Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Results
2.1. Patients
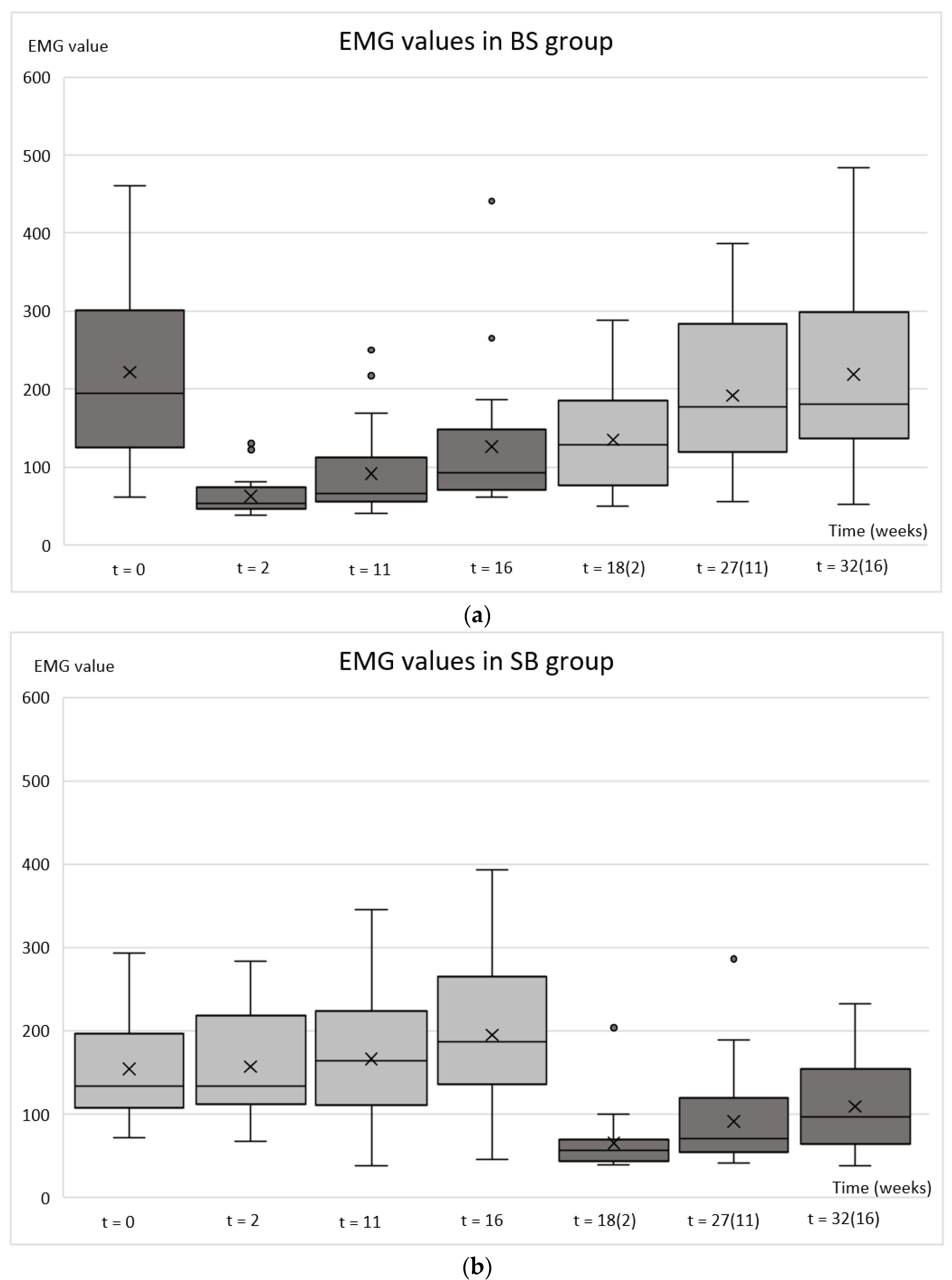
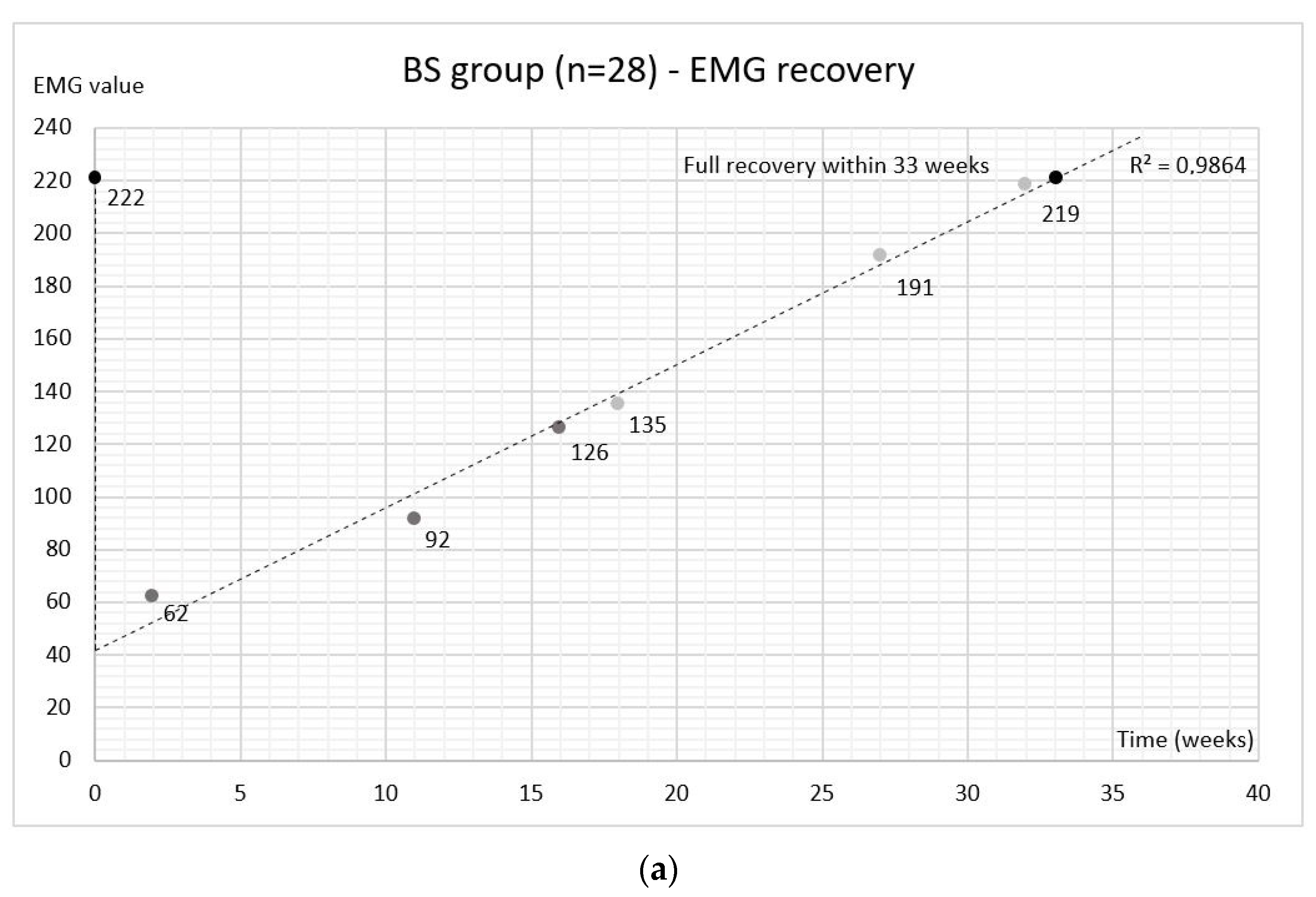
2.2. Electromyography (EMG)
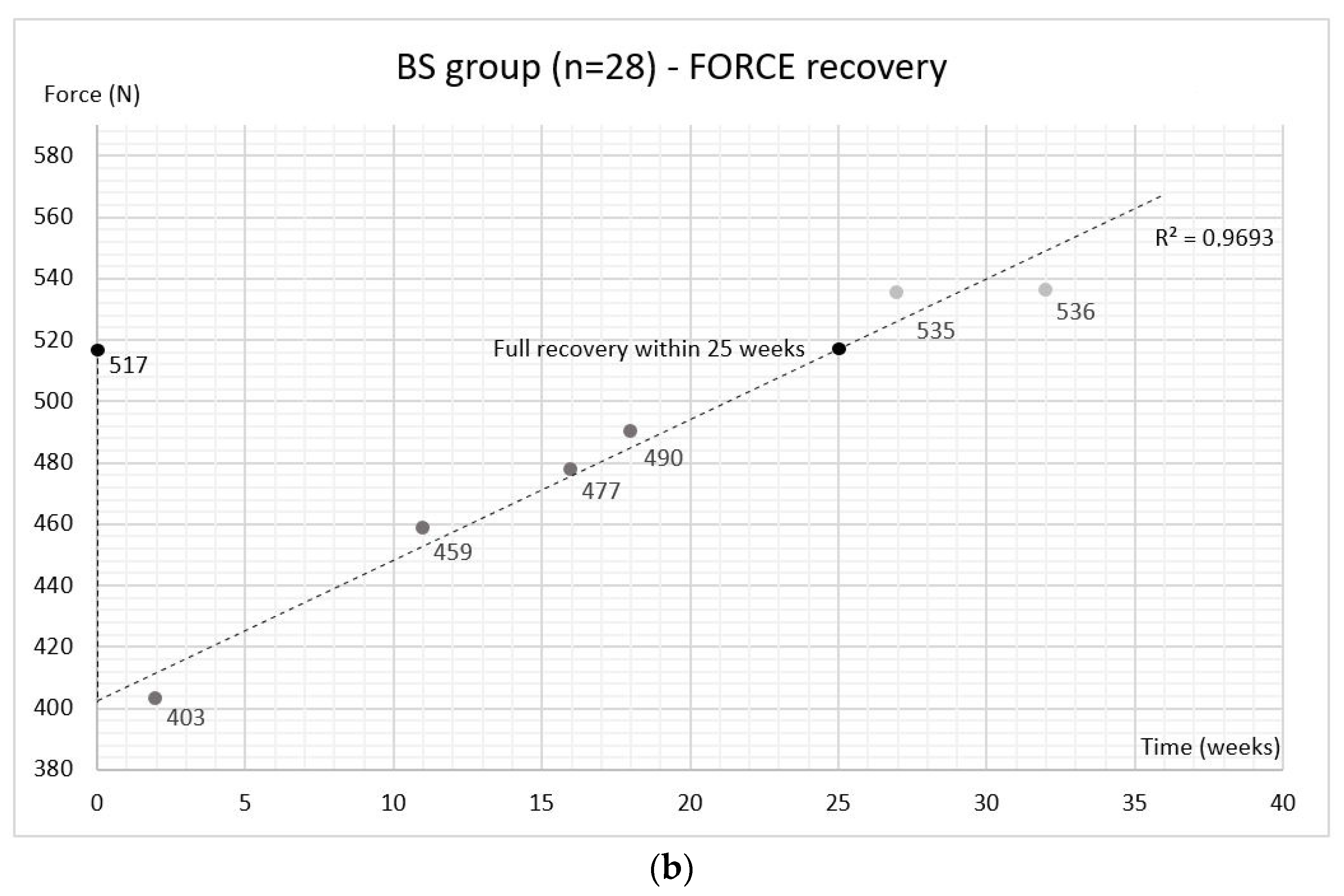
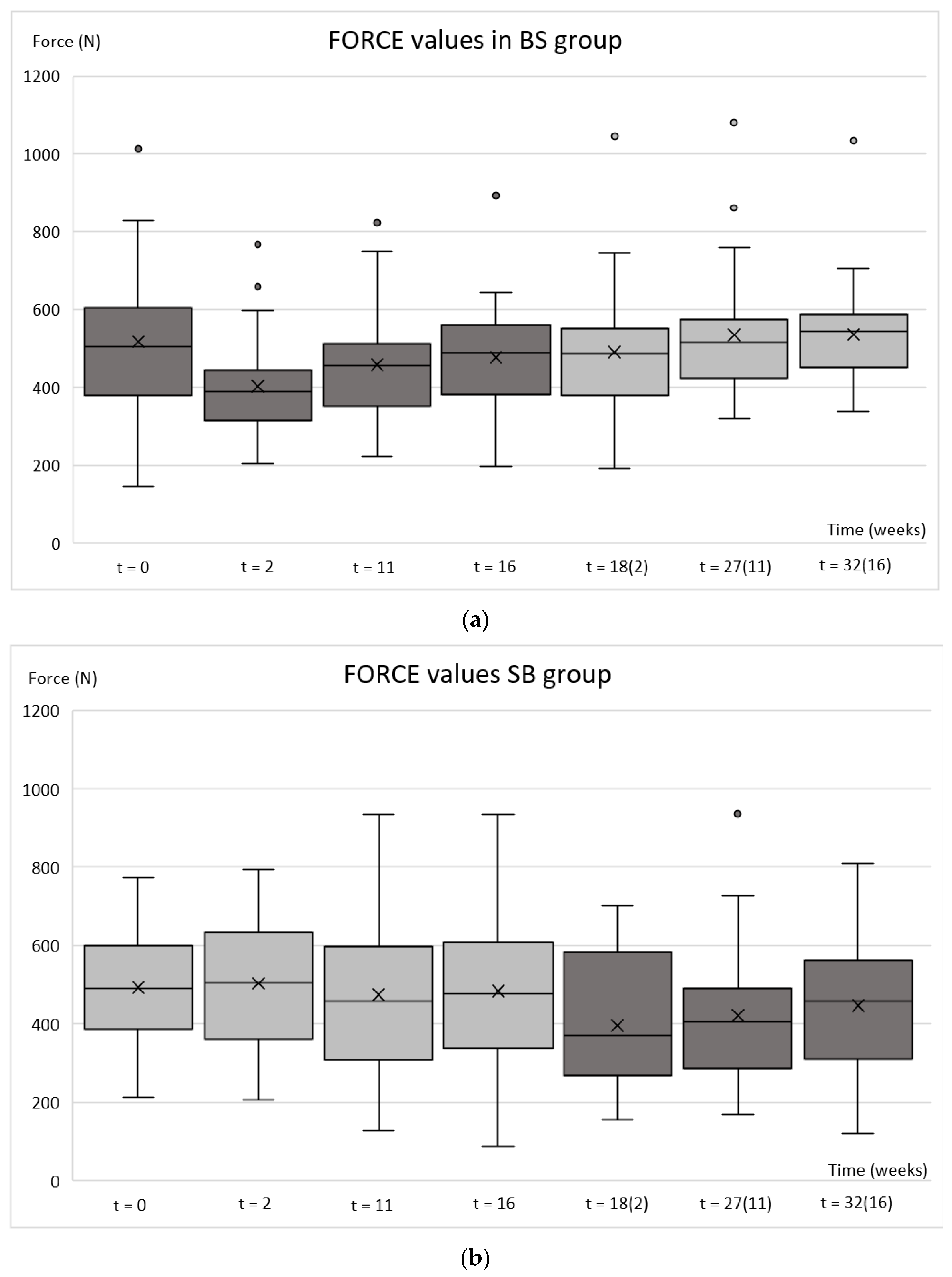
2.3. Bite Force
2.4. Harm
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Experimental Protocol
5.3. Injections
5.4. Treatment Outcomes
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manfredini, D.; Ahlberg, J.; Winocur, E.; Guarda-Nardini, L.; Lobbezoo, F. Correlation of RDC/TMD axis I diagnoses and axis II pain-related disability. A multicenter study. Clin. Oral Investig. 2011, 15, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, S.; Benoliel, R. Chronic orofacial pain. J. Neural Transm. 2020, 127, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Fricton, J.R. The relationship of temporomandibular disorders and fibromyalgia: Implications for diagnosis and treatment. Curr. Pain Headache Rep. 2004, 8, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Guarda-Nardini, L.; Stecco, A.; Stecco, C.; Masiero, S.; Manfredini, D. Myofascial pain of the jaw muscles: Comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio 2012, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Lerner, M.Z.; Blitzer, A. IncobotulinumtoxinA Injection for Temporomandibular Joint Disorder. Ann. Otol. Rhinol. Laryngol. 2017, 126, 328–333. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Poluha, R.L.; Lora, V.M.; Araújo Oliveira Ferreira, D.M.; Stuginski-Barbosa, J.; Bonjardim, L.R.; Cury, A.A.D.B.; Conti, P.C.R. Botulinum toxin type A applications for masticatory myofascial pain and trigeminal neuralgia: What is the evidence regarding adverse effects? Clin. Oral Investig. 2019, 23, 3411–3421. [Google Scholar] [CrossRef]
- la Fleur, P.; Adams, A. Botulinum Toxin for Temporomandibular Disorders: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines [Internet]; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020.
- Serrera-Figallo, M.A.; Ruiz-de-León-Hernández, G.; Torres-Lagares, D.; Castro-Araya, A.; Torres-Ferrerosa, O.; Hernández-Pacheco, E.; Gutierrez-Perez, J.L. Use of Botulinum Toxin in Orofacial Clinical Practice. Toxins 2020, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, P.S.; Lowe, N.J. Botulinum toxin types A and B: Comparison of efficacy, duration, and dose-ranging studies for the treatment of facial rhytides and hyperhidrosis. Clin. Dermatol. 2004, 22, 34–39. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, S.G.; Kim, Y.J.; Han, J.Y.; Choi, S.H.; Lee, S.I. Electrophysiologic change and facial contour following botulinum toxin A injection in square faces. Plast. Reconstr. Surg. 2007, 120, 769–778. [Google Scholar] [CrossRef]
- Balanta-Melo, J.; Toro-Ibacache, V.; Kupczik, K.; Buvinic, S. Mandibular Bone Loss after Masticatory Muscles Intervention with Botulinum Toxin: An Approach from Basic Research to Clinical Findings. Toxins 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, A.; Kün-Darbois, J.D.; Bertin, H.; Corre, P.; Chappard, D. Mandibular bone effects of botulinum toxin injections in masticatory muscles in adult. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.C.; Liu, Z.J.; Rafferty, K.L.; Keith, A.; Tamasas, B.; Kaiyala, K.; Herring, S.W. Botulinum toxin in the masseter muscle: Lingering effects of denervation. Anat. Rec. 2022, 305, 1215–1230. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, R.H.; Park, J.B. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast. Reconstr. Surg. 2010, 125, 1693–1705. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Alvarez-Pinzon, N.; Muñoz-Lora, V.R.M.; Vieira Peroni, L.; Farias Gomes, A.; Sánchez-Ayala, A.; Haiter-Neto, F.; Manfredini, D.; Rizzatti-Barbosa, C.M. Efficacy and Safety of Botulinum Toxin Type A on Persistent Myofascial Pain: A Randomized Clinical Trial. Toxins 2020, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- De La Torre Canales, G.; Câmara-Souza, M.B.; Poluha, R.L.; Grillo, C.M.; Conti, P.C.R.; Sousa, M.D.L.R.; Rodrigues Garcia, R.C.M.; Rizzatti-Barbosa, C.M. Botulinum toxin type A and acupuncture for masticatory myofascial pain: A randomized clinical trial. J. Appl. Oral Sci. 2021, 29, e20201035. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, C.; Gur, O.H.; Kurkcu, M.; Sertdemir, Y.; Guler-Uysal, F.; Uysal, H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J. Oral Maxillofac. Surg. 2008, 66, 1644–1651. [Google Scholar] [CrossRef]
- Zhang, L.D.; Liu, Q.; Zou, D.R.; Yu, L.F. Occlusal force characteristics of masseteric muscles after intramuscular injection of botulinum toxin A(BTX—A)for treatment of temporomandibular disorder. Br. J. Oral Maxillofac. Surg. 2016, 54, 736–740. [Google Scholar] [CrossRef]
- Jadhao, V.A.; Lokhande, N.; Habbu, S.G.; Sewane, S.; Dongare, S.; Goyal, N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J. Dent. Res. 2017, 28, 493–497. [Google Scholar] [CrossRef]
- Pihut, M.; Wisniewska, G.; Majewski, P.; Gronkiewicz, K.; Majewski, S. Measurement of occlusal forces in the therapy of functional disorders with the use of botulinum toxin type A. J. Physiol. Pharmacol. 2009, 60 (Suppl. 8), 113–116. [Google Scholar]
- Bettis, T.; Kim, B.J.; Hamrick, M.W. Impact of muscle atrophy on bone metabolism and bone strength: Implications for muscle-bone crosstalk with aging and disuse. Osteoporos. Int. 2018, 29, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.G.; Tadinada, A.; Bradshaw, J.M.; Janal, M.N.; Sirois, D.A.; Chan, K.C.; Lurie, A.G. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: A pilot study. J. Oral Rehabil. 2014, 41, 555–563. [Google Scholar] [CrossRef]
- Buvinic, S.; Balanta-Melo, J.; Kupczik, K.; Vásquez, W.; Beato, C.; Toro-Ibacache, V. Muscle-Bone Crosstalk in the Masticatory System: From Biomechanical to Molecular Interactions. Front. Endocrinol. 2021, 11, 606947. [Google Scholar] [CrossRef]
- Bueno, C.H.; Pereira, D.D.; Pattussi, M.P.; Grossi, P.K.; Grossi, M.L. Gender differences in temporomandibular disorders in adult populational studies: A systematic review and meta-analysis. J. Oral Rehabil. 2018, 45, 720–729. [Google Scholar] [CrossRef]
- Johansson, A.; Unell, L.; Carlsson, G.E.; Söderfeldt, B.; Halling, A. Gender difference in symptoms related to temporomandibular disorders in a population of 50-year-old subjects. J. Orofac. Pain 2003, 17, 29–35. [Google Scholar] [PubMed]
- Yekkalam, N.; Wänman, A. Associations between craniomandibular disorders, sociodemographic factors and self-perceived general and oral health in an adult population. Acta Odontol. Scand. 2014, 72, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, T.V.; Glenny, A.M.; Worthington, H.V. Systematic review of population-based epidemiological studies of orofacial pain. J. Dent. 2001, 29, 451–467. [Google Scholar] [CrossRef]
- van Selms, M.K.; Lobbezoo, F.; Visscher, C.M.; Naeije, M. Myofascial temporomandibular disorder pain, parafunctions and psychological stress. J. Oral Rehabil. 2008, 35, 45–52. [Google Scholar] [CrossRef]
- Ahlberg, J.P.; Kovero, O.A.; Hurmerinta, K.A.; Zepa, I.; Nissinen, M.J.; Könönen, M.H. Maximal bite force and its association with signs and symptoms of TMD, occlusion, and body mass index in a cohort of young adults. Cranio 2003, 21, 248–252. [Google Scholar] [CrossRef]
- Zhang, H.; Lian, Y.; Xie, N.; Chen, C.; Zheng, Y. Single-dose botulinum toxin type a compared with repeated-dose for treatment of trigeminal neuralgia: A pilot study. J. Headache Pain 2017, 18, 81. [Google Scholar] [CrossRef] [Green Version]
- de Lima, M.C.; Rizzatti Barbosa, C.M.; Duarte Gavião, M.B.; Ferreira Caria, P.H. Is low dose of botulinum toxin effective in controlling chronic pain in sleep bruxism, awake bruxism, and temporomandibular disorder? Cranio 2021, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lian, Y.; Ma, Y.; Chen, Y.; He, C.; Xie, N.; Wu, C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain 2014, 15, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purentaelimistön kipu ja toimintahäiriöt (TMD). Current Care Guidelines. Working Group Set up by the Finnish Medical Society Duodecim and the Finnish Dental Society Apollonia; The Finnish Medical Society Duodecim: Helsinki, Finland, 2021. [Google Scholar]
- Kemppainen, P.; Waltimo, A.; Waltimo, T.; Könönen, M.; Pertovaara, A. Differential effects of noxious conditioning stimulation of the cheek by capsaicin on human sensory and inhibitory masseter reflex responses evoked by tooth pulp stimulation. J. Dent. Res. 1997, 76, 1561–1568. [Google Scholar] [CrossRef]
- Suvinen, T.I.; Malmberg, J.; Forster, C.; Kemppainen, P. Postural and dynamic masseter and anterior temporalis muscle EMG repeatability in serial assessments. J. Oral Rehabil. 2009, 36, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, V.; Kämppi, A.; Teronen, O.; Kemppainen, P. Comprehensive evaluation of botulinum toxin treatment outcomes of a patient with persistent myofascial orofacial pain. Clin. Case Rep. 2021, 9, e04731. [Google Scholar] [CrossRef] [PubMed]

| Sociodemographic Characteristics | % (n) |
|---|---|
| Gender | |
| Male (age 33.8 ± 8.5 [22–51]) | 17.5 (10) |
| Female (age 39.2 ± 10.5 [24–64]) | 82.5 (47) |
| All (age 38.2 ± 10.4 [22–64]) | 100.0 (57) |
| Education | |
| High school | 5.3 (3) |
| Vocational school | 24.6 (14) |
| Polytechnic | 26.3 (15) |
| University | 38.6 (22) |
| Not reported | 5.3 (3) |
| Occupation | |
| Student | 15.8 (9) |
| Employed (Entrepreneur) | 75.4 (43) |
| Working at home | 3.5 (2) |
| Not reported | 5.3 (3) |
| Marital status | |
| Married | 35.1 (20) |
| Cohabiting | 24.6 (14) |
| Divorced | 15.8 (9) |
| Never married | 19.3 (11) |
| Not reported | 5.3 (3) |
| Diagnostic Characteristics | % (n) |
|---|---|
| DC/TMD diagnosis | |
| Myalgia | 46.4(52) |
| Myofascial pain with referral | 18.8 (21) |
| Headache attributed to TMD | 21.4 (24) |
| Arthralgia right | 2.7 (3) |
| Arthralgia left | 7.1 (8) |
| Other * | 3.6 (4) |
| Pain chronicity | |
| 1 to <5 years | 24.6 (14) |
| 5 to <10 years | 24.6 (14) |
| ≥10 years | 47.4 (27) |
| Not reported | 3.5 (2) |
| Pain frequency | |
| Persistent | 40.4 (23) |
| Recurrent | 54.4 (31) |
| One-time | 1.8 (1) |
| Not reported | 3.5 (2) |
| p Values * | ||||||
|---|---|---|---|---|---|---|
| Time Range (Weeks) | ||||||
| 0–2 | 0–11 | 0–16 | 0–18 (2) | 0–27 (11) | 0–32 (16) | |
| EMG BS group | 0.000 | 0.000 | 0.000 | 0.001 | 0.122 | 0.994 |
| EMG SB group | 0.582 | 0.079 | 0.005 | <0.001 | <0.001 | <0.001 |
| FORCE BS group | <0.001 | 0.002 | 0.167 | 0.109 | 0.838 | 0.885 |
| FORCE SB group | 0.084 | 0.922 | 0.945 | <0.001 | 0.025 | 0.520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitnikova, V.; Kämppi, A.; Teronen, O.; Kemppainen, P. Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial. Toxins 2022, 14, 545. https://doi.org/10.3390/toxins14080545
Sitnikova V, Kämppi A, Teronen O, Kemppainen P. Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial. Toxins. 2022; 14(8):545. https://doi.org/10.3390/toxins14080545
Chicago/Turabian StyleSitnikova, Victoria, Antti Kämppi, Olli Teronen, and Pentti Kemppainen. 2022. "Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial" Toxins 14, no. 8: 545. https://doi.org/10.3390/toxins14080545
APA StyleSitnikova, V., Kämppi, A., Teronen, O., & Kemppainen, P. (2022). Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial. Toxins, 14(8), 545. https://doi.org/10.3390/toxins14080545





