Modification of Deoxynivalenol by a Fungal Laccase Paired with Redox Mediator TEMPO
Abstract
:1. Introduction
2. Results
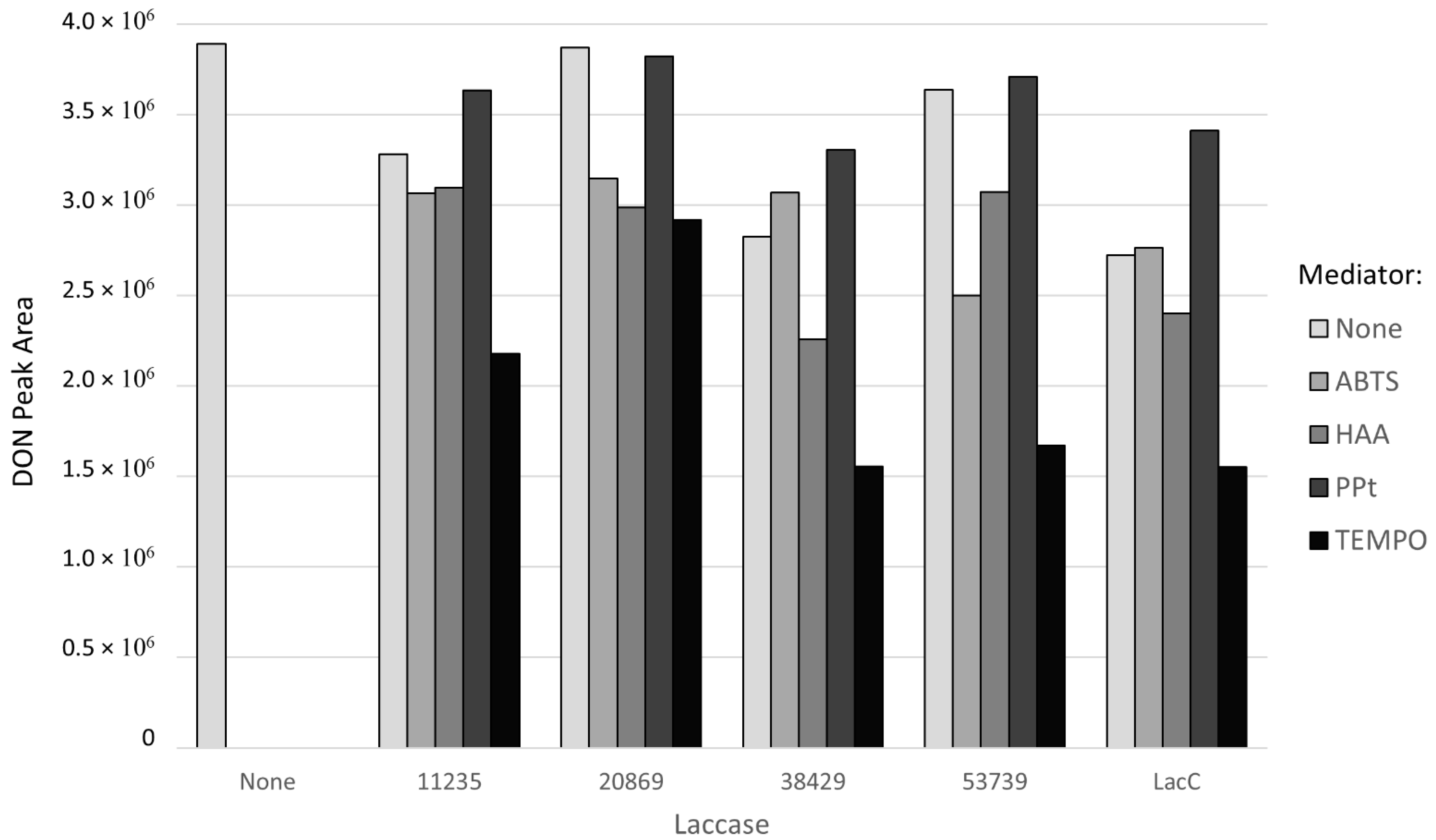
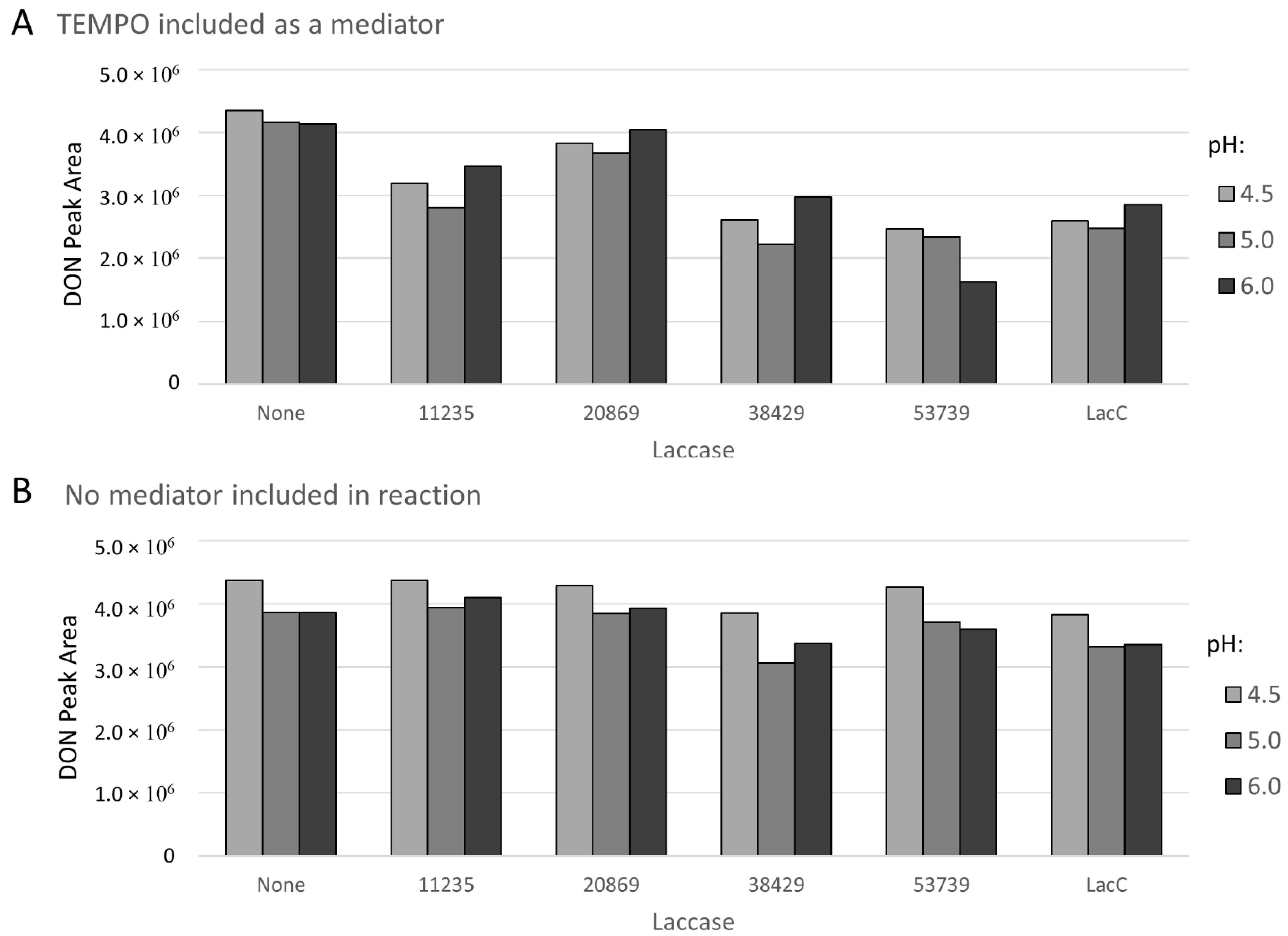
2.1. Preliminary Screening of Laccase Enzymes and Chemical Mediators
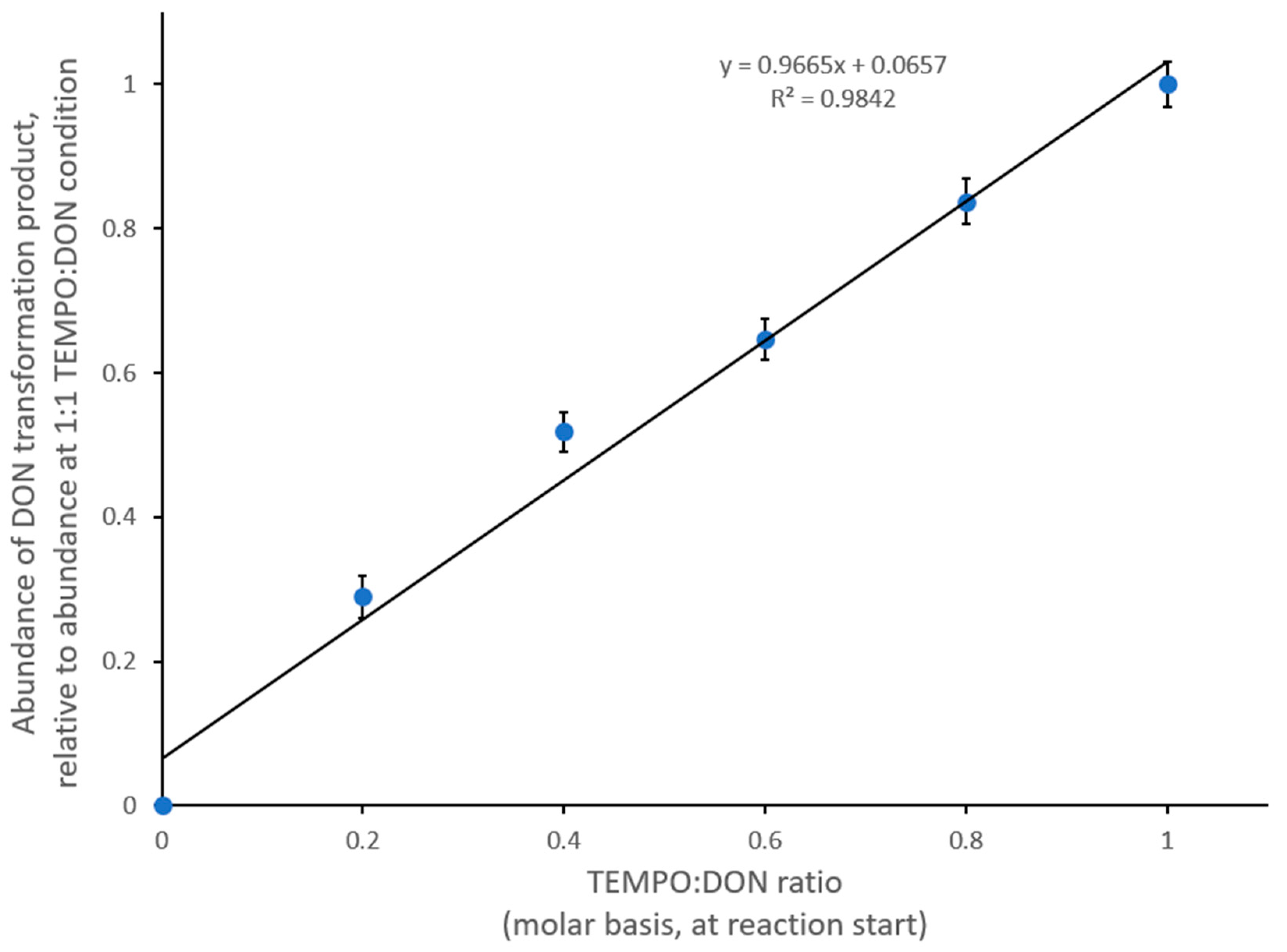
2.2. Appearance of New Gas Chromatography/Mass Spectrometry Peaks
2.3. Liquid Chromatography/Mass Spectrometry
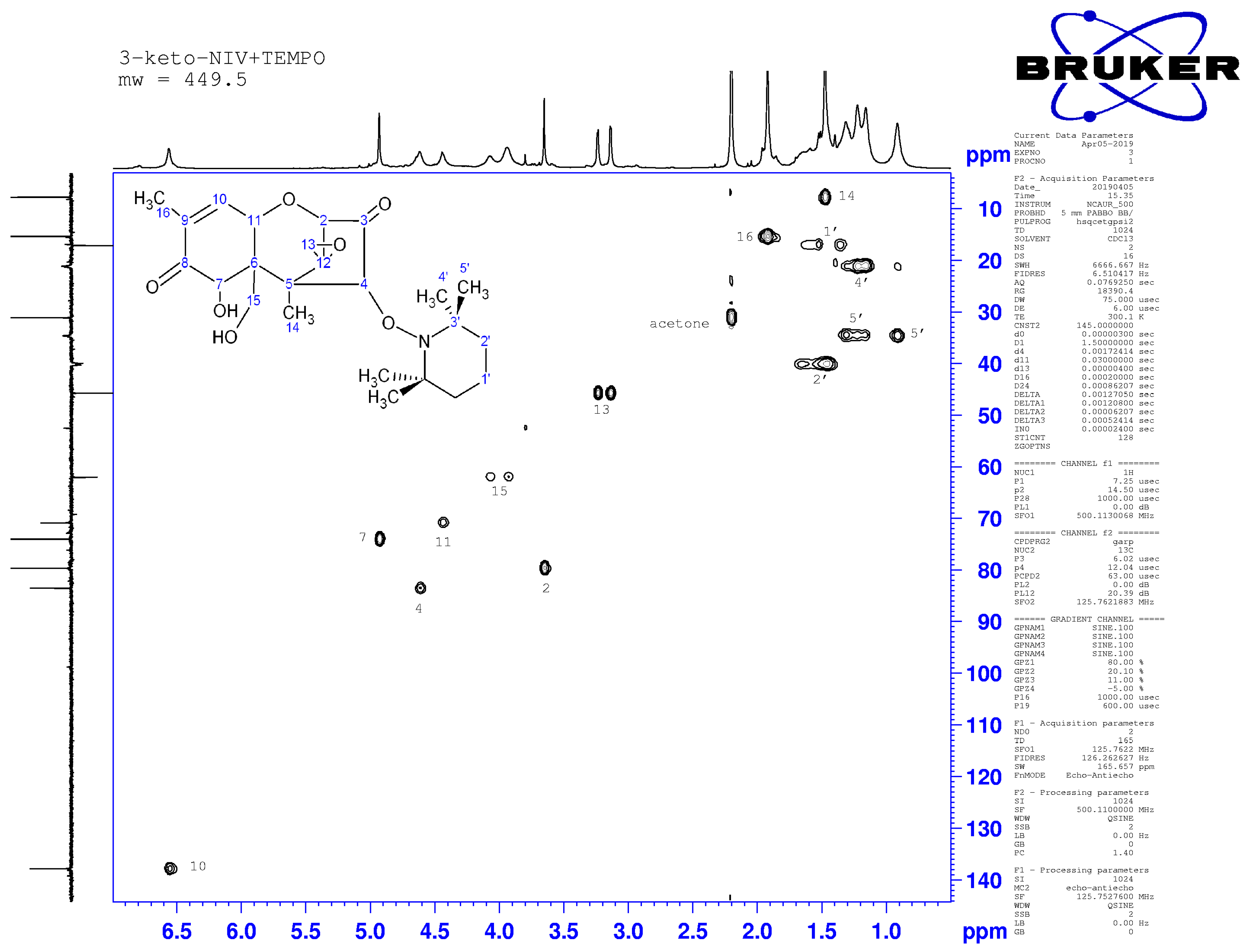
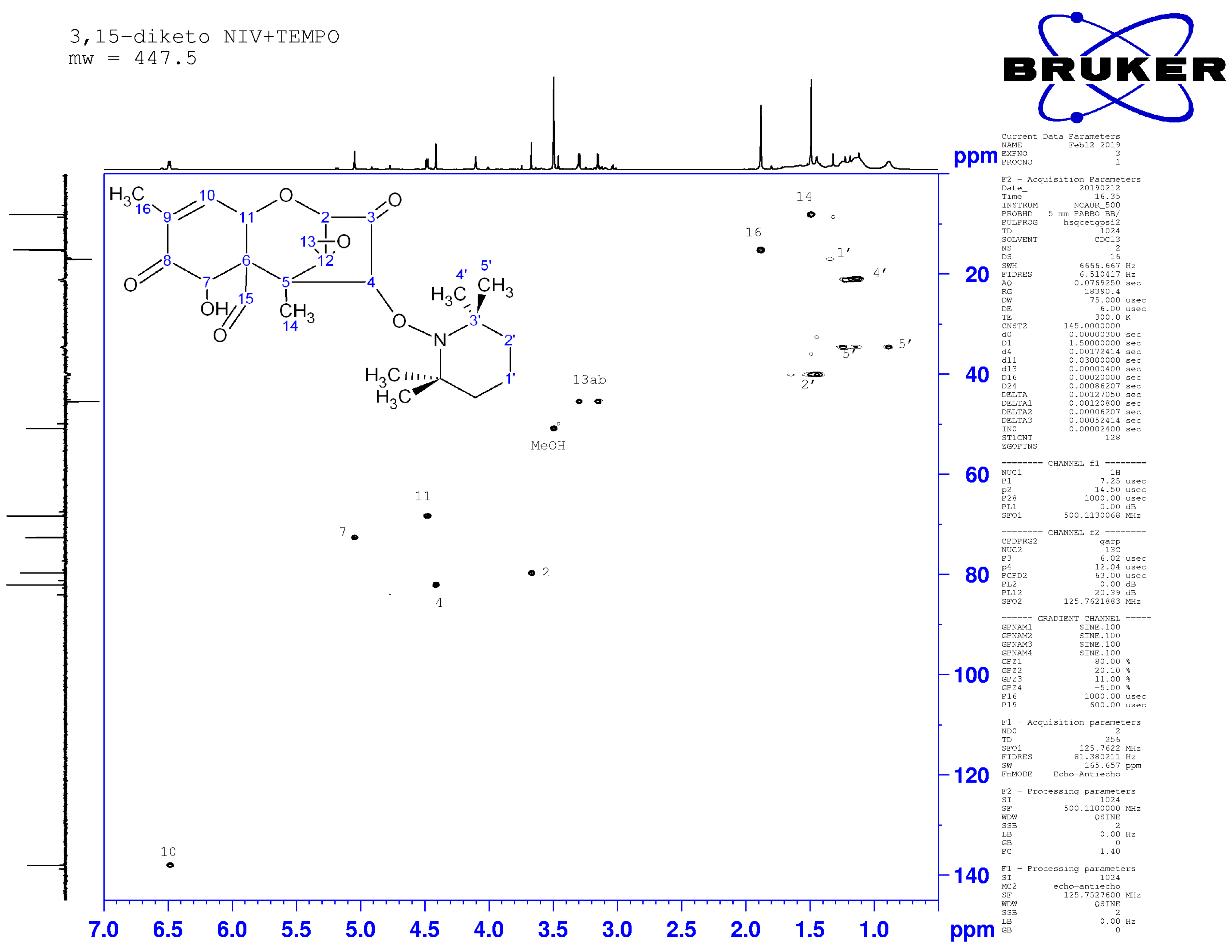
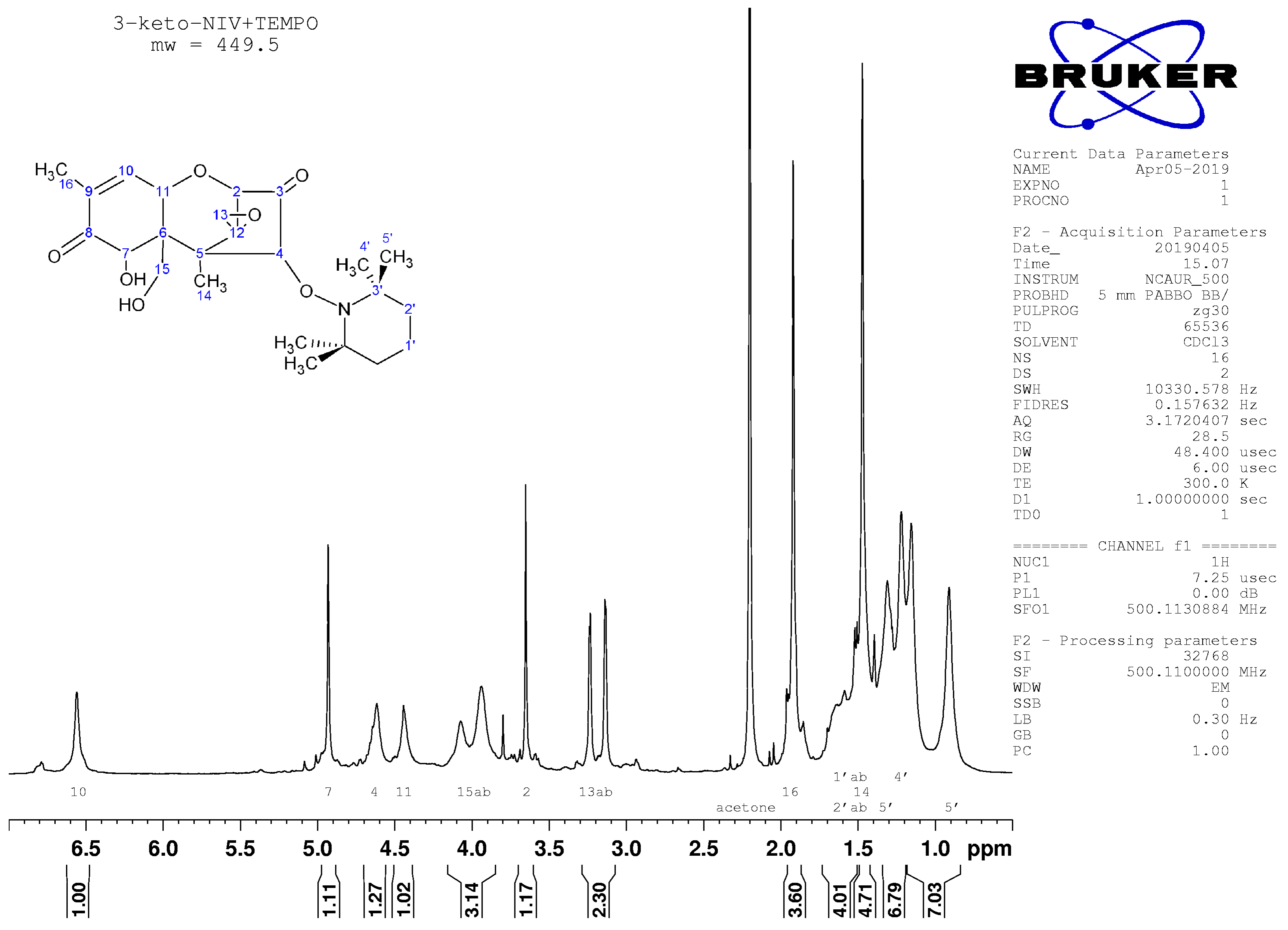
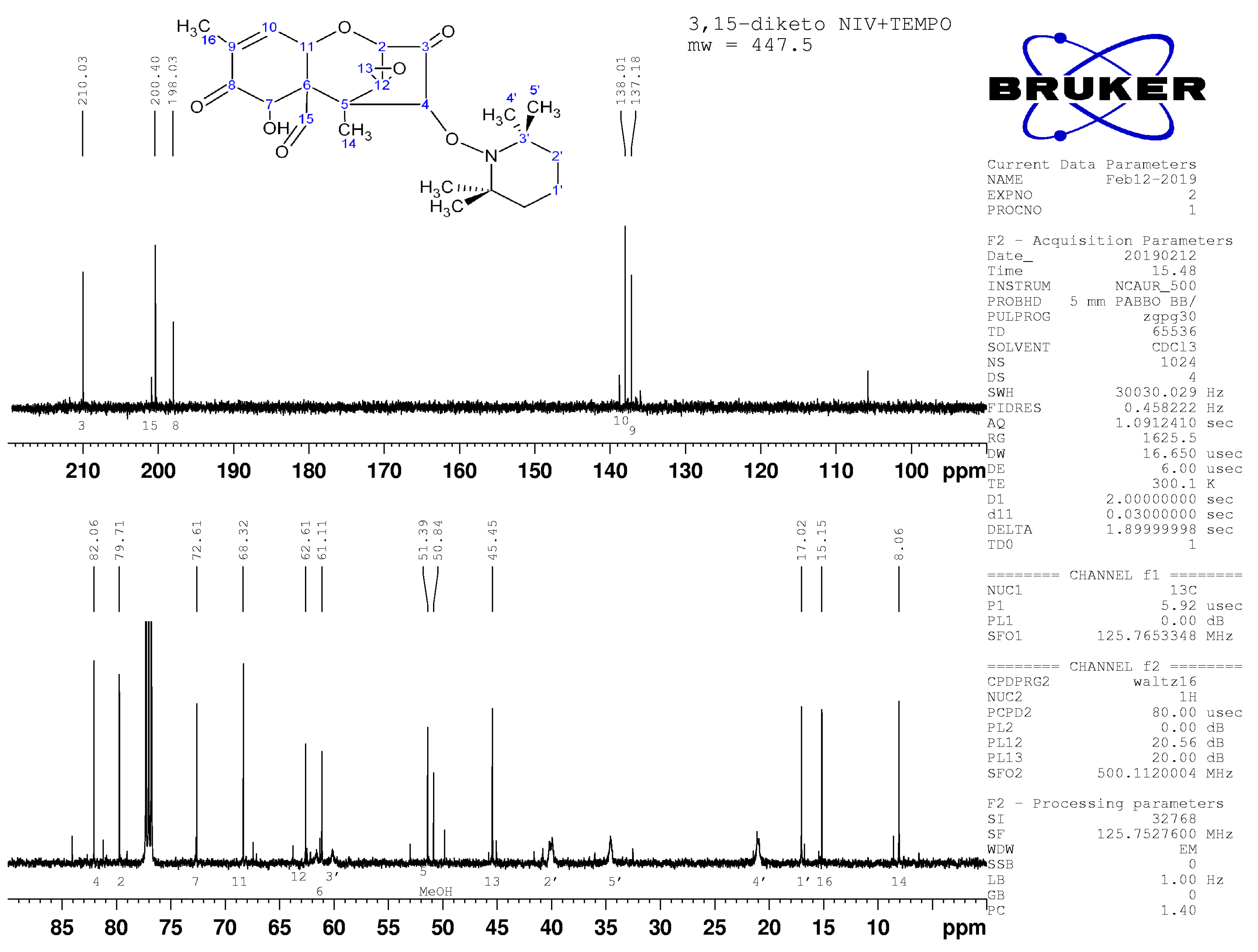
2.4. Nuclear Magnetic Resonance Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Development of the Laccase-Mediator System
4.2. Characterization of Transformation Products
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Schmale, D.G., III; Munkvold, G.P. Mycotoxins in Crops: A Threat to Human and Domestic Animal Health. Available online: https://www.apsnet.org/edcenter/disimpactmngmnt/topc/Mycotoxins/Pages/EconomicImpact.aspx (accessed on 8 July 2022).
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 235–287. [Google Scholar]
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.-S.; Kurtzman, C.P.; McCormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium mycotoxins: A trans-disciplinary overview. Can. J. Plant Pathol. 2018, 40, 161–171. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Wagacha, J.; Muthomi, J. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef]
- Pestka, J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Reverberi, M.; Salustri, M.; Masi, P.; Cavella, S. Technological properties of durum wheat semolina treated by heating and UV irradiation for reduction of mycotoxin content. J. Food Process Eng. 2019, 42, e13006. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2019, 126, 1149–1160. [Google Scholar] [CrossRef]
- Guan, S.; He, J.; Young, J.C.; Zhu, H.; Li, X.-Z.; Ji, C.; Zhou, T. Transformation of trichothecene mycotoxins by microorganisms from fish digesta. Aquaculture 2009, 290, 290–295. [Google Scholar] [CrossRef]
- Fuchs, E.; Binder, E.M.; Heidler, D.; Krska, R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002, 19, 379–386. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Liuzzi, V.; Logrieco, A.; Mulè, G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Rich, J.O.; Anderson, A.M.; Berhow, M.A. Laccase-mediator catalyzed conversion of model lignin compounds. Biocatal. Agric. Biotechnol. 2016, 5, 111–115. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trend Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Zucca, P.; Cocco, G.; Sollai, F.; Sanjust, E. Fungal laccases as tools for biodegradation of industrial dyes. Biocatalysis 2015, 1, 82–108. [Google Scholar] [CrossRef]
- Camarero, S.; Ibarra, D.; Martínez, M.J.; Martínez, Á.T. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl. Environ. Microbiol. 2005, 71, 1775–1784. [Google Scholar] [CrossRef]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef]
- Strong, P.J.; Claus, H. Laccase: A review of its past and its future in bioremediation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 373–434. [Google Scholar] [CrossRef]
- Mikolasch, A.; Schauer, F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl. Microbiol. Biotechnol. 2009, 82, 605–624. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Banu, I.; Lupu, A.; Aprodu, I. Degradation of zearalenone by laccase enzyme. Sci. Study Res. 2013, 14, 79. [Google Scholar]
- Loi, M.; Fanelli, F.; Zucca, P.; Liuzzi, V.; Quintieri, L.; Cimmarusti, M.; Monaci, L.; Haidukowski, M.; Logrieco, A.; Sanjust, E. Aflatoxin B1 and M1 degradation by Lac2 from Pleurotus pulmonarius and redox mediators. Toxins 2016, 8, 245. [Google Scholar] [CrossRef]
- Savard, M.E.; Blackwell, B.A.; Grenhalgh, R. An 1H nuclear magnetic resonance study of derivatives of 3-hydroxy-12,13-epoxytrichothec-9-enes. J. Chem. 1987, 65, 2254. [Google Scholar] [CrossRef]
- Xiong, S.; Li, X.; Zhao, C.; Gao, J.; Yuan, W.; Zhang, J. The degradation of deoxynivalenol by using electrochemical oxidation with graphite electrodes and the toxicity assessment of degradation products. Toxins 2019, 11, 478. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Zhou, T. Addressing the mycotoxin deoxynivalenol contamination with soil-derived bacterial and enzymatic transformations targeting the C3 carbon. World Mycotoxin J. 2018, 11, 101–111. [Google Scholar]
- Carere, J.; Hassan, Y.I.; Lepp, D.; Zhou, T. The enzymatic detoxification of the mycotoxin deoxynivalenol: Identification of DepA from the DON epimerization pathway. Microb. Biotechnol. 2018, 11, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Yang, R.; Zhou, T.; Boland, G.J.; Scott, P.M.; Bondy, G.S. An epimer of deoxynivalenol: Purification and structure identification of 3-epi-deoxynivalenol. Food Addit. Contam. 2015, 32, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Ikunaga, Y.; Sato, I.; Grond, S.; Numaziri, N.; Yoshida, S.; Yamaya, H.; Hiradate, S.; Hasegawa, M.; Toshima, H.; Koitabashi, M.; et al. Nocardioides sp. strain WSN05-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl. Microbiol. Biotechnol. 2011, 89, 419–427. [Google Scholar]
- Miller, D.J.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; McCormick, S.P.; Muehlbauer, G.J. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Mol. Plant-Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [CrossRef]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Frohlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef]
- Ito, M.; Sato, I.; Ishizaka, M.; Yoshida, S.; Koitabashi, M.; Yoshida, S.; Tsushima, S. Bacterial cytochrome P450 system catabolizing the Fusarium toxin deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1619–1628. [Google Scholar] [CrossRef]
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Xing, L.; Gao, L.; Chen, Q.; Pei, H.; Di, Z.; Xiao, J.; Wang, H.; Ma, L.; Chen, P.; Cao, A.; et al. Over-expressing a UDP-glucosyltransferase gene (Ta-UGT3) enhances fusarium head blight resistance of wheat. Plant Growth Regul. 2018, 84, 561–571. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Li, Y.-X.; Sheldon, R.A. Stabilities and rates in the laccase/TEMPO-catalyzed oxidation of alcohols. Biocatal. Biotransform. 2006, 24, 443–448. [Google Scholar] [CrossRef]
- Kobakhidze, A.; Elisashvili, V.; Corvini, P.F.-X.; Čvančarová, M. Biotransformation of ritalinic acid by laccase in the presence of mediator TEMPO. New Biotechnol. 2018, 43, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhu, C.; de Lange, C.F.; Zhou, T.; He, J.; Yu, H.; Gong, J.; Young, J.C. Efficacy of detoxification of deoxynivalenol-contaminated corn by Bacillus sp. LS100 in reducing the adverse effects of the mycotoxin on swine growth performance. Food Addit. Contam. 2011, 28, 894–901. [Google Scholar]
- Li, X.-Z.; Hassan, Y.I.; Lepp, D.; Zhu, Y.; Zhou, T. 3-keto-DON, but not 3-epi-DON, retains the in planta toxicological potential after the enzymatic biotransformation of deoxynivalenol. Int. J. Mol. Sci. 2022, 23, 7230. [Google Scholar] [CrossRef]
- Larson, T.M.; Anderson, A.M.; Rich, J.O. Combinatorial evaluation of laccase-mediator system in the oxidation of veratryl alcohol. Biotechnol. Lett. 2013, 35, 225–231. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanakhat, H.; McCormick, S.P.; Busman, M.; Rich, J.O.; Bakker, M.G. Modification of Deoxynivalenol by a Fungal Laccase Paired with Redox Mediator TEMPO. Toxins 2022, 14, 548. https://doi.org/10.3390/toxins14080548
Shanakhat H, McCormick SP, Busman M, Rich JO, Bakker MG. Modification of Deoxynivalenol by a Fungal Laccase Paired with Redox Mediator TEMPO. Toxins. 2022; 14(8):548. https://doi.org/10.3390/toxins14080548
Chicago/Turabian StyleShanakhat, Hina, Susan P. McCormick, Mark Busman, Joseph O. Rich, and Matthew G. Bakker. 2022. "Modification of Deoxynivalenol by a Fungal Laccase Paired with Redox Mediator TEMPO" Toxins 14, no. 8: 548. https://doi.org/10.3390/toxins14080548
APA StyleShanakhat, H., McCormick, S. P., Busman, M., Rich, J. O., & Bakker, M. G. (2022). Modification of Deoxynivalenol by a Fungal Laccase Paired with Redox Mediator TEMPO. Toxins, 14(8), 548. https://doi.org/10.3390/toxins14080548





