Molecular Basis of TcdR-Dependent Promoter Activity for Toxin Production by Clostridioides difficile Studied by a Heterologous Reporter System
Abstract
1. Introduction
2. Results
2.1. Construction of B. subtilis Heterologous Reporter System for Studying the Promoter Activity Controlled by TcdR
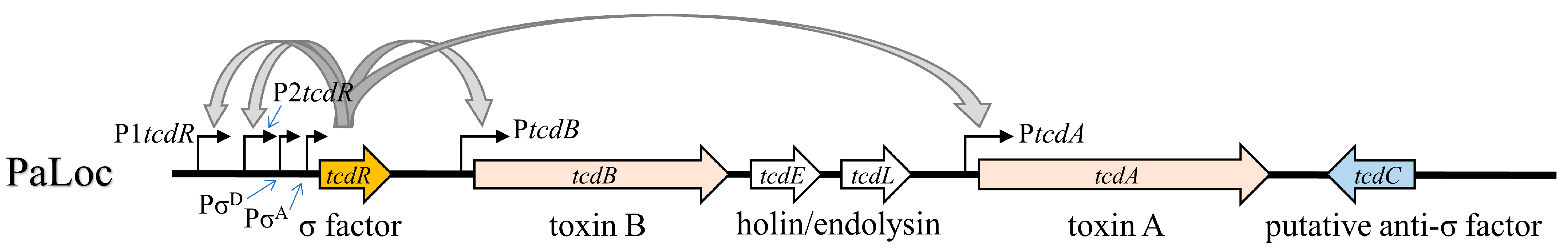
2.2. Strength of Different TcdR-Dependent Promoters in the PaLoc
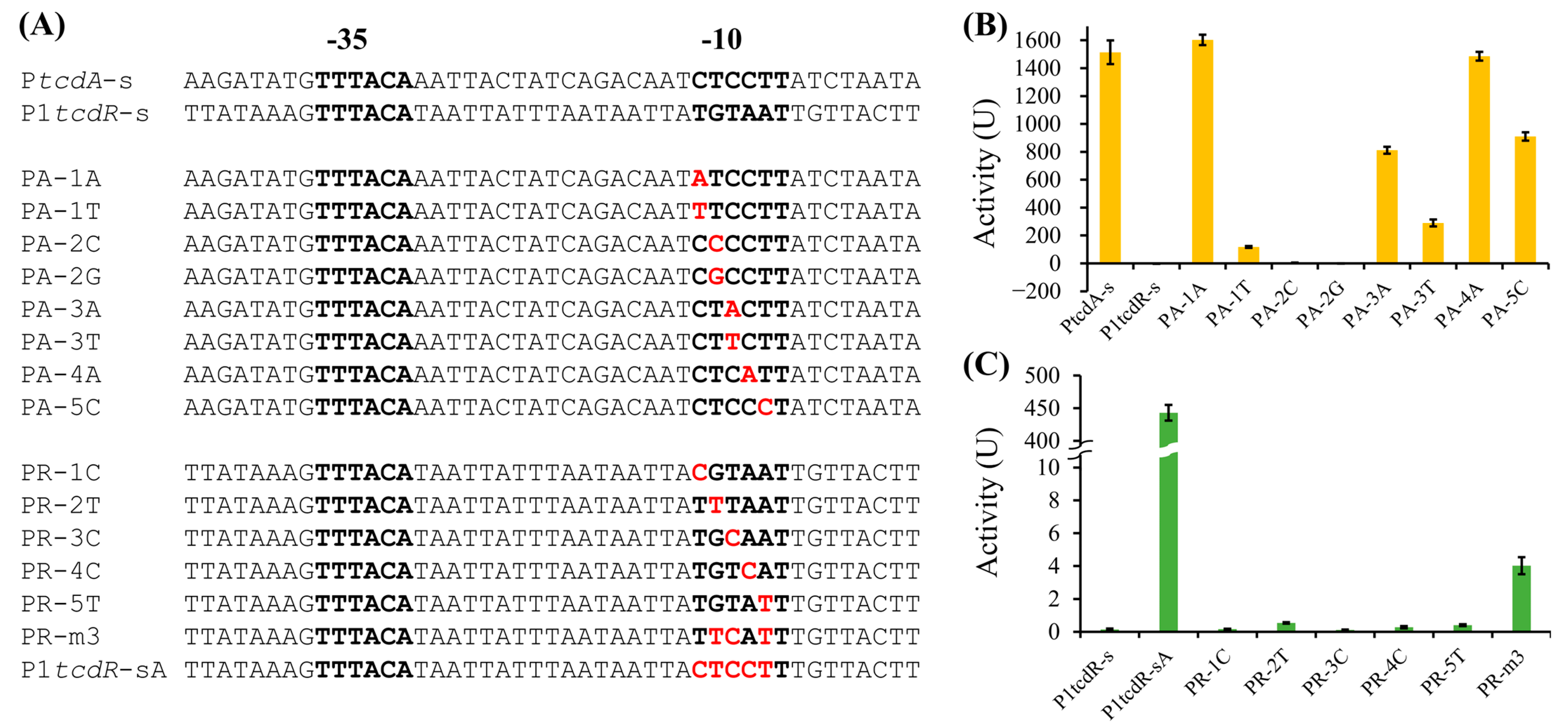
2.3. The -10 Elements Determine the Activity Difference of TcdR-Dependent Promoters
2.4. Key Nucleotide Residues in the -10 Elements of PtcdA and P1tcdR
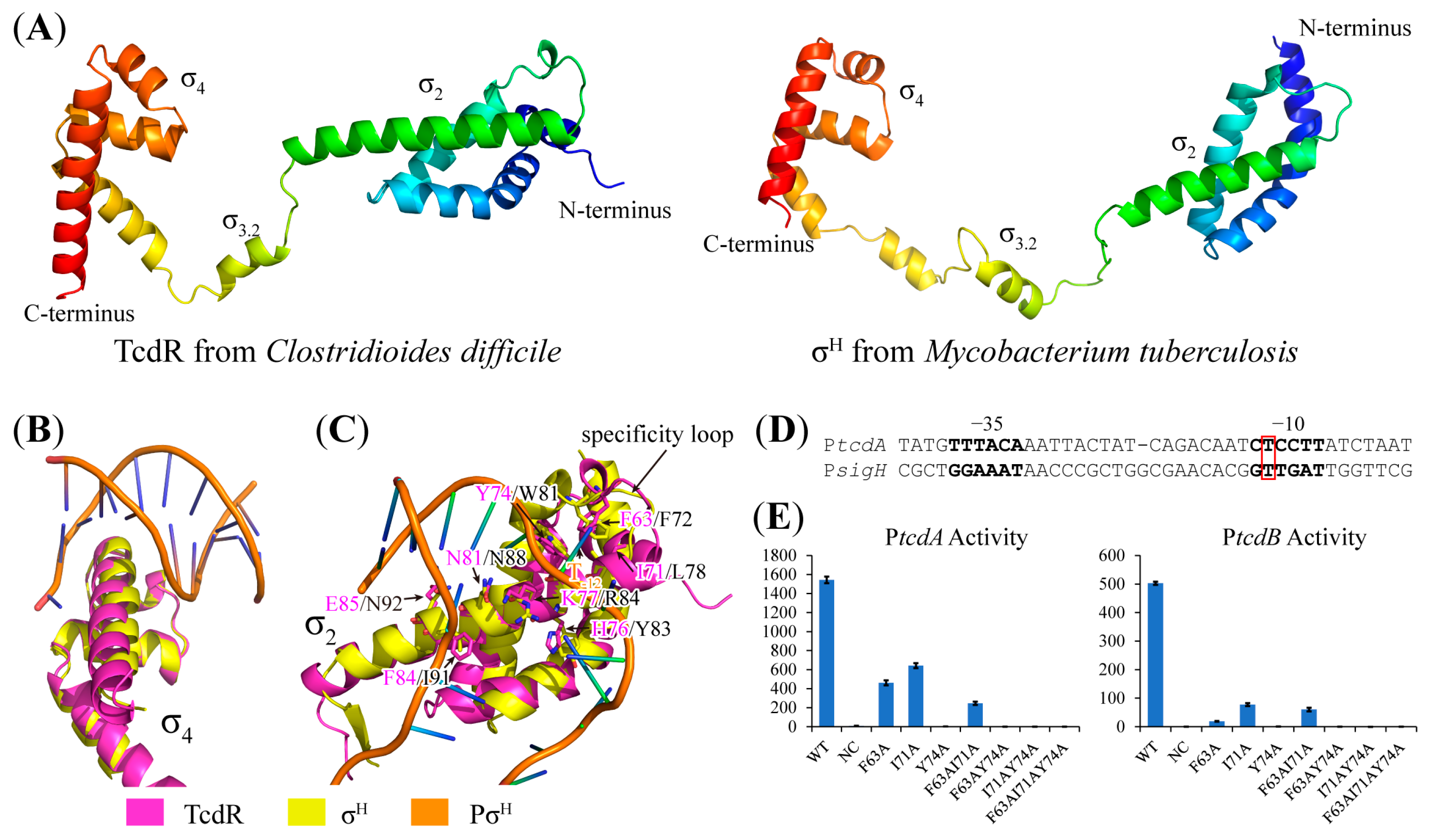
2.5. Structural Basis of TcdR-Dependent Promoter Recognition
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains, Growth Medium, and Culture Conditions
5.2. Construction of B. subtilis Strains
5.3. β-Galactosidase Activity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dureja, C.; Olaitan, A.O.; Hurdle, J.G. Mechanisms and impact of antimicrobial resistance in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 66, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E.; Lamont, J.T. Clostridium difficile Infection: A Worldwide Disease. Gut Liver 2014, 8, 1–6. [Google Scholar] [CrossRef]
- Martin-Verstraete, I.; Peltier, J.; Dupuy, B. The Regulatory Networks That Control Clostridium difficile Toxin Synthesis. Toxins 2016, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile toxins: Mechanisms of action and antitoxin therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Govind, R. Regulation of Clostridioides difficile toxin production. Curr. Opin. Microbiol. 2022, 65, 95–100. [Google Scholar] [CrossRef]
- Govind, R.; Dupuy, B. Secretion of Clostridium difficile toxins A and B requires the holin-like protein TcdE. PLoS Pathog. 2012, 8, e1002727. [Google Scholar] [CrossRef]
- Mehner-Breitfeld, D.; Rathmann, C.; Riedel, T.; Just, I.; Gerhard, R.; Overmann, J.; Brüser, T. Evidence for an Adaptation of a Phage-Derived Holin/Endolysin System to Toxin Transport in Clostridioides difficile. Front. Microbiol. 2018, 9, 2446. [Google Scholar] [CrossRef]
- Mani, N.; Dupuy, B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. USA 2001, 98, 5844–5849. [Google Scholar] [CrossRef]
- Dupuy, B.; Matamouros, S. Regulation of toxin and bacteriocin synthesis in Clostridium species by a new subgroup of RNA polymerase σ-factors. Res. Microbiol. 2006, 157, 201–205. [Google Scholar] [CrossRef]
- Matamouros, S.; England, P.; Dupuy, B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol. Microbiol. 2007, 64, 1274–1288. [Google Scholar] [CrossRef]
- Oliveira Paiva, A.M.; de Jong, L.; Friggen, A.H.; Smits, W.K.; Corver, J. The C-Terminal Domain of Clostridioides difficile TcdC Is Exposed on the Bacterial Cell Surface. J. Bacteriol. 2020, 202, e00771-19. [Google Scholar] [CrossRef]
- Mani, N.; Lyras, D.; Barroso, L.; Howarth, P.; Wilkins, T.; Rood, J.I.; Sonenshein, A.L.; Dupuy, B. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 2002, 184, 5971–5978. [Google Scholar] [CrossRef]
- El Meouche, I.; Peltier, J.; Monot, M.; Soutourina, O.; Pestel-Caron, M.; Dupuy, B.; Pons, J.L. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS ONE 2013, 8, e83748. [Google Scholar] [CrossRef] [PubMed]
- Edwards Adrianne, N.; Anjuwon-Foster Brandon, R.; McBride Shonna, M. RstA Is a Major Regulator of Clostridioides difficile Toxin Production and Motility. mBio 2019, 10, e01991-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Synthetic Biology Toolbox and Chassis Development in Bacillus subtilis. Trends Biotechnol. 2019, 37, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact 2020, 19, 173. [Google Scholar] [CrossRef]
- Muñoz-Gutiérrez, I.; Ortiz de Ora, L.; Rozman Grinberg, I.; Garty, Y.; Bayer, E.A.; Shoham, Y.; Lamed, R.; Borovok, I. Decoding Biomass-Sensing Regulons of Clostridium thermocellum Alternative Sigma-I Factors in a Heterologous Bacillus subtilis Host System. PLoS ONE 2016, 11, e0146316. [Google Scholar] [CrossRef]
- Härtl, B.; Wehrl, W.; Wiegert, T.; Homuth, G.; Schumann, W. Development of a New Integration Site within the Bacillus subtilis Chromosome and Construction of Compatible Expression Cassettes. J. Bacteriol. 2001, 183, 2696–2699. [Google Scholar] [CrossRef]
- Dupuy, B.; Sonenshein, A.L. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 1998, 27, 107–120. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.; Zhuang, N.; Wang, T.; Zhang, Y. Structural basis for transcription initiation by bacterial ECF σ factors. Nat. Commun. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Helmann, J.D. The extracytoplasmic function (ECF) sigma factors. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2002; Volume 46, pp. 47–110. [Google Scholar]
- Gruber, T.M.; Gross, C.A. Multiple Sigma Subunits and the Partitioning of Bacterial Transcription Space. Annu. Rev. Microbiol. 2003, 57, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.M.; Castro, T.L.d.P.; Carvalho, R.D.d.O.; Seyffert, N.; Silva, A.; Miyoshi, A.; Azevedo, V. σECF factors of gram-positive bacteria. Virulence 2014, 5, 587–600. [Google Scholar] [CrossRef]
- Karlsson, S.; Dupuy, B.; Mukherjee, K.; Norin, E.; Burman, L.G.; Åkerlund, T. Expression of Clostridium difficile Toxins A and B and Their Sigma Factor TcdD Is Controlled by Temperature. Infect. Immun. 2003, 71, 1784–1793. [Google Scholar] [CrossRef]
- Ortiz de Ora, L.; Lamed, R.; Liu, Y.-J.; Xu, J.; Cui, Q.; Feng, Y.; Shoham, Y.; Bayer, E.A.; Muñoz-Gutiérrez, I. Regulation of biomass degradation by alternative σ factors in cellulolytic clostridia. Sci. Rep. 2018, 8, 11036. [Google Scholar] [CrossRef] [PubMed]
- Ora, L.O.d.; Muñoz-Gutiérrez, I.; Bayer, E.A.; Shoham, Y.; Lamed, R.; Borovok, I. Revisiting the Regulation of the Primary Scaffoldin Gene in Clostridium thermocellum. Appl. Environ. Microbiol. 2017, 83, e03088-16. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.-J.; Cui, Q. Research progress in cellulosomes and their applications in synthetic biology. Synth. Biol. J. 2022, 3, 138–154. [Google Scholar] [CrossRef]
- Ho, T.D.; Ellermeier, C.D. PrsW Is Required for Colonization, Resistance to Antimicrobial Peptides, and Expression of Extracytoplasmic Function σ Factors in Clostridium difficile. Infect. Immun. 2011, 79, 3229–3238. [Google Scholar] [CrossRef]
- Soutourina, O.; Dubois, T.; Monot, M.; Shelyakin, P.V.; Saujet, L.; Boudry, P.; Gelfand, M.S.; Dupuy, B.; Martin-Verstraete, I. Genome-Wide Transcription Start Site Mapping and Promoter Assignments to a Sigma Factor in the Human Enteropathogen Clostridioides difficile. Front. Microbiol. 2020, 11, 1939. [Google Scholar] [CrossRef]
- El-Mowafi, S.A.; Sineva, E.; Alumasa, J.N.; Nicoloff, H.; Tomsho, J.W.; Ades, S.E.; Keiler, K.C. Identification of Inhibitors of a Bacterial Sigma Factor Using a New High-Throughput Screening Assay. Antimicrob. Agents Chemother. 2015, 59, 193–205. [Google Scholar] [CrossRef]
- Bai, H.; Bo, X.; Wang, S. Targeting Bacterial RNA Polymerase σ70 for Development of Broadspectrum Antisense Antibacterials. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Vojcic, L.; Despotovic, D.; Martinez, R.; Maurer, K.-H.; Schwaneberg, U. An efficient transformation method for Bacillus subtilis DB104. Appl. Microbiol. Biotechnol. 2012, 94, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Radeck, J.; Kraft, K.; Bartels, J.; Cikovic, T.; Dürr, F.; Emenegger, J.; Kelterborn, S.; Sauer, C.; Fritz, G.; Gebhard, S.; et al. The Bacillus BioBrick Box: Generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J. Biol. Eng. 2013, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Hair, S.M.; Baerman, E.A.; Fujita, M.; Igoshin, O.A.; Tabor, J.J. Optogenetic control of Bacillus subtilis gene expression. Nat. Commun. 2019, 10, 3099. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, J.; Chen, C.; Liu, Y.-J.; Cui, Q.; Hong, W.; Chen, Z.; Feng, Y.; Cui, G. Molecular Basis of TcdR-Dependent Promoter Activity for Toxin Production by Clostridioides difficile Studied by a Heterologous Reporter System. Toxins 2023, 15, 306. https://doi.org/10.3390/toxins15050306
Zhang X, Li J, Chen C, Liu Y-J, Cui Q, Hong W, Chen Z, Feng Y, Cui G. Molecular Basis of TcdR-Dependent Promoter Activity for Toxin Production by Clostridioides difficile Studied by a Heterologous Reporter System. Toxins. 2023; 15(5):306. https://doi.org/10.3390/toxins15050306
Chicago/Turabian StyleZhang, Xinyue, Jie Li, Chao Chen, Ya-Jun Liu, Qiu Cui, Wei Hong, Zhenghong Chen, Yingang Feng, and Guzhen Cui. 2023. "Molecular Basis of TcdR-Dependent Promoter Activity for Toxin Production by Clostridioides difficile Studied by a Heterologous Reporter System" Toxins 15, no. 5: 306. https://doi.org/10.3390/toxins15050306
APA StyleZhang, X., Li, J., Chen, C., Liu, Y.-J., Cui, Q., Hong, W., Chen, Z., Feng, Y., & Cui, G. (2023). Molecular Basis of TcdR-Dependent Promoter Activity for Toxin Production by Clostridioides difficile Studied by a Heterologous Reporter System. Toxins, 15(5), 306. https://doi.org/10.3390/toxins15050306





