Evaluation of the Toxicity of Chemical and Biogenic Insecticides to Three Outbreaking Insects in Desert Steppes of Northern China
Abstract
:1. Introduction
2. Results
2.1. Toxicity of Insecticides to Oedaleus asiaticus from the Three Regions Studied
2.2. Toxicity of Insecticides to Myrmeleotettix palpalis from SZWQ and XHQ
2.3. Toxicity of Insecticides to Galeruca daurica from SZWQ and XHQ
3. Discussion
4. Conclusions
5. Materials and Methods
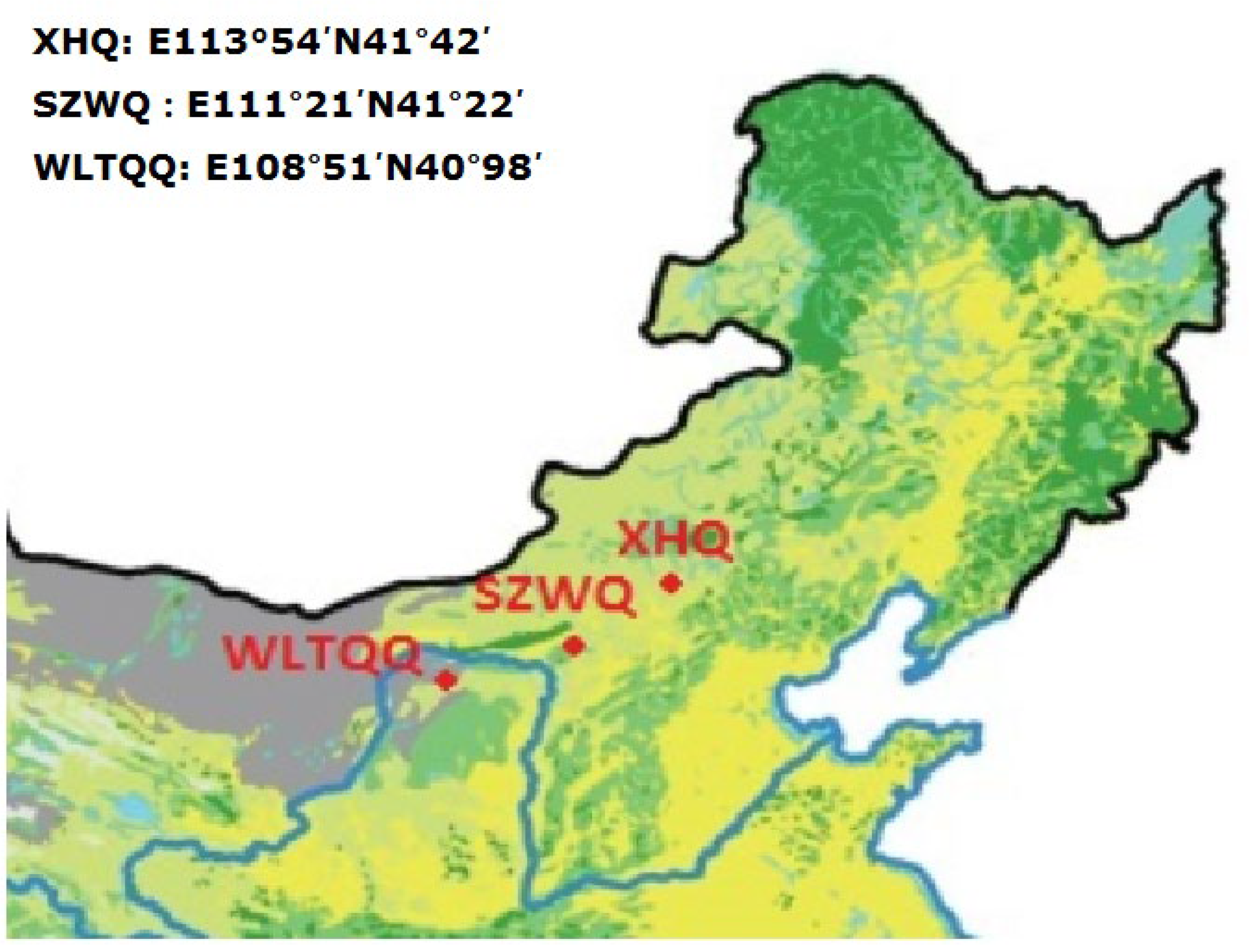
5.1. Insects
5.2. Insecticides and Chemicals
5.3. Bioassay
5.3.1. Topical Application
5.3.2. Leaf-Dip Bioassay
5.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wang, X.; Bai, M.; Shaw, J.J. Decrease in carabid beetles in grasslands of northwestern China: Further evidence of insect biodiversity loss. Insects 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fu, B.; Wei, W.; Yu, X.; Sun, R. Major ecosystems in China: Dynamics and challenges for sustainable management. Environ. Manag. 2011, 48, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, J.; Yue, T. Grassland degradation restoration and constructing green ecological protective screen. In Remote Sensing Monitoring and Evaluation of Degraded Grassland in China; Zhou, W., Li, J., Yue, T., Eds.; Springer Geography: Singapore, 2020; pp. 125–138. [Google Scholar]
- Zuo, X.; Zhao, H.; Zhao, X.; Guo, Y.; Yun, J.; Wang, S.; Miyasaka, T. Vegetation pattern variation, soil degradation and their relationship along a grassland desertification gradient in Horqin Sandy Land, northern China. Environ. Geol. 2009, 58, 1227–1237. [Google Scholar] [CrossRef]
- Han, D.; Wang, G.; Xue, B.; Liu, T.; Yinglan, A.; Xu, X. Evaluation of semiarid grassland degradation in North China from multiple perspectives. Ecol. Eng. 2018, 112, 41–50. [Google Scholar] [CrossRef]
- Wenhua, L. Degradation and restoration of forest ecosystems in China. For. Ecol. Manag. 2004, 201, 33–41. [Google Scholar] [CrossRef]
- Kang, L.; Han, X.; Zhang, Z.; Sun, O.J. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B 2007, 362, 997–1008. [Google Scholar] [CrossRef]
- Peters, D.P. Plant species dominance at a grassland-shrubland ecotone: An individual-based gap dynamics model of herbaceous and woody species. Ecol. Model. 2002, 152, 5–32. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Du, G.; Hu, G.; Zhang, Y.; Tu, X.; Zhang, Z. An analysis of the possible migration routes of Oedaleus decorus asiaticus Bey-Bienko (Orthoptera: Acrididae) from Mongolia to China. Insects 2022, 13, 72. [Google Scholar] [CrossRef]
- Wang, Y.P.; Tu, X.B.; Lin, P.J.; Li, S.; Xu, C.M.; Wang, X.Q.; Reynolds, D.R.; Chapman, J.; Zhang, Z.H.; Hu, G. Migratory take-off behavior of the Mongolian grasshopper Oedaleus asiaticus. Insects 2020, 11, 416. [Google Scholar] [CrossRef]
- Le Gall, M.; Overson, R.; Cease, A. A global review on locusts (Orthoptera: Acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol. 2019, 7, 263. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ba, L. Ecology of meadow steppe in northeast China. Rangel. J. 2008, 30, 247–254. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, X.R.; Pang, B.P. Reference gene selection and evaluation for expression analysis using qRT-PCR in Galeruca daurica (Joannis). Bull. Entomol. Res. 2017, 107, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Huang, X.; Li, S.; Hao, K.; Chang, B.H.; Tu, X.; Pang, B.; Zhang, Z. Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2019, 112, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.B.; Zhang, X.Y.; Wu, H.H.; Zhang, M.; Ma, E.B.; Zhang, J.Z. Susceptibility and potential biochemical mechanism of Oedaleus asiaticus to beta-cypermethrin and deltamethrin in the Inner Mongolia, China. Pestic. Biochem. Physiol. 2016, 132, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Wang, G.; Lowry, A.; Huang, W.; Dong, Y.; Shang, S.; Luke, B. The effects of vegetation type on Oedaleus decorus asiaticus (Orthoptera: Acrididae) oviposition and hatching success. Environ. Entomol. 2021, 50, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Lomer, C.J.; Bateman, R.P.; Dent, D.; De Groote, H.; Douro--Kpindou, O.K.; Kooyman, C.; Ouambama, Z.; Peveling, R.; Kooyman, C.; Thomas, M. Development of strategies for the incorporation of biological pesticides into the integrated management of locusts and grasshoppers. Agric. For. Entomol. 1999, 1, 71–88. [Google Scholar] [CrossRef]
- Liu, C.Z.; Yang, Y.B.; Ma, L.X. Spatial patterns of Myrmeleotettix palpalis and their application. Pratac. Sci. 2000, 17, 42–43. [Google Scholar]
- Li, Z.W. Studies on the biological characteristics of myrmeleotettix palpalis on bayinbulake grasslands. Plant Prot. 2008, 34, 131–132. [Google Scholar]
- Liu, C.Z.; Feng, G.H. Studies on the bionomics of Myrmeleotettix palpali. Acta Phytophyl. Sin. 1999, 26, 153–156. [Google Scholar]
- Cao, K.L.; Wang, Y.; Gao, Y.F.; Tan, S.Q.; Shi, W.P. Regulatory effects of vegetation on the behavior and population of grasshoppers. J. Plant Prot. 2021, 48, 54–59. [Google Scholar]
- Kang, L.; Chen, Y.L. Trophic niche of steppe grasshop- pers. Acta Entomol. Sin. 1994, 37, 178–189. [Google Scholar]
- Kang, L.; Chen, Y.L. Multidimensional analysis of resource utilization in assemblages of rangeland grasshoppers. Entomol. Sin. 1994, 1, 264–282. [Google Scholar]
- Tan, Y.; Zhang, Y.; Huo, Z.J.; Zhou, X.R.; Pang, B.P. Molecular cloning of heat shock protein 10 (Hsp10) and 60 (Hsp60) cDNAs from Galeruca daurica (Coleoptera: Chrysomelidae) and their expression analysis. Bull. Entomol. Res. 2018, 108, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.F.; Gao, S.J.; Wang, H.C.; Li, L.; Li, Y.Y.; Tan, Y.; Pang, B.P. MicroRNA let-7-5p targets the juvenile hormone primary response gene Krüppel homolog 1 and regulates reproductive diapause in Galeruca daurica. Insect Biochem. Mol. Biol. 2022, 142, 103727. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, L.; Li, N.; Li, Y.Y.; Pang, B.P. Expression profiling and functional analysis of candidate odorant receptors in Galeruca daurica. Insects 2022, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Li, L.; Li, Y.Y.; Tan, Y.; Pang, B.P. Evaluation of reference genes for miRNA expression analysis in Galeruca daurica (Coleoptera: Chrysomelidae) using qRT-PCR. Entomol. Res. 2021, 51, 393–402. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Stepnowski, P.; Gołębiowski, M. The use of insecticides to control insect pests. ISJ—Invert. Surviv. J. 2016, 13, 210–220. [Google Scholar]
- Liu, J.W.; Zhang, Y.J.; Li, Y.J.; Wang, D.L.; Hou, F.J. Overview of grassland and its development in China. Proc. Int. Grassl. Congr. Int. Rangel. Congr. 2008, 6, 3–10. [Google Scholar]
- Magor, J.I.; Lecoq, M.; Hunter, D.M. Preventive control and desert locust plagues. Crop Prot. 2008, 27, 1527–1533. [Google Scholar] [CrossRef]
- Carruthers, R.I.; Onsager, J.A. Perspective on the use of exotic natural enemies for biological control of pest grasshoppers (Orthoptera: Acrididae). Environ. Entomol. 1993, 22, 885–903. [Google Scholar] [CrossRef]
- Cease, A.J.; Elser, J.J.; Ford, C.F.; Hao, S.; Kang, L.; Harrison, J.F. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 2012, 335, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.G.; Tu, X.B.; Liu, X.G.; Zhang, L.; Zhang, Y.; Zhu, L.Z.; Zhang, Y.Q.; Jiang, I.H. Advances and prospects of pesticides registered for controlling locusts and grasshoppers in China. Mod. Agrochem. 2021, 20, 1–7. [Google Scholar]
- Cao, G.; Jia, M.; Zhao, X.; Wang, L.; Tu, X.; Wang, G.; Nong, X.; Zhang, Z. Effects of chlorantraniliprole on detoxification enzymes activities in Locusta migratoria L. J. Asia-Pac. Entomol. 2017, 20, 741–746. [Google Scholar] [CrossRef]
- Matthews, G.; Bateman, R.; Miller, P. Pesticide Application Methods; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Guo, K.U.N.; Hao, S.G.; Sun, O.J.; Kang, L.E. Differential responses to warming and increased precipitation among three contrasting grasshopper species. Glob. Change Biol. 2009, 15, 2539–2548. [Google Scholar] [CrossRef]
- Dutcher, J.D. A review of resurgence and replacement causing pest outbreaks in IPM. Gen. Concepts Integr. Pest Dis. Manag. 2007, 1, 27–43. [Google Scholar]
- Silva, A.P.B.; Santos, J.M.M.; Martins, A.J. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids-a review. Parasites Vectors 2014, 7, 450. [Google Scholar] [CrossRef] [Green Version]
- Jesser, E.; Yeguerman, C.; Gili, V.; Santillan, G.; Murray, A.P.; Domini, C.; Werdin-González, J.O. Optimization and characterization of essential oil nanoemulsions using ultrasound for new ecofriendly insecticides. ACS Sustain. Chem. Eng. 2020, 8, 7981–7992. [Google Scholar] [CrossRef]
- China Pesticide. Available online: http://www.chinapesticide.org.cn/hysj/index.jhtml (accessed on 2 July 2022).
- Li, H.; Zhang, H.; Pang, B.; Jing, C. Toxicities of four pyrethroids and their inhibition to ATPase in Oedaleus asiaticus. Plant Prot. 2014, 40, 90–94. [Google Scholar]
- Bao, L.I.; Xiao-Mei, W.U.; Zhang, A.G.; Jun-Hu, S.U.; Zhou, E.F.; Liu, Q.; Yan, K.J.; Wang, H.P.; Yao, J.G.; Zhao, M.Q. Control efficiency of cypermethrin 4.5%EC on mixed population of grassland locusts. Grassl. Turf 2013, 2, 13–17. [Google Scholar]
- Pilz, C.; Keller, S.; Kuhlmann, U.; Toepfer, S. Comparative efficacy assessment of fungi, nematodes and insecticides to control western corn rootworm larvae in maize. BioControl 2009, 54, 671–684. [Google Scholar] [CrossRef]
- Hunter, D.M.; Milner, R.J.; Spurgin, P.A. Aerial treatment of the Australian plague locust, Chortoicetes terminifera (Orthoptera: Acrididae) with Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Bull. Entomol. Res. 2001, 91, 93–99. [Google Scholar] [PubMed]
- Li, S.; Xu, C.; Du, G.; Wang, G.; Tu, X.; Zhang, Z. Synergy in efficacy of Artemisia sieversiana crude extract and Metarhizium anisopliae on resistant Oedaleus asiaticus. Front. Physiol. 2021, 12, 642893. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.M.; Donzelli, B.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef]
- Guo, Y.; An, Z.; Shi, W. Control of grasshoppers by combined application of Paranosema locustae and an insect growth regulator (IGR) (cascade) in rangelands in China. J. Econ. Entomol. 2012, 105, 1915–1920. [Google Scholar] [CrossRef]
- Chowański, S.; Kudlewska, M.; Marciniak, P.; Rosiński, G. Synthetic insecticides-is there an alternative? Pol. J. Environ. Stud. 2014, 23, 291–302. [Google Scholar]
- Sparks, T.C.; Crouse, G.D.; Durst, G. Natural products as insecticides: The biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag. Sci. 2001, 57, 896–905. [Google Scholar] [CrossRef]
- Michaud, J.P.; Grant, A.K. IPM-compatibility of foliar insecticides for citrus: Indices derived from toxicity to beneficial insects from four orders. J. Insect Sci. 2003, 3, 18. [Google Scholar] [CrossRef]
- Dubey, N.K.; Kumar, A.; Singh, P.; Shukla, R. Exploitation of natural compounds in eco-friendly management of plant pests. In Recent Developments in Management of Plant Diseases; Gisi, U., Chet, I., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 181–198. [Google Scholar]
- Costa, E.M.; Araujo, E.L.; Maia, A.V.; Silva, F.E.; Bezerra, C.E.; Silva, J.G. Toxicity of insecticides used in the Brazilian melon crop to the honey bee Apis mellifera under laboratory conditions. Apidologie 2014, 45, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Hamaidia, K.; Soltani, N. Methoxyfenozide, a molting hormone agonist, affects autogeny capacity, oviposition, fecundity, and fertility in Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.R.; Dhadialla, T.S.; Hunter, R.; Jansson, R.K.; Jany, C.S.; Lidert, Z.; Slawecki, R.A. The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag. Sci. 2001, 57, 115–119. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Helmy, Y.S.; Helmy, W.S. Toxicity and biochemical impact of methoxyfenozide/pinetoram mixture on susceptible and methoxyfenozide-selected strains of Spodoptera littoralis (Lepidoptera: Noctuidae). Sci. Reports 2022, 12, 6974. [Google Scholar]
- Abbott, W.S.A. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Robertson, J.L.; Savin, N.E.; Preisler, H.K.; Russell, R.M. Bioassays with Arthropods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849323317. [Google Scholar]
| Insecticide | Chemical Structure | Year | Population | LC50 (95% FL) [ng a.i./Adult] | Slope ± SE | X2 (df a) | RTI b (95% FL) |
|---|---|---|---|---|---|---|---|
| Phoxim | 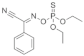 | 2021 | SZWQ | 301.58 [232.18–374.85] | 2.50 ± 0.37 | 0.41 (3) | 1 |
| 2021 | XHQ | 369.96 [289.96–460.10] | 2.42 ± 0.37 | 0.25 (3) | 1.23 | ||
| 2022 | WLTQQ | 281.90 [214.42–348.85] | 2.89 ± 0.46 | 0.65 (3) | 0.93 | ||
| Methomyl | 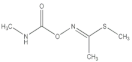 | 2021 | SZWQ | 940.13 [798.65–1102.83] | 4.52 ± 0.82 | 0.74 (3) | 1 |
| 2021 | XHQ | 954.24 [783.35–1163.26] | 3.26 ± 0.57 | 0.99 (3) | 1.02 | ||
| 2022 | WLTQQ | 906.55 [764.05–1061.96] | 4.80 ± 0.92 | 0.46 (3) | 0.96 | ||
| Imidacloprid |  | 2021 | SZWQ | 292.89 [67.35–591.22] | 0.97 ± 0.18 | 1.42 (3) | 1 |
| 2021 | XHQ | 717.91 [531.21–924.09] | 2.03 ± 0.31 | 0.23 (5) | 2.45 | ||
| 2022 | WLTQQ | 462.59 [331.11–612.17] | 1.80 ± 0.29 | 0.85 (3) | 1.58 | ||
| λ-cyhalothrin | 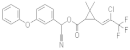 | 2021 | SZWQ | 334.90 [178.72–533.40] | 0.99 ± 0.17 | 0.53 (4) | 1 |
| 2021 | XHQ | 878.04 [560.79–1281.50] | 1.39 ± 0.26 | 0.48 (3) | 2.62 | ||
| 2022 | WLTQQ | 546.12 [317.84–788.50] | 1.48 ± 0.28 | 0.66 (4) | 1.63 | ||
| β-cypermeth- rin | 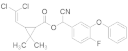 | 2021 | SZWQ | 213.48 [110.20–370.33] | 1.06 ± 0.23 | 0.57 (4) | 1 |
| 2021 | XHQ | 286.34 [208.80–405.16] | 2.10 ± 0.49 | 0.65 (4) | 1.68 | ||
| 2022 | WLTQQ | 174.95 [89.08–281.70] | 1.37 ± 0.29 | 0.67 (4) | 0.82 | ||
| Chlorantrani- liprole |  | 2021 | SZWQ | 1116.63 [375.05–4949.58] | 1.01 ± 0.17 | 2.10 (4) | 1 |
| 2021 | XHQ | 1017.52 [605.26–1709.82] | 1.04 ± 0.21 | 0.85 (4) | 0.91 | ||
| 2022 | WLTQQ | 435.50 [171.75–827.09] | 1.04 ± 0.18 | 1.05 (4) | 0.39 | ||
| Avermectin c |  | 2021 | SZWQ | 11.99 μg [9.50–15.70] | 1.99 ± 0.32 | 0.50 (4) | 1 |
| 2021 | XHQ | 15.09 μg [12.10–19.82] | 2.34 ± 0.42 | 0.05 (4) | 1.26 | ||
| Spinosad | 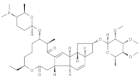 | 2021 | SZWQ | 488.06 [301.24–730.80] | 1.44 ± 0.27 | 0.68 (4) | 1 |
| 2021 | XHQ | 571.48 [388.62–892.36] | 1.20 ± 0.18 | 0.34 (5) | 1.17 | ||
| 2022 | WLTQQ | 578.99 [386.96–842.43] | 1.54 ± 0.26 | 0.92 (4) | 1.18 | ||
| Matrine | 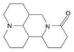 | 2022 | SZWQ | 1702.98 [455.41–2149.16] | 3.16 ± 0.85 | 0.38 (3) | 1 |
| 2022 | XHQ | 1147.01 [748.61–1464.61] | 2.73 ± 0.62 | 0.81 (3) | 0.67 | ||
| 2022 | WLTQQ | 1727.01 [1129.87–2221.80] | 2.87 ± 0.80 | 0.34 (3) | 1.01 | ||
| Azadirachtin | 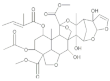 | 2022 | SZWQ | 1811.89 [1179.77–2393.49] | 2.54 ± 0.75 | 0.51 (3) | 1 |
| 2022 | XHQ | 1198.20 [451.57–1820.36] | 2.53 ± 0.52 | 1.10 (3) | 0.66 | ||
| 2022 | WLTQQ | 1069.17 [710.83–1412.23] | 2.50 ± 0.63 | 0.46 (3) | 0.59 | ||
| Rotenone | 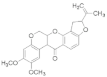 | 2022 | SZWQ | 750.63 [494.29–1015.93] | 2.08 ± 0.44 | 0.98 (3) | 1 |
| 2022 | XHQ | 930.94 [552.91–1622.65] | 1.17 ± 0.28 | 0.62 (3) | 1.24 | ||
| 2022 | WLTQQ | 737.61 [420.29–1028.81] | 2.10 ± 0.52 | 0.73 (3) | 0.98 |
| Insecticide | Year | Population | LC50 (95% FL) [ng a.i./Adult] | Slope ± SE | X2(df a) | RTI b (95% FL) |
|---|---|---|---|---|---|---|
| Phoxim | 2021 | SZWQ | 216.72 [134.98–290.42] | 2.03 ± 0.40 | 1.05 (6) | 1 |
| 2022 | XHQ | 247.89 [163.68–309.71] | 3.67 ± 0.66 | 1.03 (4) | 1.14 | |
| Methomyl | 2021 | SZWQ | 200.95 [126.87–263.76] | 3.30 ± 0.55 | 1.31 (5) | 1 |
| 2022 | XHQ | 164.64 [121.86–200.89] | 2.88 ± 0.52 | 0.44 (4) | 0.82 | |
| Imidacloprid | 2021 | SZWQ | 101.94 [61.44–172.82] | 1.10 ± 0.21 | 0.78 (3) | 1 |
| 2022 | XHQ | 109.99 [87.76–133.16] | 3.39 ± 0.55 | 0.10 (3) | 1.08 | |
| λ-cyhalothrin | 2021 | SZWQ | 77.73 [55.00–98.52] | 2.68 ± 0.50 | 0.21 (3) | 1 |
| 2022 | XHQ | 88.39 [66.06–114.89] | 2.11 ± 0.32 | 0.22 (3) | 1.14 | |
| β-cypermethrin | 2021 | SZWQ | 279.73 [87.10–684.14] | 1.44 ± 0.22 | 2.51 (4) | 1 |
| 2022 | XHQ | 215.54 [95.76–376.44] | 1.59 ± 0.27 | 1.55 (4) | 0.77 | |
| Chlorantraniliprole | 2021 | SZWQ | 278.06 [118.76–447.50] | 2.58 ± 0.48 | 1.30 (3) | 1 |
| 2022 | XHQ | 339.89 [257.88–424.45] | 2.23 ± 0.31 | 0.83 (4) | 1.22 | |
| Avermectin c | 2021 | SZWQ | 2.17 μg [1.33–3.16] | 1.80 ± 0.27 | 1.28 (5) | 1 |
| 2022 | XHQ | 3.03 μg [2.35–3.84] | 1.96 ± 0.34 | 0.35 (4) | 1.40 | |
| Spinosad | 2021 | SZWQ | 35.45 [23.65–48.43] | 1.78 ± 0.32 | 0.28 (4) | 1 |
| 2022 | XHQ | 49.96 [32.71–70.96] | 1.69 ± 0.33 | 0.10 (4) | 1.41 | |
| Matrine | 2021 | SZWQ | 526.71 [386.95–665.46] | 2.37 ± 0.38 | 0.42 (5) | 1 |
| 2022 | XHQ | 749.73 [438.05–1249.35] | 1.16 ± 0.30 | 1.09 (4) | 1.42 | |
| Azadirachtin | 2021 | SZWQ | 924.86 [720.98–1137.62] | 2.78 ± 0.56 | 0.56 (3) | 1 |
| 2022 | XHQ | 662.80 [404.34–918.39] | 2.09 ± 0.63 | 0.66 (3) | 0.72 | |
| Rotenone | 2022 | SZWQ | 337.50 [198.57–469.59] | 1.85 ± 0.40 | 0.77 (4) | 1 |
| 2022 | XHQ | 198.20 [104.75–282.40] | 1.92 ± 0.40 | 0.60 (5) | 0.59 |
| Insecticide | Year | Population | LC50 (95% FL) [mg/L] | Slope ± SE | X2(df a) | RTI b (95% FL) |
|---|---|---|---|---|---|---|
| Phoxim | 2022 | SZWQ | 2.91 [1.53–4.74] | 1.39 ± 0.27 | 0.20 (3) | 1 |
| 2022 | XHQ | 0.66 [0.26–1.24] | 1.09 ± 0.23 | 0.72 (3) | 0.26 | |
| Methomyl | 2022 | SZWQ | 40.77 [27.30–52.25] | 2.99 ± 0.80 | 0.54 (3) | 1 |
| 2022 | XHQ | 31.91 [22.55–39.91] | 3.12 ± 0.69 | 0.79 (3) | 0.78 | |
| Imidacloprid | 2022 | SZWQ | 0.17 [0.10–0.31] | 0.97 ± 0.15 | 0.59 (3) | 1 |
| 2022 | XHQ | 0.16 [0.07–0.32] | 0.85 ± 0.17 | 0.28 (4) | 6.82 | |
| λ-cyhalothrin | 2022 | SZWQ | 0.73 [0.46–1.09] | 1.69 ± 0.28 | 0.66 (3) | 1 |
| 2022 | XHQ | 0.56 [0.30–0.92] | 1.24 ± 0.22 | 0.25 (3) | 0.77 | |
| β-cypermethrin | 2022 | SZWQ | 1.57 [0.91–2.61] | 1.41 ± 0.27 | 0.55 (3) | 1 |
| 2022 | XHQ | 1.06 [0.55–1.86] | 1.16 ± 0.24 | 0.58 (3) | 0.68 | |
| Chlorantraniliprole | 2021 | SZWQ | 56.12 [42.69–71.81] | 2.03 ± 0.27 | 0.27 (4) | 1 |
| 2021 | XHQ | 38.17 [12.27–105.19] | 1.40 ± 0.20 | 2.65 (4) | 0.68 | |
| Methoxyfenozide c * | 2021 | SZWQ | 3205.64 [2088.01–4319.27] | 2.80 ± 0.59 | 1.12 (4) | 1 |
| 2021 | XHQ | 3802.80 [3008.44–4738.47] | 3.53 ± 1.19 | 0.04 (3) | 1.19 | |
| Spinosad | 2022 | SZWQ | 50.10 [33.78–64.40] | 3.00 ± 0.66 | 0.30 (3) | 1 |
| 2022 | XHQ | 28.12 [8.64–39.70] | 2.46 ± 0.81 | 0.06 (2) | 0.56 | |
| Matrine | 2021 | SZWQ | 2267.18 [902.28–3977.09] | 1.78 ± 0.35 | 1.11 (4) | 1 |
| 2021 | XHQ | 2269.82 [1384.18–3492.35] | 1.45 ± 0.26 | 0.67 (4) | 4.41 | |
| Azadirachtin | 2021 | SZWQ | 3456.99 [1991.59–5242.97] | 1.60 ± 0.42 | 0.20 (3) | 1 |
| 2021 | XHQ | 2982.66 [1788.12–4133.05] | 1.91 ± 0.38 | 0.10 (4) | 0.86 | |
| Rotenone | 2021 | SZWQ | 751.73 [529.19–1050.16] | 1.91 ± 0.38 | 0.82 (3) | 1 |
| 2021 | XHQ | 660.39 [350.54–1096.59] | 2.17 ± 0.34 | 1.18 (3) | 1.44 |
 .
.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ren, H.; Sun, F.; Shen, T.; Yuan, S.; Gao, X.; Tan, Y. Evaluation of the Toxicity of Chemical and Biogenic Insecticides to Three Outbreaking Insects in Desert Steppes of Northern China. Toxins 2022, 14, 546. https://doi.org/10.3390/toxins14080546
Zhang W, Ren H, Sun F, Shen T, Yuan S, Gao X, Tan Y. Evaluation of the Toxicity of Chemical and Biogenic Insecticides to Three Outbreaking Insects in Desert Steppes of Northern China. Toxins. 2022; 14(8):546. https://doi.org/10.3390/toxins14080546
Chicago/Turabian StyleZhang, Wenbing, Hao Ren, Feilong Sun, Tingting Shen, Shuai Yuan, Xiwu Gao, and Yao Tan. 2022. "Evaluation of the Toxicity of Chemical and Biogenic Insecticides to Three Outbreaking Insects in Desert Steppes of Northern China" Toxins 14, no. 8: 546. https://doi.org/10.3390/toxins14080546
APA StyleZhang, W., Ren, H., Sun, F., Shen, T., Yuan, S., Gao, X., & Tan, Y. (2022). Evaluation of the Toxicity of Chemical and Biogenic Insecticides to Three Outbreaking Insects in Desert Steppes of Northern China. Toxins, 14(8), 546. https://doi.org/10.3390/toxins14080546





