; Matsuda, Y.; Yamashita, A.; Isomoto, A.; et al. The habu genome reveals accelerated evolution of venom protein genes. Sci. Rep. 2018, 8, 11300. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Oda-Ueda, N.; Hisata, K.; Nakamura, H.; Chijiwa, T.; Hattori, S.; Isomoto, A.; Yugeta, H.; Yamasaki, S.; Fukumaki, Y.; et al. Alternative mRNA Splicing in Three Venom Families Underlying a Possible Production of Divergent Venom Proteins of the Habu Snake, Protobothrops flavoviridis. Toxins 2019, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Dowell, N.L.; Giorgianni, M.W.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. The Deep Origin and Recent Loss of Venom Toxin Genes in Rattlesnakes. Curr. Biol. 2016, 26, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Koludarov, I. How the toxin got its toxicity. Front. Pharmacol. 2020, 11, 574925. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Madaras, F.; Turnbull, R.K.; Morley, T.; Dunstan, N.; Allen, L.; Kuchel, T.; Mirtschin, P.; Hodgson, W.C. Comparative Studies of the Venom of a New Taipan Species, Oxyuranus temporalis, with Other Members of Its Genus. Toxins 2014, 6, 1979–1995. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef]
- Tasoulis, T.; Silva, A.; Veerati, P.C.; Baker, M.; Hodgson, W.C.; Dunstan, N.; Isbister, G.K. Intra-Specific Venom Variation in the Australian Coastal Taipan Oxyuranus scutellatus. Toxins 2020, 12, 485. [Google Scholar] [CrossRef]
- Reeks, T.; Lavergne, V.; Sunagar, K.; Jones, A.; Undheim, E.; Dunstan, N.; Fry, B.; Alewood, P.F. Deep venomics of the Pseudonaja genus reveals inter- and intra-specific variation. J. Proteom. 2016, 133, 20–32. [Google Scholar] [CrossRef]
- Birrell, G.W.; Earl, S.; Masci, P.P.; de Jersey, J.; Wallis, T.P.; Gorman, J.J.; Lavin, M.F. Molecular diversity in venom from the Australian Brown snake, Pseudonaja textilis. Mol. Cell Proteom. 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Hay, C.; Arbuckle, K.; Jackson, T.N.W.; Bos, M.H.A.; op den Brouw, B.; Debono, J.; Allen, L.; Dunstan, N.; Morley, T.; et al. Coagulotoxic effects by brown snake (Pseudonaja) and taipan (Oxyuranus) venoms, and the efficacy of a new antivenom. Toxicol. Vitr. 2019, 58, 97–109. [Google Scholar] [CrossRef]
- Flight, S.; Mirtschin, P.; Masci, P.P. Comparison of Active Venom Components between Eastern Brown Snakes Collected from South Australia and Queensland. Ecotoxicology 2006, 15, 133–141. [Google Scholar] [CrossRef]
- Skejić, J.; Hodgson, W.C. Population Divergence in Venom Bioactivities of Elapid Snake Pseudonaja textilis: Role of Procoagulant Proteins in Rapid Rodent Prey Incapacitation. PLoS ONE 2013, 8, e63988. [Google Scholar] [CrossRef]
- McCleary, R.J.R.; Sridharan, S.; Dunstan, N.L.; Mirtschin, P.J.; Kini, R.M. Proteomic comparisons of venoms of long-term captive and recently wild-caught Eastern brown snakes (Pseudonaja textilis) indicate venom does not change due to captivity. J. Proteom. 2016, 144, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Skejic, J.; Steer, D.L.; Dunstan, N.; Hodgson, W.C. Venoms of related mammal-eating species of taipans (Oxyuranus) and brown snakes (Pseudonaja) differ in composition of toxins involved in mammal poisoning. bioRxiv 2018, 378141. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; Page, C.B.; Buckley, N.A.; Brown, S.G.A.; O’Leary, M.A.; Isbister, G.K. The Australian Snakebite Project, 2005–2015 (ASP-20). Med. J. Aust. 2017, 207, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Jensen, S.; Nimorakiotakis, B.; Winkel, K. Snakebite in Papua New Guinea. In Venomous Bites and Stings in Papua New Guinea: A Treatment Guide for Health Workers and Doctors; University of Melbourne: Melbourne, Australia, 2005; pp. 5–32. [Google Scholar]
- Williams, D.J.; Jensen, S.D.; Nimorakiotakis, B.; Müller, R.; Winkel, K.D. Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon 2007, 49, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. Constraints, Allometry, and Adaptation: Food Habits and Reproductive Biology of Australian Brownsnakes (Pseudonaja: Elapidae). Herpetologica 1989, 45, 195–207. [Google Scholar]
- Shine, R. Habitats, diets, and sympatry in snakes: A study from Australia. Can. J. Zool. 1977, 55, 1118–1128. [Google Scholar] [CrossRef]
- Broad, A.; Sutherland, S.; Coulter, A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Solving the ‘Brown snake paradox’: In vitro characterisation of Australasian snake presynaptic neurotoxin activity. Toxicol. Lett. 2012, 210, 318–323. [Google Scholar] [CrossRef]
- Viala, V.L.; Hildebrand, D.; Trusch, M.; Fucase, T.M.; Sciani, J.M.; Pimenta, D.C.; Arni, R.K.; Schlüter, H.; Betzel, C.; Mirtschin, P.; et al. Venomics of the Australian eastern brown snake (Pseudonaja textilis): Detection of new venom proteins and splicing variants. Toxicon 2015, 107, 252–265. [Google Scholar] [CrossRef]
- Cipriani, V.; Debono, J.; Goldenberg, J.; Jackson, T.N.W.; Arbuckle, K.; Dobson, J.; Koludarov, I.; Li, B.; Hay, C.; Dunstan, N.; et al. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 53–60. [Google Scholar] [CrossRef]
- Birrell, G.W.; Earl, S.T.H.; Wallis, T.P.; Masci, P.P.; de Jersey, J.; Gorman, J.J.; Lavin, M.F. The Diversity of Bioactive Proteins in Australian Snake Venoms * S. Mol. Cell. Proteom. 2007, 6, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.; Donnellan, S.C.; Hutchinson, M.N.; Hutchinson, R.G. A phylogenetic analysis of Pseudonaja (Hydrophiinae, Elapidae, Serpentes) based on mitochondrial DNA sequences. Mol. Phylogenetics Evol. 2005, 37, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.L.; Lee, M.S.; Leys, R.; Foster, R.; Scott Keogh, J. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (Hydrophiinae): Evidence from seven genes for rapid evolutionary radiations. J. Evol. Biol. 2008, 21, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; den Brouw, B.o.; Dashevsky, D.; Gloria, A.; Youngman, N.J.; Watson, E.; Green, P.; Hay, C.; Dunstan, N.; Allen, L.; et al. Clinical implications of convergent procoagulant toxicity and differential antivenom efficacy in Australian elapid snake venoms. Toxicol. Lett. 2019, 316, 171–182. [Google Scholar] [CrossRef]
- Jackson, T.N.; Sunagar, K.; Undheim, E.A.; Koludarov, I.; Chan, A.H.; Sanders, K.; Ali, S.A.; Hendrikx, I.; Dunstan, N.; Fry, B.G. Venom down under: Dynamic evolution of Australian elapid snake toxins. Toxins 2013, 5, 2621–2655. [Google Scholar] [CrossRef]
- Patterson, M.; Wolfe, A.K.; Fleming, P.A.; Bateman, P.W.; Martin, M.L.; Sherratt, E.; Warburton, N.M. Ontogenetic shift in diet of a large elapid snake is facilitated by allometric change in skull morphology. Evol. Ecol. 2022, 36, 489–509. [Google Scholar] [CrossRef]
- Mason, A.J.; Holding, M.L.; Rautsaw, R.M.; Rokyta, D.R.; Parkinson, C.L.; Gibbs, H.L. Venom Gene Sequence Diversity and Expression Jointly Shape Diet Adaptation in Pitvipers. Mol. Biol. Evol. 2022, 39, msac082. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Fry, B.G. A tricky trait: Applying the fruits of the “function debate” in the philosophy of biology to the “venom debate” in the science of toxinology. Toxins 2016, 8, 263. [Google Scholar] [CrossRef]
- Calderón-Celis, F.; Cid-Barrio, L.; Encinar, J.R.; Sanz-Medel, A.; Calvete, J.J. Absolute venomics: Absolute quantification of intact venom proteins through elemental mass spectrometry. J. Proteom. 2017, 164, 33–42. [Google Scholar] [CrossRef]
- Calderón-Celis, F.; Diez-Fernández, S.; Costa-Fernández, J.M.; Encinar, J.R.; Calvete, J.J.; Sanz-Medel, A. Elemental Mass Spectrometry for Absolute Intact Protein Quantification without Protein-Specific Standards: Application to Snake Venomics. Anal. Chem. 2016, 88, 9699–9706. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Thiel, J.; Alonso, L.L.; Slagboom, J.; Dunstan, N.; Wouters, R.M.; Modahl, C.M.; Vonk, F.J.; Jackson, T.N.W.; Kool, J. Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.). Toxins 2023, 15, 74. https://doi.org/10.3390/toxins15010074
van Thiel J, Alonso LL, Slagboom J, Dunstan N, Wouters RM, Modahl CM, Vonk FJ, Jackson TNW, Kool J. Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.). Toxins. 2023; 15(1):74. https://doi.org/10.3390/toxins15010074
Chicago/Turabian Stylevan Thiel, Jory, Luis L. Alonso, Julien Slagboom, Nathan Dunstan, Roel M. Wouters, Cassandra M. Modahl, Freek J. Vonk, Timothy N. W. Jackson, and Jeroen Kool. 2023. "Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.)" Toxins 15, no. 1: 74. https://doi.org/10.3390/toxins15010074
APA Stylevan Thiel, J., Alonso, L. L., Slagboom, J., Dunstan, N., Wouters, R. M., Modahl, C. M., Vonk, F. J., Jackson, T. N. W., & Kool, J. (2023). Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.). Toxins, 15(1), 74. https://doi.org/10.3390/toxins15010074





