CDT of Clostridioides difficile Induces MLC-Dependent Intestinal Barrier Dysfunction in HT-29/B6 Epithelial Cell Monolayers
Abstract
1. Introduction
2. Results
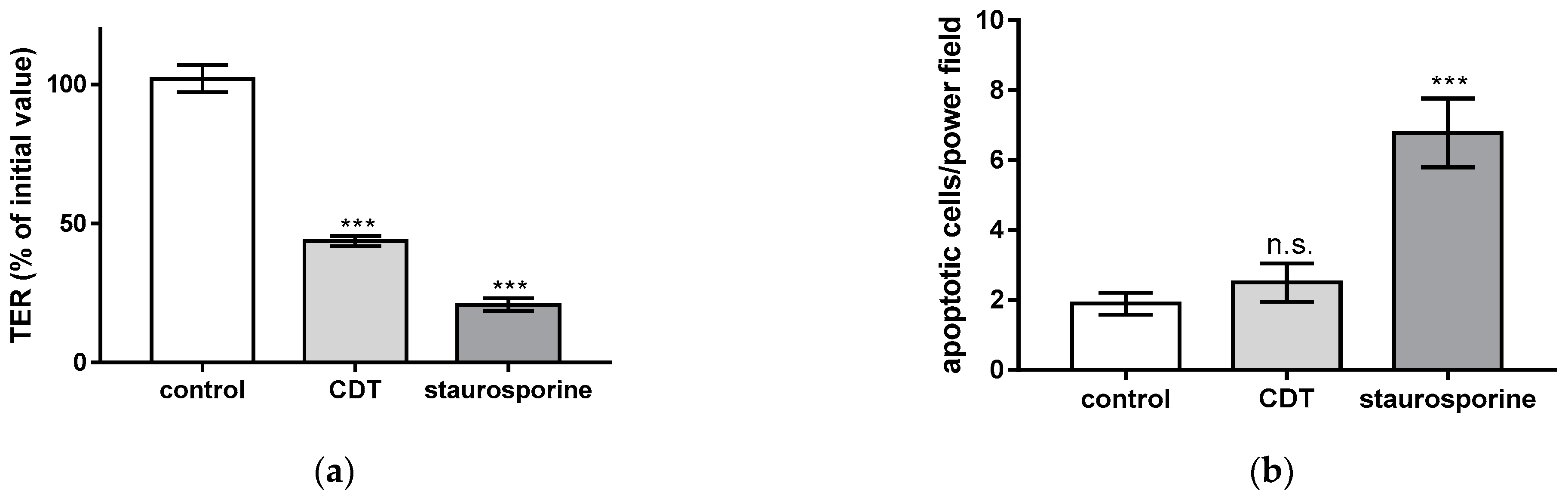
2.1. CDT Impairs Epithelial Barrier Integrity of HT-29/B6 Monolayers
2.2. Necrosis Induction and the Rate of Apoptotic Epithelial Cells Are Unchanged after Treatment with Sublethal Concentrations of CDT
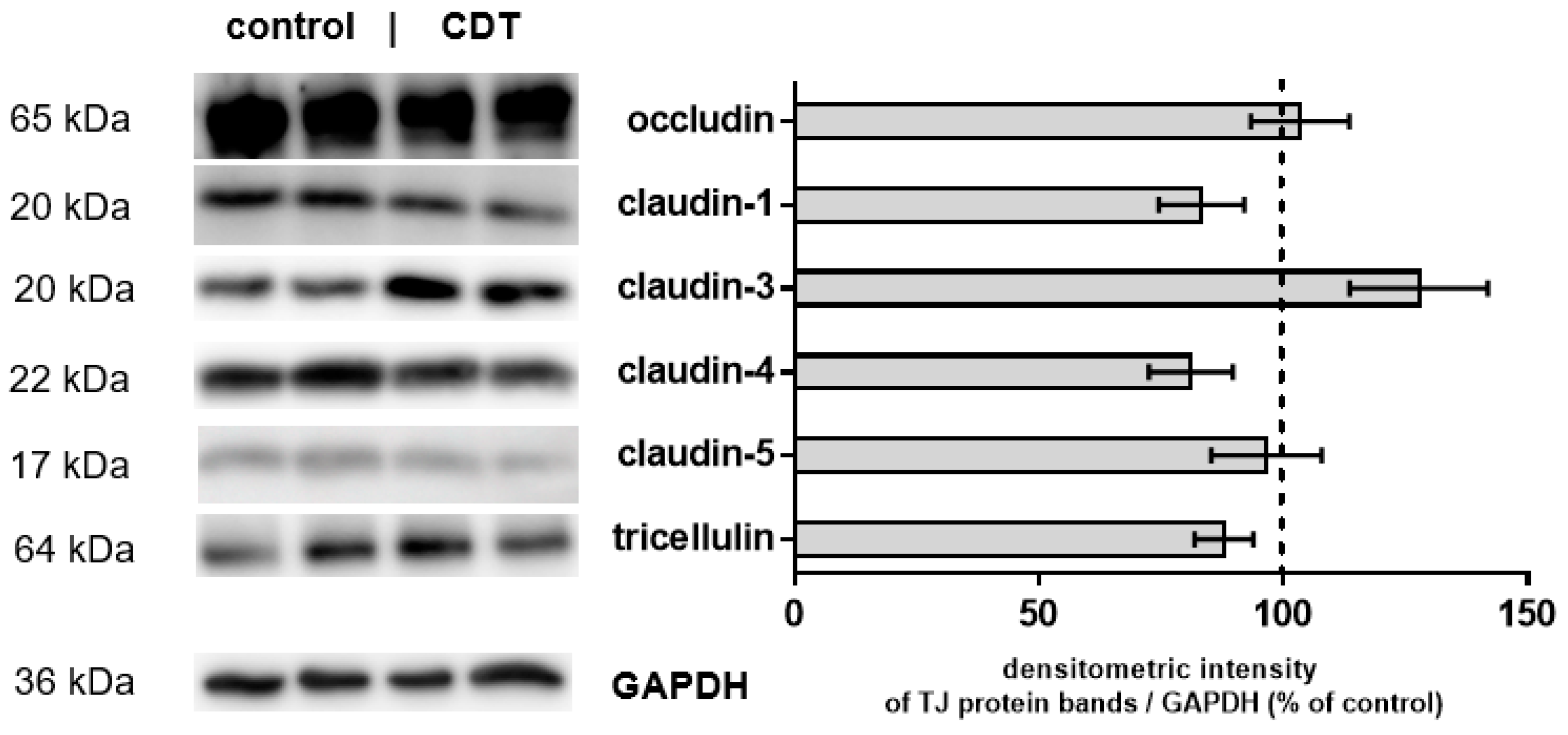
2.3. Tight Junction Protein Expression after 24 h of Low-Dose CDT Treatment in HT-29/B6 Monolayers
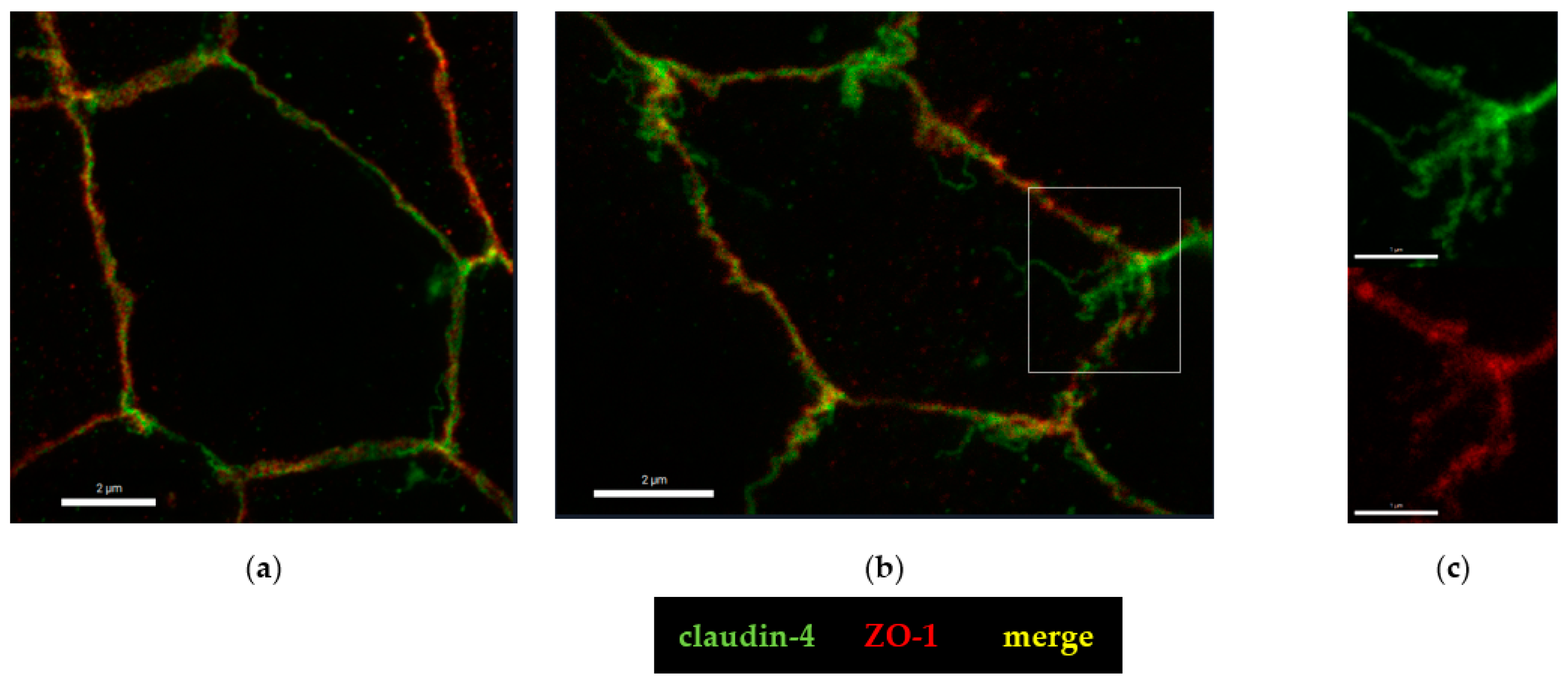
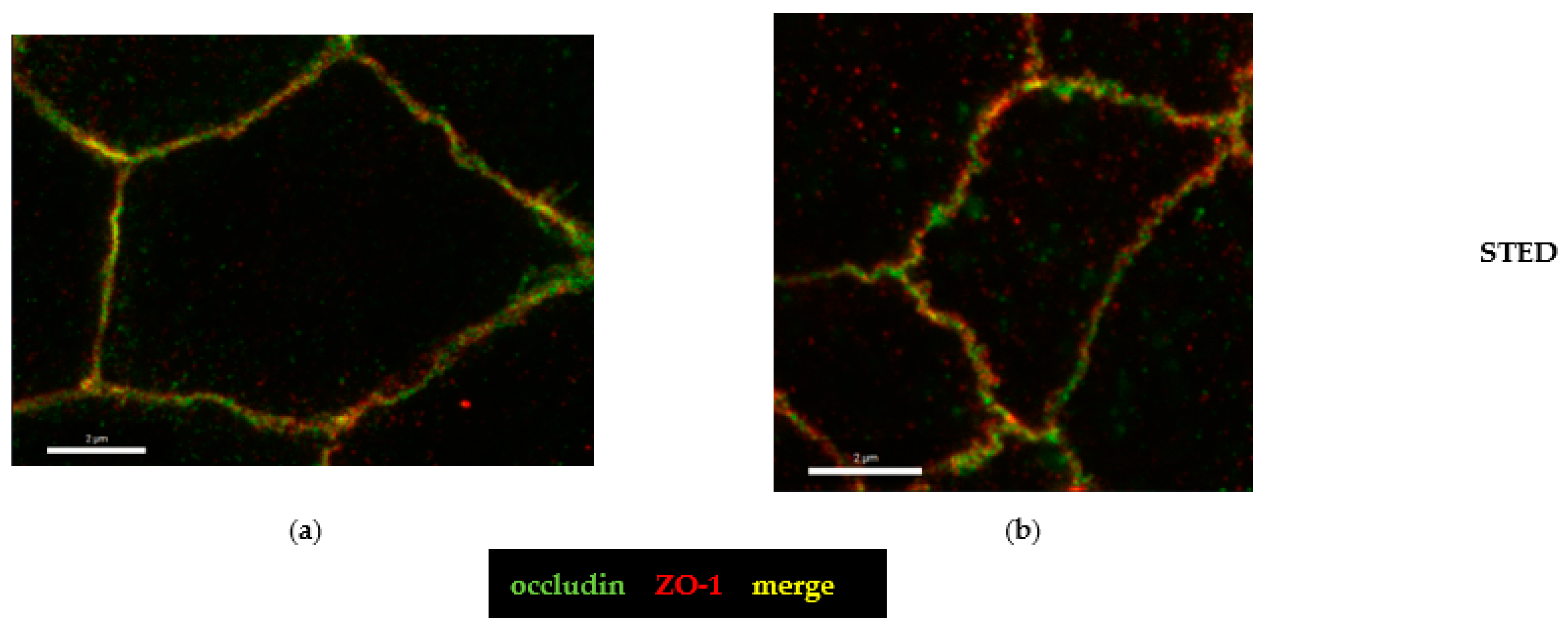
2.4. Microstructural Analysis by Super-Resolution Microscopy of CDT Impact on the Tight Junction
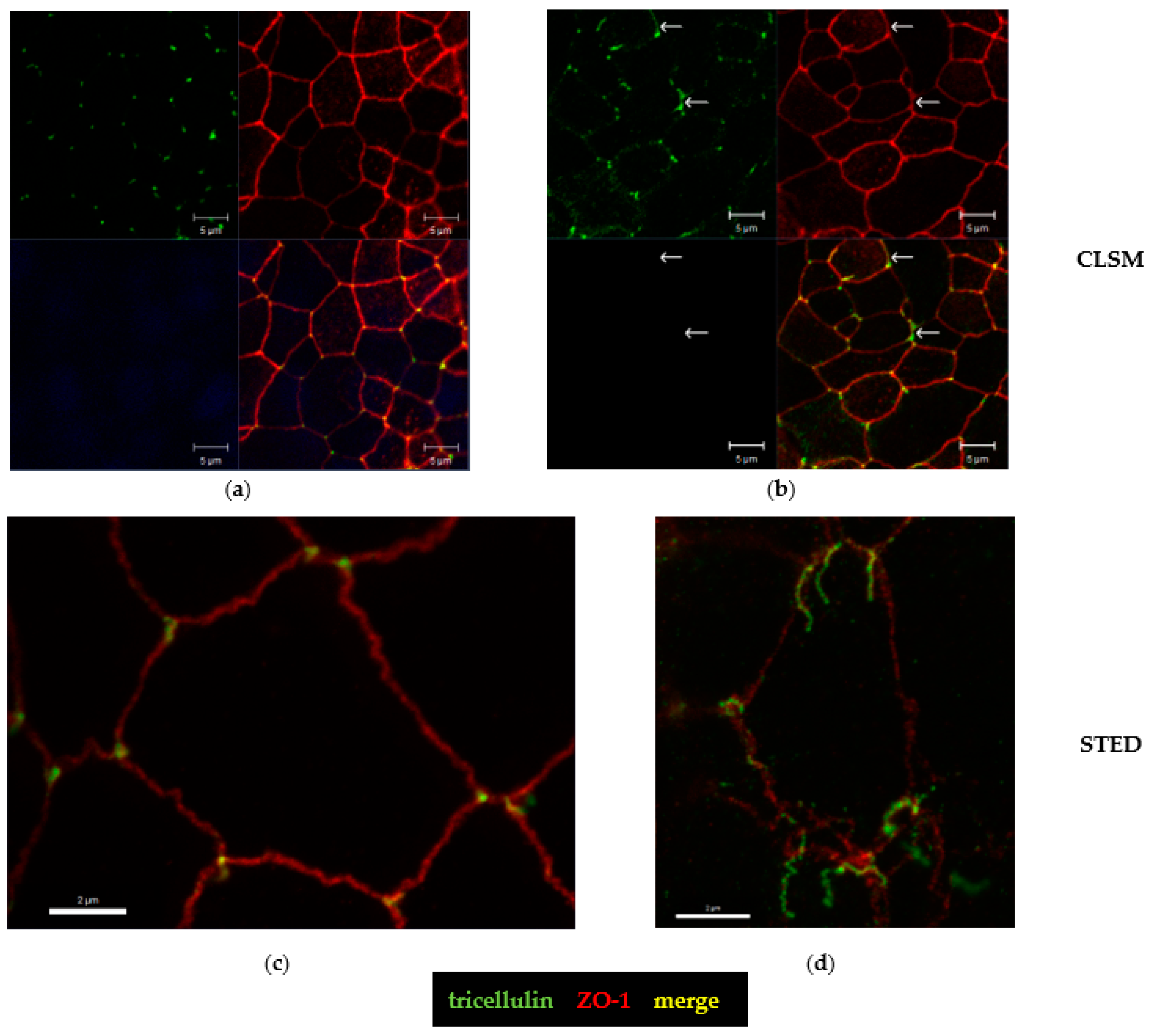
2.5. Tricellular Tight Junction Delocalization in CDT-Treated Epithelial Cells
2.6. Disinhibition of the Barrier Defect of CDT by Specific Myosin Light-Chain Kinase Inhibitor PIK
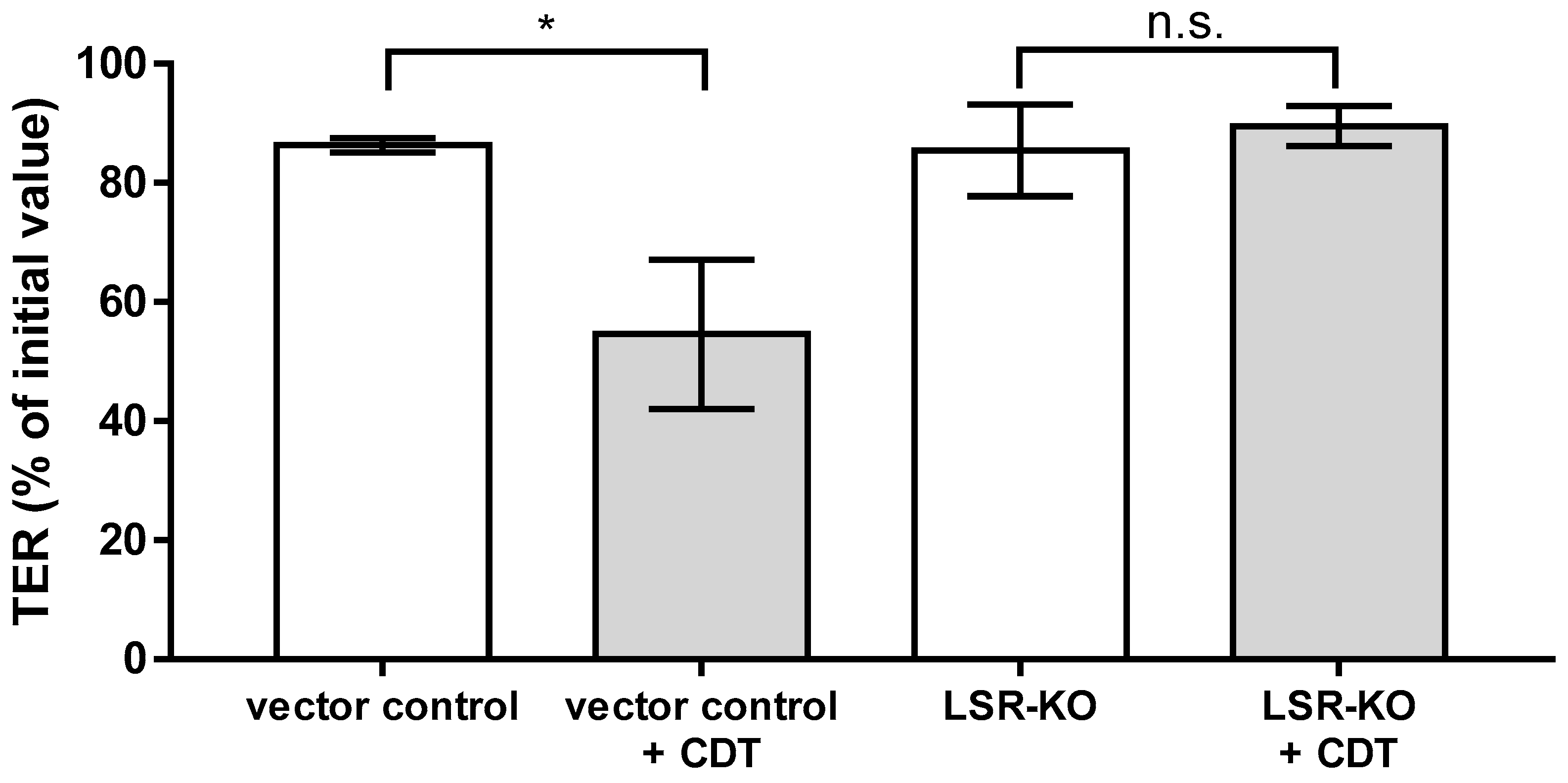
2.7. Lipolysis-Stimulated Lipoprotein Receptor (LSR) Dependence of Barrier Defect of CDT
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Toxin Treatment and Functional Characterization of Epithelial Barrier Function
4.3. Cytotoxicity Tests
4.4. Western Blot Analysis
4.5. Immunostaining and Super-Resolution STED Microscopy
4.6. Inhibitor Studies
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium Difficile Colitis: Pathogenesis and Host Defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Guery, B.; Galperine, T.; Barbut, F. Clostridioides Difficile: Diagnosis and Treatments. BMJ 2019, 366, l4609. [Google Scholar] [CrossRef]
- López-Cárdenas, S.; Torres-Martos, E.; Mora-Delgado, J.; Sánchez-Calvo, J.M.; Santos-Peña, M.; Zapata López, Á.; Dolores López-Prieto, M.; Pérez-Cortés, S.; Carlos Alados, J. The Prognostic Value of Toxin B and Binary Toxin in Clostridioides Difficile Infection. Gut Microbes 2021, 13, 1884516. [Google Scholar] [CrossRef] [PubMed]
- Young, M.K.; Leslie, J.L.; Madden, G.R.; Lyerly, D.M.; Carman, R.J.; Lyerly, M.W.; Stewart, D.B.; Abhyankar, M.M.; Petri, W.A. Binary Toxin Expression by Clostridioides Difficile Is Associated with Worse Disease. Open Forum Infect. Dis. 2022, 9, ofac001. [Google Scholar] [CrossRef]
- Aktories, K.; Schwan, C.; Jank, T. Annual Review of Microbiology Clostridium Difficile Toxin Biology. Annu. Rev. Microbiol. 2017, 71, 281–307. [Google Scholar] [CrossRef]
- Gülke, I.; Pfeifer, G.; Liese, J.; Fritz, M.; Hofmann, F.; Aktories, K.; Barth, H. Characterization of the Enzymatic Component of the ADP-Ribosyltransferase Toxin CDTa from Clostridium Difficile. Infect. Immun. 2001, 69, 6004–6011. [Google Scholar] [CrossRef]
- Martínez-Meléndez, A.; Cruz-López, F.; Morfin-Otero, R.; Maldonado-Garza, H.J.; Garza-González, E. An Update on Clostridioides Difficile Binary Toxin. Toxins 2022, 14, 305. [Google Scholar] [CrossRef]
- Stieglitz, F.; Gerhard, R.; Pich, A. The Binary Toxin of Clostridioides Difficile Alters the Proteome and Phosphoproteome of HEp-2 Cells. Front. Microbiol. 2021, 12, 725612. [Google Scholar] [CrossRef]
- Jin, Y.; Blikslager, A.T. The Regulation of Intestinal Mucosal Barrier by Myosin Light Chain Kinase/Rho Kinases. Int. J. Mol. Sci. 2020, 21, 3550. [Google Scholar] [CrossRef]
- Nusrat, A.; von Eichel-Streiber, C.; Turner, J.R.; Verkade, P.; Madara, J.L.; Parkos, C.A. Clostridium Difficile Toxins Disrupt Epithelial Barrier Function by Altering Membrane Microdomain Localization of Tight Junction Proteins. Infect. Immun. 2001, 69, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Günzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin Forms a Barrier to Macromolecules in Tricellular Tight Junctions without Affecting Ion Permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef]
- Krug, S.M.; Bojarski, C.; Fromm, A.; Lee, I.M.; Dames, P.; Richter, J.F.; Turner, J.R.; Fromm, M.; Schulzke, J.D. Tricellulin Is Regulated via Interleukin-13-Receptor A2, Affects Macromolecule Uptake, and Is Decreased in Ulcerative Colitis. Mucosal Immunol. 2018, 11, 345–356. [Google Scholar] [CrossRef]
- Bjarnason, I.; Ward, K.; Peters, T.J. The Leaky Gut of Alcoholism: Possible Route of Entry for Toxic Compounds. Lancet 1984, 323, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Schulz, E.; Günzel, D.; Bojarski, C.; Lee, I.F.M.; John, L.J.; Wiegand, S.; Janßen, T.; Wieler, L.H.; Dobrindt, U.; et al. α-Haemolysin of Escherichia Coli in IBD: A Potentiator of Inflammatory Activity in the Colon. Gut 2014, 63, 1893–1901. [Google Scholar] [CrossRef]
- Bergann, T.; Fromm, A.; Borden, S.A.; Fromm, M.; Schulzke, J.D. Glucocorticoid receptor is indispensable for physiological responses to aldosterone in epithelial Na+ channel induction via the mineralocorticoid receptor in a human colonic cell line. Eur. J. Cell Biol. 2011, 90, 432–439. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and Other Tight Junction Proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar] [CrossRef] [PubMed]
- Kaak, J.L.; Lobo de Sá, F.D.; Turner, J.R.; Schulzke, J.D.; Bücker, R. Unraveling the Intestinal Epithelial Barrier in Cyanotoxin Microcystin-Treated Caco-2 Cell Monolayers. Ann. N. Y. Acad. Sci. 2022, 1516, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Krug, S.M.; Rosenthal, R.; Günzel, D.; Fromm, A.; Zeitz, M.; Chakraborty, T.; Fromm, M.; Epple, H.J.; Schulzke, J.D. Aerolysin from Aeromonas Hydrophila Perturbs Tight Junction Integrity and Cell Lesion Repair in Intestinal Epithelial HT-29/B6 Cells. J. Infect. Dis. 2011, 204, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.E.; Graham, W.V.; Siccardi, D.; Turner, J.R.; Mrsny, R.J. A Strategy to Identify Stable Membrane-Permeant Peptide Inhibitors of Myosin Light Chain Kinase. Pharm. Res. 2005, 22, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium Difficile Binary Toxin CDT: Mechanism, Epidemiology, and Potential Clinical Importance. Gut Microbes 2013, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.G.; Pradhan, K.; Fleming, J.M.; Samy, R.P.; Barth, H.; Popoff, M.R. Clostridium and Bacillus Binary Enterotoxins: Bad for the Bowels, and Eukaryotic Being. Toxins 2014, 6, 2626–2656. [Google Scholar] [CrossRef]
- Landenberger, M.; Nieland, J.; Roeder, M.; Nørgaard, K.; Papatheodorou, P.; Ernst, K.; Barth, H. The Cytotoxic Effect of Clostridioides Difficile Pore-Forming Toxin CDTb. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183603. [Google Scholar] [CrossRef]
- Payne, A.M.; Zorman, J.; Horton, M.; Dubey, S.; ter Meulen, J.; Vora, K.A. Caspase Activation as a Versatile Assay Platform for Detection of Cytotoxic Bacterial Toxins. J. Clin. Microbiol. 2013, 51, 2970–2976. [Google Scholar] [CrossRef]
- Shashikanth, N.; France, M.M.; Xiao, R.; Haest, X.; Rizzo, H.E.; Yeste, J.; Reiner, J.; Turner, J.R. Tight Junction Channel Regulation by Interclaudin Interference. Nat. Commun. 2022, 13, 3780. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; Tsukita, S.; Mostov, K.E. Loss of Occludin Affects Tricellular Localization of Tricellulin. Mol. Biol. Cell 2008, 19, 4687–4693. [Google Scholar] [CrossRef]
- Buschmann, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.; Wang, Y.; Lingaraju, A.; Zha, J.; Abbott, E.; McAuley, E.M.; et al. Occludin OCEL-Domain Interactions Are Required for Maintenance and Regulation of the Tight Junction Barrier to Macromolecular Flux. Mol. Biol. Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Bücker, R.; Boehm, M.; Zarzecka, U.; Tegtmeyer, N.; Sticht, H.; Schulzke, J.D.; Backert, S. Campylobacter Jejuni Enters Gut Epithelial Cells and Impairs Intestinal Barrier Function through Cleavage of Occludin by Serine Protease HtrA. Gut Pathog. 2019, 11, 4. [Google Scholar] [CrossRef]
- Krug, S.M. Contribution of the Tricellular Tight Junction to Paracellular Permeability in Leaky and Tight Epithelia. Ann. N. Y. Acad. Sci. 2017, 1397, 219–230. [Google Scholar] [CrossRef]
- Lyon, S.A.; Hutton, M.L.; Rood, J.I.; Cheung, J.K.; Lyras, D. CdtR Regulates TcdA and TcdB Production in Clostridium Difficile. PLoS Pathog. 2016, 12, e1005758. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhang, J.; Meraner, P.; Tovaglieri, A.; Wu, X.; Gerhard, R.; Zhang, X.; Stallcup, W.B.; Miao, J.; He, X.; et al. Frizzled Proteins Are Colonic Epithelial Receptors for C. Difficile Toxin B. Nature 2016, 538, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, H.; Cai, C.; Zhu, S.; Zhou, Y.; Yang, X.; He, R.; Li, C.; Guo, S.; Li, S.; et al. Chondroitin Sulfate Proteoglycan 4 Functions as the Cellular Receptor for Clostridium Difficile Toxin B. Cell Res. 2015, 25, 157–168. [Google Scholar] [CrossRef]
- Hemmasi, S.; Czulkies, B.A.; Schorch, B.; Veit, A.; Aktories, K.; Papatheodorou, P. Interaction of the Clostridium Difficile Binary Toxin CDT and Its Host Cell Receptor, Lipolysis-Stimulated Lipoprotein Receptor (LSR). J. Biol. Chem. 2015, 290, 14031–14044. [Google Scholar] [CrossRef]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR Defines Cell Corners for Tricellular Tight Junction Formation in Epithelial Cells. J. Cell Sci. 2011, 124, 548–555. [Google Scholar] [CrossRef]
- Marquardt, I.; Jakob, J.; Scheibel, J.; Hofmann, J.D.; Klawonn, F.; Neumann-Schaal, M.; Gerhard, R.; Bruder, D.; Jänsch, L. Clostridioides Difficile Toxin CDT Induces Cytotoxic Responses in Human Mucosal-Associated Invariant T (MAIT) Cells. Front. Microbiol. 2021, 12, 752549. [Google Scholar] [CrossRef]
- Nibbering, B.; Gerding, D.N.; Kuijper, E.J.; Zwittink, R.D.; Smits, W.K. Host Immune Responses to Clostridioides Difficile: Toxins and Beyond. Front. Microbiol. 2021, 12, 804949. [Google Scholar] [CrossRef] [PubMed]
- Geric, B.; Carman, R.J.; Rupnik, M.; Genheimer, C.W.; Sambol, S.P.; Lyerly, D.M.; Gerding, D.N.; Johnson, S. Binary Toxin-Producing, Large Clostridial Toxin-Negative Clostridium Difficile Strains Are Enterotoxic but Do Not Cause Disease in Hamsters. J. Infect. Dis. 2006, 193, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Cartman, S.T.; Minton, N.P. Both, Toxin A and Toxin B, Are Important in Clostridium Difficile Infection. Gut Microbes 2011, 2, 252–255. [Google Scholar] [CrossRef]
- Ernst, K.; Landenberger, M.; Nieland, J.; Nørgaard, K.; Frick, M.; Fois, G.; Benz, R.; Barth, H. Characterization and Pharmacological Inhibition of the Pore-Forming Clostridioides Difficile Cdtb Toxin. Toxins 2021, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.; Zakrzewski, S.S.; Eichner, M.; Schulz, E.; Günzel, D.; Pieper, R.; Rosenthal, R.; Barmeyer, C.; Bleich, A.; Dobrindt, U.; et al. Zinc Treatment Is Efficient against Escherichia Coli α-Haemolysin-Induced Intestinal Leakage in Mice. Sci. Rep. 2017, 7, 45649. [Google Scholar] [CrossRef]
- Shen, L.; Black, E.D.; Witkowski, E.D.; Lencer, W.I.; Guerriero, V.; Schneeberger, E.E.; Turner, J.R. Myosin Light Chain Phosphorylation Regulates Barrier Function by Remodeling Tight Junction Structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Torres, C.; Krug, S.M.; Rosenthal, R.; Fromm, M. Angulin-1 (LSR) Affects Paracellular Water Transport, However Only in Tight Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 7827. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.A.; Tatge, H.; Schneider, C.; Ruschig, M.; Hust, M.; Barton, J.; Thiemann, S.; Fühner, V.; Russo, G.; Gerhard, R. The Binary Toxin CDT of Clostridium Difficile as a Tool for Intracellular Delivery of Bacterial Glucosyltransferase Domains. Toxins 2018, 10, 225. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heils, L.; Schneemann, M.; Gerhard, R.; Schulzke, J.-D.; Bücker, R. CDT of Clostridioides difficile Induces MLC-Dependent Intestinal Barrier Dysfunction in HT-29/B6 Epithelial Cell Monolayers. Toxins 2023, 15, 54. https://doi.org/10.3390/toxins15010054
Heils L, Schneemann M, Gerhard R, Schulzke J-D, Bücker R. CDT of Clostridioides difficile Induces MLC-Dependent Intestinal Barrier Dysfunction in HT-29/B6 Epithelial Cell Monolayers. Toxins. 2023; 15(1):54. https://doi.org/10.3390/toxins15010054
Chicago/Turabian StyleHeils, Lucas, Martina Schneemann, Ralf Gerhard, Jörg-Dieter Schulzke, and Roland Bücker. 2023. "CDT of Clostridioides difficile Induces MLC-Dependent Intestinal Barrier Dysfunction in HT-29/B6 Epithelial Cell Monolayers" Toxins 15, no. 1: 54. https://doi.org/10.3390/toxins15010054
APA StyleHeils, L., Schneemann, M., Gerhard, R., Schulzke, J.-D., & Bücker, R. (2023). CDT of Clostridioides difficile Induces MLC-Dependent Intestinal Barrier Dysfunction in HT-29/B6 Epithelial Cell Monolayers. Toxins, 15(1), 54. https://doi.org/10.3390/toxins15010054





