Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges
Abstract
1. Introduction
2. Principles and Harmonised Guidance Document for the Risk Assessment of Combined Exposure to Multiple Chemicals
2.1. Defining the Concept of “Mixtures” and the Three Types of Mixtures
2.2. Principles of Risk Assessment of Combined Exposure to Multiple Chemicals Using Component-Based Approaches
2.3. Harmonised Guidance Document at the European Food Safety Authority: MIXTOX
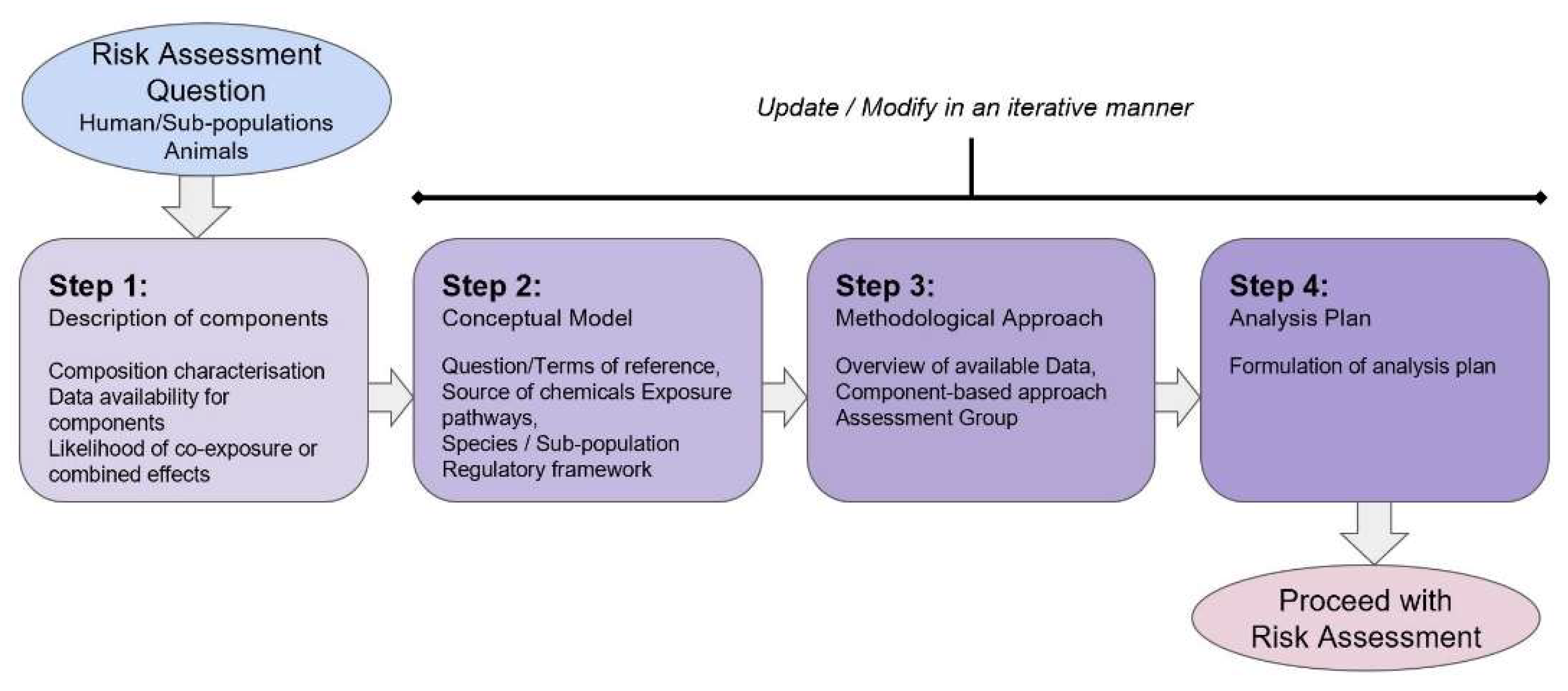
2.3.1. Problem Formulation
- Step 1 deals with describing the components of the mixture and defines whether a combined exposure assessment is required according to the requestor’s question or the Terms of Reference. If so, the mixture composition needs to be characterised both qualitatively and quantitatively (e.g., if the mixture is intentional, is the composition poorly, partially or well-defined; if unintentional or coincidental, are the components and correlation between the components known?). At this stage, data availability is considered, including exposure in the species or population as well as hazard information, together with the likelihood of combined effects.
- Step 2 aims to develop a conceptual model to frame the RA itself, define data needs and suitable methods to be applied in subsequent assessment steps. The conceptual model is also the starting point for the assessment plan and the mathematical formulations of the models to be used during the exposure and the hazard assessment phases. At this stage, the identification of the origin or source of the chemical components of the mixture, the transfer pathway from the source to the target, the exposure pattern and the target populations and life stage exposed [17] can be included.
- Step 3 sets the method to be applied according to the exposure and toxicological data availability and can also be revisited depending on the outcome of the preliminary assessment.
- Step 4 provides an analysis plan to proceed with the RA process itself. The analysis plan may be modified, in the light of available new evidence, making it an iterative process.
2.3.2. Exposure Assessment
- Step 1: a list of the components within the assessment group is produced according to the grouping criteria (e.g., exposure, hazard, etc.) discussed in Section 3. Toxicologists are consulted to retrieve information on the relative potencies of individual chemicals and for defining the time frame when co-occurrence of exposures are relevant for the RA. For chronic and sub-chronic exposures, the co-occurrence timeframe for combined toxicity elicitation may vary depending on the kinetic profiles of the chemicals [8,18].
- Step 2: chemical occurrence data are collected and assembled, taking into account the plausibility of co-occurrence of individual components. When occurrence data specific to the target population are not available, data gaps can be filled from the available datasets on other populations that define the ratios and correlations between components. Occurrence data for each chemical need to originate from monitoring studies that use accurate and precise analytical methods. The data should, for example, note when concentration data are below the limit of detection or limit of quantification, as this could lead to left-censored data distributions that require specific considerations and corrections.
- Step 3: occurrence and consumption data are combined to estimate exposure through the use of appropriate tools depending on data availability and the selected methodology for risk characterisation [11,12]. Step 3 also foresees the calculation of potency-adjusted exposures starting from the toxicological advice provided in Step 1.
- Step 4: a summary report of exposure data with a comprehensive list of assumptions and uncertainties is produced. Specific exposure assessments may be performed for individual chemicals covered by an existing risk assessment or a defined legal framework.
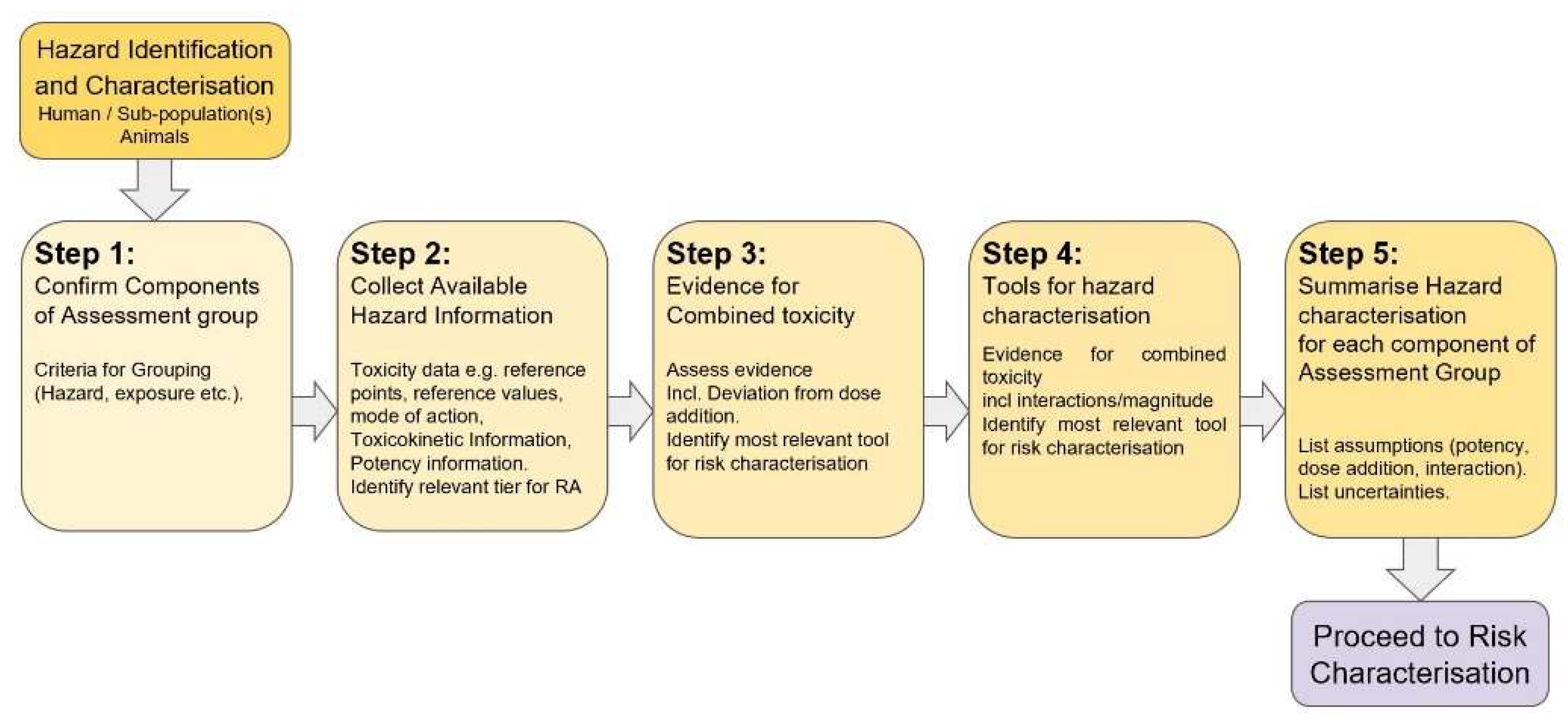
2.3.3. Hazard Identification and Characterisation
- Step 2: the relevant entry tier [11,12] for the assessment is decided based on the purpose of the assessment and the available data. Hazard information is collected for each individual chemical and may include toxicity data, reference points, reference values, mechanistic data, toxicokinetic information and relative potency information. In case of data-poor situations, a list of possibilities to fill data gaps is identified.
- Step 3 assesses the evidence for independent action between individual chemicals of the assessment groups and the potential for interactions [18]. Within step 3, the most appropriate approach for risk characterisation is defined.
- In Step 4, for each individual component of the assessment group, reference point and uncertainty factors are derived to obtain appropriate reference values through the relevant tier. Such reference values can be used for individual components of the whole group (equivalents of an index chemical).
- Step 5 summarises the hazard metrics for individual components and lists assumptions and uncertainties.
2.3.4. Risk Characterisation
2.3.5. Template for Summarising a Risk Assessment of Combined Exposure to Multiple Chemicals Using a Component-Based Approach
3. Scientific Criteria for Grouping Chemicals into Assessment Groups
3.1. Hazard-Driven Criteria for Grouping Chemicals
3.2. Prioritisation Methods
4. Applications in the Human Health and Animal Health Areas
4.1. Human Health Area
4.1.1. Risk Assessment of Multiple Pesticide Residues in Food
4.1.2. Combined Risk Assessment of Multiple Phthalates Using Biomonitoring Data
- Interindividual variation in daily dose for the surveyed individuals varied by factors of one thousand to three thousand, depending on the phthalate.
- Children ages 6–18 had slightly larger exposures than adults on a body weight basis.
- There was no significant difference in exposure with ethnicity or gender.
- The risk predictions of the RPF and the hazard index approaches were similar.
- Only 21 of the 2663 individuals surveyed in the 2013–2014 cycle had a value of HI greater than one, suggesting that combined exposures of the six phthalates were a potential concern for less than 1% of the surveyed individuals.
- A study of the earlier cycles found that risks posed by the phthalates had declined from 2005 to 2014, largely as a result of the displacement of more toxic phthalates by less toxic phthalates.
- Only three of the six phthalates drive the hazard index values for individuals with HI values greater than one. Any future study of toxicological interactions between phthalates should focus on these phthalates.
- The differences between the largest hazard quotient, hazard index and the maximum cumulative ratio declined from 3 to 1.3 in individuals with larger HI values. This indicates that the differences between risk estimates based on response addition would be similar to those from dose addition for the individuals most at risk.
4.2. Animal Health Area
4.2.1. Multiple Chemicals in Essential Oils
4.2.2. Multiple Mycotoxins in Maize
4.2.3. Multiple Pesticides in Bees
5. Future Challenges, Recommendations and Conclusions
- Develop and maintain open-source curated databases for exposure and hazard assessment of multiple chemicals, including production, use, occurrence, consumption data, TK and toxicity in the human health, animal health and ecological areas. This will allow development, implementation and testing of the relevance of NAM-based methods, such as in silico tools in the human health and animal health area. Such open-access databases on parent compounds, metabolites associated with critical and noncritical toxicological effects, mechanistic data and TK data will support grouping, refinement of assessment groups using MoA and AOP information and the development of predictive in silico models.
- For exposure assessment: (a) develop analytical methods with a broad scope, such as nontarget chemical analysis, for simultaneously characterising concentrations of a large number of chemicals in food, feed and drinking water; (b) develop guidance for use of probabilistic methods in exposure assessment for both single and multiple chemicals; (c) develop guidance for the generation and use of biomonitoring data in exposure assessment; and (d) develop databases on human dietary and occupational exposure to multiple chemicals. In addition, recent biomonitoring programmes, such as the European research Horizon 2020 project, HBM4EU, allowed health-based guidance values to be derived from epidemiological data as human biomonitoring guidance values. It is foreseen that, in the near future, such results can also contribute to integrate biomonitoring data and epidemiological data in these databases for specific European and other world populations.
- For hazard identification and characterisation: (a) develop approaches for better integration of high throughput, in vitro and omics data generated as NAM-based datasets, as explored worldwide in translational research and Horizon 2020 programmes; (b) apply, test and implement OECD Harmonised Templates (OHT) under the OECD harmonised guidelines to support NAMs implementation and improve grouping using mechanistic data of multiple chemicals. In this context, the OHT 201 template provides means to structure intermediate effect/mechanistic data from NAM-based methods (in silico and in vitro) and integrate them in the assessment [1,2,3]; and (c) apply and implement generic physiologically based TK and TK-TD models integrating internal dose in CBAs. Examples include models developed at US-EPA and EFSA, including Httk and TKplate published on EFSA knowledge junction, respectively [113]; and (d) apply and implement biologically based models as NAMs handling TK, TD or TK-TD interactions for predicting likelihood, dose dependencies and uncertainty factors in the case of synergisms and antagonisms. A recent example is provided by the physiologically based TK-TD model investigating melamine–cyanuric acid synergism in rainbow trout [24].
- For risk characterisation: (a) testing NAM-based methods through case studies is needed and (b) the use of default threshold values for risk metrics to prioritise chemicals should be further tested depending on regulatory context, number of chemicals under consideration in the assessment and data availability.
- With regards to international scientific co-operation, further improvement between regulatory agencies, member states and international agencies is warranted through data sharing, harmonisation of methods and practice, as well as training of staff and experts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Scientific Committee; Dorne, J.-L.C.; Hogstrand, C.; Dujardin, B.; Kass, G.E.; Liem, A.D.; Tarazona, J.; Machera, K.; Robinson, T.; Manini, P.; et al. EFSA International Workshop on RA of Combined Exposure to Multiple Chemicals. EFSA Support. Publ. 2022, 19, 7422E. [Google Scholar]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H.; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [PubMed]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Hernandez-Jerez, A.; Bennekou, S.H.; Halldorsson, T.I.; Koutsoumanis, K.P.; Lambré, C.; et al. Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals. EFSA J. 2021, 19, e07033. [Google Scholar]
- EC (European Commission). Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No. 793/93 and Commission Regulation (EC) No. 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC; Article 3 of the REACH; EC (European Commission): Brussels, Belgium.
- EC (European Commission). Regulation (EC) No. 1272/2008 of the European Parliament and of the Council of 16December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No. 1907/2006; Article 2 of the CLP; EC (European Commission): Brussels, Belgium.
- EC (European Commission). Communication from the Commission to the Council-the Combination Effects of Chemicals; EC (European Commission): Brussels Belgium.
- Karman, C.C.; Smit, M.G. Whole Effluent Toxicity Data and Discharge Volumes to Assess the Likelihood that Environmental Risks of Offshore Produced Water Discharges Are Adequately Controlled. Integr. Environ. Assess. Manag. 2019, 15, 584–595. [Google Scholar] [CrossRef] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residue). Guidance on the Use of Probabilistic Methodology for Modelling Dietary Exposure to Pesticide Residues. EFSA J. 2012, 10, 2839. [Google Scholar]
- Berry, M.R. Advances in dietary exposure research at the United States Environmental Protection Agency-National Exposure Research Laboratory. J. Expo. Anal. Environ. Epidemiol. 1997, 7, 3–16. [Google Scholar] [PubMed]
- Fromme, H.; Gruber, L.; Schlummer, M.; Wolz, G.; Böhmer, S.; Angerer, J.; Mayer, R.; Liebl, B.; Bolte, G. Intake of phthalates and di(2-ethylhexyl)adipate: Results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ. Int. 2007, 33, 1012–1020. [Google Scholar] [CrossRef]
- Meek, M.E.; Boobis, A.R.; Crofton, K.M.; Heinemeyer, G.; Raaij, M.V.; Vickers, C. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul Toxicol Pharm. 2011, 60, S1–S14. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Cooperation and Development). Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals, Series on Testing and Assessment No. 296, Environment, Healthand Safety Division, Environment Directorate. 2018. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/considerations-for-assessing-the-risks-of-combined-exposure-to-multiple-chemicals.pdf. (accessed on 25 August 2022).
- Teeguarden, J.G.; Tan, Y.-M.; Edwards, S.W.; Leonard, J.A.; Anderson, K.A.; Corley, R.A.; Kile, M.L.; Simonich, S.M.; Stone, D.; Tanguay, R.L.; et al. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ. Sci. Technol. 2016, 50, 4579–4586. [Google Scholar] [CrossRef]
- Price, P.S.; Jarabek, A.M.; Burgoon, L.D. Organizing mechanism-related information on chemical interactions using a framework based on the aggregate exposure and adverse outcome pathways. Environ. Int. 2020, 138, 105673. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA J. 2017, 15, e04971. [Google Scholar] [PubMed]
- EFSA (European Food Safety Authority). Editorial: Increasing robustness, transparency and openness of scientific assessments. EFSA J. 2015, 13, e13031. [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residue). Scientific Opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA J. 2014, 12, 3589. [Google Scholar]
- EFSA (European Food Safety Authority). International Frameworks Dealing with Human Risk Assessment of Combined Exposure to Multiple Chemicals. EFSA J. 2013, 11, 3313. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on Ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Polycyclic Aromatic Hydrocarbons in Food-Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- Bhat, V.S.; Meek, M.E.B.; Valcke, M.; English, C.; Boobis, A.; Brown, R. Evolution of chemical-specific adjustment factors (CSAF) based on recent international experience; increasing utility and facilitating regulatory acceptance. Crit. Rev. Toxicol. 2017, 47, 729–749. [Google Scholar] [CrossRef]
- Tebby, C.; Brochot, C.; Dorne, J.L.; Beaudouin, R. Investigating the interaction between melamine and cyanuric acid using a Physiologically-Based Toxicokinetic model in rainbow trout. Toxicol. Appl. Pharmacol. 2019, 370, 184–195. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Cooperation and Development). Guidance Document on the Characterisation, Validation and Reporting of Physiologically Based Kinetic (PBK) Models for Regulatory Purposes, OECD Series on Testing and Assessment, No. 331, Environment, Health and Safety, Environment Directorate. 2021. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/guidance-document-on-the-characterisation-validation-and-reporting-of-physiologically-based-kinetic-models-for-regulatory-purposes.pdf (accessed on 25 August 2022).
- Haddad, S.; Béliveau, M.; Tardif, R.; Krishnan, K. A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol. Sci. 2001, 63, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Bois, F.Y. A mechanistic modeling framework for predicting metabolic interactions in complex mixtures. Environ. Health Perspect 2011, 119, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, A.; Berggren, E.; Bessems, J.; Bopp, S.; Linden, S.; Worth, A. Assessment of Mixtures: Review of Regulatory Requirements and Guidance; Publications Office of the European Union: Luxembourg, 2017; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC90601 (accessed on 25 August 2022).
- Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Quignot, N.; Wiecek, W.; Amzal, B.; Dorne, J.L. The Yin-Yang of CYP3A4: A Bayesian meta-analysis to quantify inhibition and induction of CYP3A4 metabolism in humans and refine uncertainty factors for mixture risk assessment. Arch. Toxicol. 2019, 93, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.C.; Reimschuessel, R.; Von Tungeln, L.S.; Olson, G.R.; Warbritton, A.R.; Hattan, D.G.; Beland, F.A.; Gamboa da Costa, G. Dose-response assessment of nephrotoxicity from a 7-day combined exposure to melamine and cyanuric acid in F344 rats. Toxicol. Sci. 2011, 119, 391–397. [Google Scholar] [CrossRef] [PubMed]
- da Costa, G.G.; Jacob, C.C.; Von Tungeln, L.S.; Hasbrouck, N.R.; Olson, G.R.; Hattan, D.G.; Reimschuessel, R.; Beland, F.A. Dose-response assessment of nephrotoxicity from a twenty-eight-day combined-exposure to melamine and cyanuric acid in F344 rats. Toxicol. Appl. Pharmacol. 2012, 262, 99–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA (European Food Safety Authority). EFSA Scientific Colloquium No. 21: Harmonisation of Human and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals. 2014. Available online: https://www.efsa.europa.eu/en/events/event/140911 (accessed on 25 August 2022).
- EFSA (European Food Safety Authority). Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2013, 11, 3295. [Google Scholar]
- EFSA Scientific Committee; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA J. 2018, 16, e05123. [Google Scholar]
- US EPA (US Environmental Protection Agency). Guidelines for Carcinogen Risk Assessment. Federal Register70(66)177650-18717. 2005. Available online: https://www.epa.gov/sites/default/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf (accessed on 25 August 2022).
- Bopp, S.K.; Kienzler, A.; Linden, S.; Richarz, A.-N.; Triebe, J.; Worth, A. Review of Case Studies on the Human and Environmental Risk Assessment of Chemical Mixtures: Identification of Priorities, Methodologies, Datagaps, Future Needs. JRC Technical Reports. 2016. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC102111 (accessed on 25 August 2022).
- OECD (Organisation for Economic Cooperation and Development). Guidance on Grouping of Chemicals, 2nd ed.; OECD Series on Testing and Assessment, No. 194; OECD Publishing: Paris, France, 2014; Available online: https://doi.org/10.1787/9789264274679-en (accessed on 25 August 2022).
- van der Voet, H.; van der Heijden, G.W.A.M.; Bos, P.M.J.; Bosgra, S.; Boon, P.E.; Muri, S.D.; Brüschweiler, B.J. A model for probabilistic health impact assessment of exposure to food chemicals. Food Chem. Toxicol. 2009, 47, 2926–2940. [Google Scholar] [CrossRef]
- Müller, A.K.; Bosgra, S.; Boon, P.E.; Voet, H.v.d.; Nielsen, E.; Ladefoged, O. Probabilistic cumulative risk assessment of anti-androgenic pesticides in food. Food Chem. Toxicol. 2009, 47, 2951–2962. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Towards an integrated environmental risk assessment of multiple stressors on bees: Review of research projects in Europe, knowledge gaps and recommendations. EFSA J. 2014, 12, 3594. [Google Scholar]
- Cedergreen, N.; Dalhoff, K.; Li, D.; Gottardi, M.; Kretschmann, A.C. Can Toxicokinetic and Toxicodynamic Modeling Be Used to Understand and Predict Synergistic Interactions between Chemicals? Environ. Sci. Technol. 2017, 51, 14379–14389. [Google Scholar] [CrossRef] [PubMed]
- Pletz, J.; Blakeman, S.; Paini, A.; Parissis, N.; Worth, A.; Andersson, A.-M.; Frederiksen, H.; Sakhi, A.K.; Thomsen, C.; Bopp, S.K. Physiologically based kinetic (PBK) modelling and human biomonitoring data for mixture risk assessment. Environ. Int. 2020, 143, 105978. [Google Scholar] [CrossRef] [PubMed]
- Price, P.S.; Han, X. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int. J. Environ. Res. Public Health 2011, 8, 2212–2225. [Google Scholar] [CrossRef]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef]
- Mumtaz, M.M.; Durkin, P.R. A weight-of-evidence approach for assessing interactions in chemical mixtures. Toxicol. Ind. Health 1992, 8, 377–406. [Google Scholar] [CrossRef]
- US EPA (US Environmental Protection Agency). Supplementary Guidance for Conducting Health Risk Assessmentof Chemical Mixtures. 2000. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533 (accessed on 25 August 2022).
- US EPA (US Environmental Protection Agency). Concepts, Methods, and Data Sources for Cumulative Healthrisk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report, 2008). Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=190187 (accessed on 25 August 2022).
- ATSDR (Agency for Toxic Substances and Disease Registry). Guidance Manual for the Assessment of JointToxic Action of Chemical Mixtures. 2004. Available online: https://stacks.cdc.gov/view/cdc/6935 (accessed on 25 August 2022).
- SCHER, SCCS and SCENIHR (Scientific Committee on Health and Environmental Risks, Scientific Committee onConsumer Safety and Scientific Committee on Emerging and Newly Identified Health Risks). Opinion onthe Toxicity and Assessment of Chemical Mixtures. 2012. Available online: https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_155.pdf (accessed on 25 August 2022).
- OECD (Organisation for Economic Co-operation and Development). Case Study on the Use of IATA for Pesticide Cumulative Risk Assessment & Assessment of Lifestage Susceptibility. Series on Testing and Assessment No. 272, Environment, Health and Safety Division, Environment Directorate. 2017. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2017)24&doclanguage=en (accessed on 25 August 2022).
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA J. 2017, 15, e04970. [Google Scholar]
- EFSA (European Food Safety Authority); Hart, A.; Maxim, L.; Siegrist, M.; Von Goetz, N.; da Cruz, C.; Merten, C.; Mosbach-Schulz, O.; Lahaniatis, M.; Smith, A.; et al. Guidance on Communication of Uncertainty in Scientific Assessments. EFSA J. 2019, 17, e05520. [Google Scholar]
- US EPA (US Environmental Protection Agency). Organophosphorus Cumulative Risk Assessment; Office of Pesticide Programs, Office of Prevention, Pesticides, and Toxic Substances: Washington, DC, USA, 2006. Available online: https://downloads.regulations.gov/EPA-HQ-OPP-2006-0618-0002/content.pdf (accessed on 25 August 2022).
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333. [Google Scholar]
- OECD. The OECD QSAR Toolbox. Available online: https://www.oecd.org/chemicalsafety/oecd-qsar-toolbox.htm (accessed on 25 August 2022).
- VEGAHUB. Available online: https://www.vegahub.eu/ (accessed on 25 August 2022).
- Benfenati, E.; Chaudhry, Q.; Gini, G.; Dorne, J.L. Integrating in silico models and read-across methods for predicting toxicity of chemicals: A step-wise strategy. Environ. Int. 2019, 131, 105060. [Google Scholar] [CrossRef]
- FAO/WHO. Expert Consultation on Dietary Risk Assessment of Chemical Mixtures (Risk Assessment of Combined Exposure to Multiple Chemicals); WHO: Geneva, Switzerland, 2019; Available online: https://www.euromixproject.eu/2019/04/29/fao-who-expert-consultation-on-dietary-risk-assessment-of-chemical-mixtures/ (accessed on 25 August 2022).
- Crépet, A.; Vasseur, P.; Jean, J.; Badot, P.M.; Nesslany, F.; Vernoux, J.P.; Feidt, C.; Mhaouty-Kodja, S. Integrating Selection and Risk Assessment of Chemical Mixtures: A Novel Approach Applied to a Breast Milk Survey. Env. Health Perspect 2022, 130, 35001. [Google Scholar] [CrossRef] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residue). Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. EFSA J. 2013, 11, 3293. [Google Scholar]
- EFSA (European Food Safety Authority); Crivellente, F.; Hart, A.; Hernandez-Jerez, A.F.; Hougaard Bennekou, S.; Pedersen, R.; Terron, A.; Wolterink, G.; Mohimont, L. Establishment of cumulative assessment groups of pesticides for their effects on the nervous system. EFSA J. 2019, 17, e05800. [Google Scholar] [PubMed]
- EFSA (European Food Safety Authority); Crivellente, F.; Hart, A.; Hernandez-Jerez, A.F.; Hougaard Bennekou, S.; Pedersen, R.; Terron, A.; Wolterink, G.; Mohimont, L. Establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA J. 2019, 17, e05801. [Google Scholar] [PubMed]
- EFSA (European Food Safety Authority); Dujardin, B.; Bocca, V. Cumulative dietary exposure assessment of pesticides that have chronic effects on the thyroid using SAS® software. EFSA J. 2019, 17, e05763. [Google Scholar] [PubMed]
- EFSA (European Food Safety Authority); Dujardin, B.; Bocca, V. Cumulative dietary exposure assessment of pesticides that have acute effects on the nervous system using SAS® software. EFSA J. 2019, 17, e05764. [Google Scholar]
- EFSA (European Food Safety Authority); Anastassiadou, M.; Choi, J.; Coja, T.; Dujardin, B.; Hart, A.; Hernandez-Jerrez, A.F.; Jarrah, S.; Lostia, A.; Machera, K.; et al. Cumulative dietary risk assessment of chronic acetylcholinesterase inhibition by residues of pesticides. EFSA J. 2021, 19, e06392. [Google Scholar]
- EFSA (European Food Safety Authority); Craig, P.S.; Dujardin, B.; Hart, A.; Hernandez-Jerez, A.F.; Hougaard Bennekou, S.; Kneuer, C.; Ossendorp, B.; Pedersen, R.; Wolterink, G.; et al. Cumulative dietary risk characterisation of pesticides that have chronic effects on the thyroid. EFSA J. 2020, 18, e06088. [Google Scholar]
- van Klaveren, J.D.; Kruisselbrink, J.W.; de Boer, W.J.; van Donkersgoed, G.; Biesebeek, J.D.T.; Sam, M.; van der Voet, H. Cumulative dietary exposure assessment of pesticides that have chronic effects on the thyroid using MCRA software. EFSA Support. Publ. 2019, 16, 1707E. [Google Scholar] [CrossRef]
- van Klaveren, J.D.; Kruisselbrink, J.W.; de Boer, W.J.; van Donkersgoed, G.; Biesebeek, J.D.T.; Sam, M.; van der Voet, H. Cumulative dietary exposure assessment of pesticides that have acute effects on the nervous system using MCRA software. EFSA Support. Publ. 2019, 16, 1708E. [Google Scholar] [CrossRef]
- te Biesebeek, J.; Sam, M.; Sprong, R.; van Donkersgoed, G.; Kruisselbrink, J.; de Boer, W.; van Lenthe, M.; van der Voet, H.; van Klaveren, J. Potential impact of prioritisation methods on the outcome of cumulative exposure assessments of pesticides. EFSA Support. Publ. 2021, 18, 6559E. [Google Scholar] [CrossRef]
- Reyes, J.M.; Price, P.S. An analysis of cumulative risks based on biomonitoring data for six phthalates using the Maximum Cumulative Ratio. Environ. Int. 2018, 112, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.; Price, P.S. Temporal Trends in Exposures to Six Phthalates from Biomonitoring Data: Implications for Cumulative Risk. Environ. Sci. Technol. 2018, 52, 12475–12483. [Google Scholar] [CrossRef] [PubMed]
- CDC (Centers for Disease Control and Prevention). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data; U.S. Department of Health and Human Services: Hyattsville, MD, USA, 2016. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 25 August 2022).
- CDC (Centers for Disease Control and Prevention). NHANES 2013–2014 Laboratory Data Overview, National Health and Nutrition Examination Survey. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2013 (accessed on 25 August 2022).
- Varshavsky, J.R.; Zota, A.R.; Woodruff, T.J. A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environ. Sci. Technol. 2016, 50, 10616–10624. [Google Scholar] [CrossRef]
- Christensen, K.L.; Makris, S.L.; Lorber, M. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul. Toxicol. Pharmacol. 2014, 69, 380–389. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Dorne, J.L.C.; Manini, P.; Hogstrand, C. Animal Health Risk assessment of multiple chemicals in essential oils for farm animals. EFSA Support. Publ. 2020, 17, 1760E. [Google Scholar]
- EFSA (European Food Safety Authority); Dorne, J.L.C.; Crépet, A.; te Biesebeek, J.D.; Machera, K.; Hogstrand, C. Human risk assessment of multiple chemicals using component-based approaches: A horizontal perspective. EFSA Support. Publ. 2020, 17, 1759E. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of an essential oil from Origanum vulgare subsp. hirtum (Link) letsw. var. Vulkan when used as a sensory additive in feed for all animal species. EFSA J. 2017, 15, e05095. [Google Scholar]
- Dorne, J.L.C.M.; Richardson, J.; Livaniou, A.; Carnesecchi, E.; Ceriani, L.; Baldin, R.; Kovarich, S.; Pavan, M.; Saouter, E.; Biganzoli, F.; et al. EFSA’s OpenFoodTox: An open source toxicological database on chemicals in food and feed and its future developments. Environ. Int. 2021, 146, 106293. [Google Scholar] [CrossRef]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of an essential oil from Origanum vulgare ssp. hirtum (Link) Ietsw. for all animal species. EFSA J. 2019, 17, e05909. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of an essential oil from Elettaria cardamomum (L.) Maton when used as a sensory additive in feed for all animal species. EFSA J. 2019, 17, e05721. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of essential oil, oleoresin and tincture from Zingiber officinale Roscoe when used as sensory additives in feed for all animal species. EFSA J. 2020, 18, e06147. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. rhizome when used as sensory additives in feed for all animal species. EFSA J. 2020, 18, e06146. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kouba, M.; Fašmon Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of feed additives consisting of expressed lemon oil and its fractions from Citrus limon (L.) Osbeck and of lime oil from Citrus aurantiifolia (Christm.) Swingle for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06548. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an essential oil from the leaves of Citrus × aurantium L. (petitgrain bigarade oil) for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06624. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kouba, M.; Fasmon Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of expressed mandarin oil from the fruit peels of Citrus reticulata Blanco for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06625. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of feed additives consisting of expressed sweet orange peel oil and its fractions from Citrus sinensis (L.) Osbeck for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06891. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of a flavonoid-rich dried extract of Citrus × aurantium L. fruit (bitter orange extract) for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06709. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kouba, M.; Fašmon Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an aqueous extract of Citrus limon (L.) Osbeck (lemon extract) for use in all animal species (Nor-Feed SAS). EFSA J. 2021, 19, e06893. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an essential oil from the fruits of Litsea cubeba (Lour.) Pers. (litsea berry oil) for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06623. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of a tincture from the bark of Cinnamomum verum J. Presl (cinnamon tincture) for use in all animal species (FEFANA asbl). EFSA J. 2021, 19, e06986. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an essential oil from Cinnamomum camphora (L.) J. Presl (camphor white oil) for use in all animal species (FEFANA asbl). EFSA J. 2022, 20, e06985. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an essential oil from the leaves of Agathosma betulina (P.J. Bergius) Pillans (buchu leaf oil) for use in all animal species (FEFANA asbl). EFSA J. 2022, 20, e07160. [Google Scholar] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an essential oil from the flowers of Cananga odorata (Lam.) Hook.f. & Thomson (ylang ylang oil) for use in all animal species (FEFANA asbl). EFSA J. 2022, 20, e07159. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of an extract of olibanum from Boswellia serrata Roxb. ex Colebr. for use in dogs and horses (FEFANA asbl). EFSA J. 2022, 20, e07158. [Google Scholar] [PubMed]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Cortiñas Abrahantes, J.; Dorne, J.L.; Battilani, P.; Toscano, P. Occurrence and Co-Occurrence of Mycotoxins in Cereal-Based Feed and Food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef]
- Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.A.; Klink, H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, e04425. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017, 15, e04655. [Google Scholar] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, e05172. [Google Scholar] [PubMed]
- Palumbo, R.; Dorne, J.-L.; Battilani, P. Human and Animal Health Risk Assessment of Mycotoxin Mixtures in Maize: From Fungal Production and Occurrence to Harmonised Risk Characterisation. Ph.D. Thesis, Università Cattolica del Sacro Cuore, Piacenza, Italy, 2018. Available online: http://hdl.handle.net/10280/73545 (accessed on 25 August 2022).
- Ioannidou, S.; Cascio, C.; Gilsenan, M.B. European Food Safety Authority open access tools to estimate dietary exposure to food chemicals. Environ. Int. 2021, 149, 106357. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, E.; Svendsen, C.; Lasagni, S.; Grech, A.; Quignot, N.; Amzal, B.; Toma, C.; Tosi, S.; Rortais, A.; Cortinas-Abrahantes, J.; et al. Investigating combined toxicity of binary mixtures in bees: Meta-analysis of laboratory tests, modelling, mechanistic basis and implications for risk assessment. Environ. Int. 2019, 133, 105256. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Sfeir, C.; Carnesecchi, E.; vanEngelsdorp, D.; Chauzat, M.P. Lethal, sublethal, and combined effects of pesticides on bees: A meta-analysis and new risk assessment tools. Sci. Total Environ. 2022, 844, 156857. [Google Scholar] [CrossRef]
- Carnesecchi, E.; Toropov, A.A.; Toropova, A.P.; Kramer, N.; Svendsen, C.; Dorne, J.L.; Benfenati, E. Predicting acute contact toxicity of organic binary mixtures in honey bees (A. mellifera) through innovative QSAR models. Sci. Total Environ. 2020, 704, 135302. [Google Scholar] [CrossRef]
- Carnesecchi, E.; Toma, C.; Roncaglioni, A.; Kramer, N.; Benfenati, E.; Dorne, J. Integrating QSAR models predicting acute contact toxicity and mode of action profiling in honey bees (A. mellifera): Data curation using open source databases, performance testing and validation. Sci. Total Environ. 2020, 735, 139243. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Certain Veterinary Drug Residues in Food: Eighty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2020. [Google Scholar]
- Grech, A.; Tebby, C.; Brochot, C.; Bois, F.Y.; Bado-Nilles, A.; Dorne, J.-L.; Quignot, N.; Beaudouin, R. Physiological Parameters for Four Fish Species (Rainbow Trout, Zebrafish, Fathead Minnow and Three-Spined Stickleback) as the Basis for the Development of Generic Physiologically-Based Kinetic Models. 2018. Available online: https://zenodo.org/record/1414332#.XEW3WDBKjIU (accessed on 25 August 2022).
- Astuto, M.C.; Di Nicola, M.R.; Tarazona, J.V.; Rortais, A.; Devos, Y.; Liem, A.K.D.; Kass, G.E.N.; Bastaki, M.; Schoonjans, R.; Maggiore, A.; et al. In Silico Methods for Environmental Risk Assessment: Principles, Tiered Approaches, Applications, and Future Perspectives. In In Silico Methods for Predicting Drug Toxicity; Benfenati, E., Ed.; Springer: New York, NY, USA, 2022; pp. 589–636. [Google Scholar]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Machera, K.; et al. A systems-based approach to the environmental risk assessment of multiple stressors in honey bees. EFSA J. 2021, 19, e06607. [Google Scholar]



| Problem Formulation | Description of the Components in the Mixture | Chemical Space to Be Covered, Composition, Data Availability for Components |
| Conceptual model | Question/Terms of Reference, Source, exposure pathways, Species/subpopulation, Regulatory framework, Other? | |
| Methodology | Overview of available data Component-based approach Principles for grouping and Assessment Group(s) | |
| Analysis plan | ||
| Exposure Assessment | Components of the assessment group | |
| Summary occurrence (concentration) data | ||
| Summary exposure | Assumptions, Exposure metrics | |
| Identify uncertainties | ||
| Hazard Identification and Hazard Characterisation | Component-based approach | |
| Reference points/Reference values | ||
| Summary hazard metrics | Assumptions combined toxicity (Dose addition, response addition, interactions) Hazard metrics | |
| Identify uncertainties | ||
| Risk Characterisation | Summary exposure and hazard metrics | |
| Risk characterisation approach | ||
| Summary risk metrics | Associated Assumptions (Dose addition, response addition, interactions), Risk metrics | |
| Overall uncertainty analysis | ||
| Interpretation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, I.; Kalian, A.D.; Di Nicola, M.R.; Dujardin, B.; Levorato, S.; Mohimont, L.; Nathanail, A.V.; Carnessechi, E.; Astuto, M.C.; Tarazona, J.V.; et al. Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges. Toxins 2023, 15, 40. https://doi.org/10.3390/toxins15010040
Cattaneo I, Kalian AD, Di Nicola MR, Dujardin B, Levorato S, Mohimont L, Nathanail AV, Carnessechi E, Astuto MC, Tarazona JV, et al. Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges. Toxins. 2023; 15(1):40. https://doi.org/10.3390/toxins15010040
Chicago/Turabian StyleCattaneo, Irene, Alexander D. Kalian, Matteo R. Di Nicola, Bruno Dujardin, Sara Levorato, Luc Mohimont, Alexis V. Nathanail, Edoardo Carnessechi, Maria Chiara Astuto, Jose V. Tarazona, and et al. 2023. "Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges" Toxins 15, no. 1: 40. https://doi.org/10.3390/toxins15010040
APA StyleCattaneo, I., Kalian, A. D., Di Nicola, M. R., Dujardin, B., Levorato, S., Mohimont, L., Nathanail, A. V., Carnessechi, E., Astuto, M. C., Tarazona, J. V., Kass, G. E. N., Liem, A. K. D., Robinson, T., Manini, P., Hogstrand, C., Price, P. S., & Dorne, J. L. C. M. (2023). Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges. Toxins, 15(1), 40. https://doi.org/10.3390/toxins15010040








