In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Instruments, Chemical, Fungi and Plants
4.2. Cancer Cell Culture and Viability Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Jianchu, X.; Rapior, S.; Jeewon, R.; Lumyong, S.; Nieg, A.G.T.; Pranami, D.; Abeywickrama, P.D.; ·Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diver. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- Barbero, M.; Artuso, E.; Prandi, C. Fungal anticancer metabolites: Synthesis towards drug discovery. Curr. Med. Chem. 2018, 25, 141–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Gopal, J.V.; Ren, S.; Chen, L.; Liu, L.; Gao, Z. Anticancer fungal natural products: Mechanisms of action and biosynthesis. Eur. J. Med. Chem. 2020, 202, 112502. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef]
- Crous, P.W.; Rossman, A.Y.; Aime, M.C.; Allen, W.C.; Burgess, T.; Groenewald, J.Z.; Castlebury, L.A. Names of phytopathogenic fungi: A Practical guide. Phytopathology 2021, 111, 1500–1508. [Google Scholar] [CrossRef]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. CMC 2018, 25, 208–252. [Google Scholar] [CrossRef]
- Balde, E.S.; Andolfi, A.; Bruyère, C.; Cimmino, A.; Lamoral-Theys, D.; Vurro, M.; Damme, M.V.; Altomare, C.; Mathieu, V.; Kiss, R.; et al. Investigations of fungal secondary metabolites with potential anticancer activity. J. Nat. Prod. 2010, 73, 969–971. [Google Scholar] [CrossRef]
- Mathieu, V.; Chantôme, A.; Lefranc, F.; Cimmino, A.; Miklos, W.; Paulitschke, V.; Mohr, T.; Maddau, L.; Kornienko, A.; Berger, W.; et al. Sphaeropsidin A shows promising activity against drug-resistant cancer cells by targeting regulatory volume increase. Cell. Mol. Life Sci. 2015, 72, 3731–3746. [Google Scholar] [CrossRef] [Green Version]
- Masi, M.; Dasari, R.; Evidente, A.; Mathieu, V.; Kornienko, A. Chemistry and biology of ophiobolin A and its congeners. Bioorg. Med. Chem. Lett. 2019, 29, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A. Fungal bioactive macrolides. Nat. Prod. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Cimmino, A.; Vurro, M.; Fracchiolla, M.; Charudattan, R. Herbicidal potential of ophiobolins produced by Drechslera gigantea. J. Agric. Food Chem. 2006, 54, 1779–1783. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranopyran-4,5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic acids C and D, phytotoxic tetrasubstituted 3-chromanonacrylic acids isolated from Cochliobolus australiensis with potential herbicidal activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef]
- Masi, M.; Di Lecce, R.; Tuzi, A.; Linaldeddu, B.T.; Montecchio, L.; Maddau, L.; Evidente, A. Hyfraxinic acid, a phytotoxic tetrasubstituted octanoic Acid, produced by the ash (Fraxinus excelsior L.) pathogen Hymenoscyphus fraxineus together with viridiol and some of its analogues. J. Agric. Food Chem. 2019, 67, 13617–13623. [Google Scholar] [CrossRef]
- Cimmino, A.; Bahmani, Z.; Masi, M.; Di Lecce, R.; Amini, J.; Abdollahzadeh, J.; Tuzi, A.; Evidente, A. Massarilactones D and H, phytotoxins produced by Kalmusia variispora, associated with grapevine trunk diseases (GTDs) in Iran. Nat. Prod. Res. 2021, 35, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef] [Green Version]
- Robeson, D.J.; Gray, G.R.; Strobel, G.A. Production of the phytotoxins radicinin and radicinol by Alternaria chrysanthemi. Phytochemistry 1982, 21, 2359–2362. [Google Scholar] [CrossRef]
- Yokota, T.; Ishikura, T.; Ozaki, A. For Sankaru-Ocean Co. Ltd. Japanese Patent NO. 420 11 997 B, 7 July 1967. [Google Scholar]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce′s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef]
- Van Quaquebeke, E.; Simon, G.; André, A.; Dewelle, J.; Yazidi, M.E.; Bruyneel, F.; Tuti, J.; Nacoulma, O.; Guissou, P.; Decaestecker, C.; et al. Identification of a Novel cardenolide (2‘‘-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: Structure−activity relationship analyses. J. Med. Chem. 2005, 48, 849–856. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calvé, B.; Wauthoz, N.; Mégalizzi, V.; Gras, T.; Bruyère, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure−activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, L.M.; Danner, A.; Gronych, J.; Wolter, M.; Stühler, K.; Grzendowski, M.; Becker, N.; Bageritz, J.; Goidts, V.; Toedt, G.; et al. Downregulation of PRDX1 by promoter hypermethylation is frequent in 1p/19q-deleted oligodendroglial tumours and increases radio- and chemosensitivity of Hs683 glioma cells in vitro. Oncogene 2012, 31, 3409–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefranc, F.; Nuzzo, G.; Hamdy, N.A.; Fakhr, I.; Moreno, Y.; Banuls, L.; Van Goietsenoven, G.; Villani, G.; Mathieu, V.; van Soest, R.; et al. In vitro pharmacological and toxicological effects of norterpene peroxides isolated from the red sea sponge Diacarnus erythraeanus on normal and cancer cells. J. Nat. Prod. 2013, 76, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Baborie, A.; Alam, F.; Joyce, K.; Moxham, M.; Sibson, R.; Crooks, D.; Husband, D.; Shenoy, A.; Brodbelt, A.; et al. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br. J. Cancer 2009, 101, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tièche, C.C.; Gao, Y.; Bührer, E.D.; Hobi, N.; Berezowska, S.A.; Wyler, K.; Froment, L.; Weis, S.; Peng, R.W.; Bruggmann, R.; et al. Tumor Initiation Capacity and Therapy Resistance Are Differential features of EMT-related subpopulations in the NSCLC Cell Line A549. Neoplasia 2019, 21, 185–196. [Google Scholar] [CrossRef]
- McGrath, E.E. The tumor necrosis factor-related apoptosis-inducing ligand and lung cancer: Still following the right TRAIL? J. Thorac. Oncol. 2011, 6, 983–987. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; You, M.; Liu, Y.J.; Ma, L.; Jin, P.P.; Zhou, R.; Zhang, Z.X.; Hua, B.; Ji, X.J.; Cheng, X.Y.; et al. Reversal of the apoptotic resistance of non-small-cell lung carcinoma towards TRAIL by natural product toosendanin. Sci. Rep. 2017, 7, 42748. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.L.; Li, J.Z.; Ma, Y.Y.; Qian, D.; Zhong, J.Y.; Jin, M.M.; Huang, P.; Che, L.Y.; Pan, B.; Wang, Y.; et al. Shikonin sensitizes A549 cells to TRAIL-induced apoptosis through the JNK, STAT3 and AKT pathways. BMC Cell Biol. 2018, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Pirker, C.; Martin de Lassalle, E.; Vernier, M.; Mijatovic, T.; DeNeve, N.; Gaussin, J.F.; Dehoux, M.; Lefranc, F.; Berger, W.; et al. The sodium pump alpha1 subunit: A disease progression-related target for metastatic melanoma treatment. J. Cell. Mol. Med. 2009, 13, 3960–3972. [Google Scholar] [CrossRef] [Green Version]
- Van Goietsenoven, G.; Hutton, J.; Becker, J.P.; Lallemand, B.; Robert, F.; Lefranc, F.; Pirker, C.; Vandenbussche, G.; Van Antwerpen, P.; Evidente, A.; et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010, 24, 4575–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalinova, A.; Chisty, L.; Kochura, D.; Garnyuk, V.; Petrova, M.; Prokofieva, D.; Yurchenko, A.; Dubovik, V.; Ivanov, A.; Smirnov, S.; et al. Isolation and bioactivity of secondary metabolites from solid culture of the fungus, “Alternaria sonchi”. Biomolecules 2020, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Phuwapraisirisan, P.; Rangsan, J.; Siripong, P.; Tip-Pyang, S. 9-epi-Viridiol, a novel cytotoxic furanosteroid from soil fungus Trichoderma virens. Nat. Prod. Res. 2006, 20, 1321–1325. [Google Scholar] [CrossRef]
- Teponno, R.B.; Noumeur, S.R.; Helaly, S.E.; Hüttel, S.; Harzallah, D.; Stadler, M. Furanones and anthranilic acid derivatives from the endophytic fungus Dendrothyrium variisporum. Molecules 2017, 22, 1674. [Google Scholar] [CrossRef] [Green Version]
- Perše, M. Cisplatin mouse models: Treatment, toxicity and translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Handayani, D.; Putri, R.A.; Ismed, F.; Hertiani, T.; Ariantari, N.P.; Proksch, P. Bioactive metabolite from marine sponge-derived fungus Cochliobolus geniculatus WR12. Rasayan J. Chem. 2020, 13, 417–422. [Google Scholar] [CrossRef]
- Schrader, T.J.; Cherry, W.; Soper, K.; Langlois, I.; Vijay, H.M. Examination of Alternaria alternata mutagenicity and effects of nitrosylation using the Ames Salmonella test. Teratog. Carcinog. Mutagen. 2001, 21, 261–274. [Google Scholar] [CrossRef]
- Schrader, T.J.; Cherry, W.; Soper, K.; Langlois, I. Further examination of the effects of nitrosylation on Alternaria alternata mycotoxin mutagenicity in vitro. Mutat. Res. 2006, 606, 61–71. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Cabras, A.; Maddau, L.; Serra, S.; Andolfi, A.; Motta, A. Viridepyronone, a New Antifungal 6-Substituted 2 H-pyran-2-one produced by Trichoderma viride. J. Agric. Food Chem. 2003, 51, 6957–6960. [Google Scholar] [CrossRef]
- Evidente, A.; Cabras, A.; Maddau, L.; Marras, F.; Andolfi, A.; Melck, D.; Motta, A. Viridenepoxydiol, a New Pentasubstituted oxiranyldecene produced by Trichoderma viride. J. Agric. Food Chem. 2006, 54, 6588–6592. [Google Scholar] [CrossRef]
- Favilla, M.; Pascale, M.; Ricelli, A.; Evidente, A.; Amalfitano, C.; Altomare, C. Inhibition of species of the Aspergillus section Nigri and ochratoxin A production in Grapes by fusapyrone. Appl. Environ. Microbiol. 2008, 74, 2248–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Calve, B.; Lallemand, B.; Perrone, C.; Lenglet, G.; Depauw, S.; Van Goietsenoven, G.; Bury, M.; Vurro, M.; Herphelin, F.; Andolfi, A.; et al. In Vitro anticancer activity, toxicity and structure–activity relationships of phyllostictine A, a natural oxazatricycloalkenone produced by the fungus Phyllosticta cirsii. Toxicol. Appl. Pharmacol. 2011, 254, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Venturi, V.; Masi, M.; Degrassi, G.; Cimmino, A.; Maddau, L.; Andolfi, A. In Vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J. Nat. Prod. 2011, 74, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Cristofaro, M.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and herbicidal activity against buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2019, 24, 3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, M.; Freda, F.; Clement, S.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic activity and structure–activity relationships of radicinin derivatives against the invasive weed buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [Green Version]
- Santoro, E.; Mazzeo, G.; Marsico, G.; Masi, M.; Longhi, G.; Superchi, S.; Evidente, A.; Abbate, S. Assignment through chiroptical methods of the absolute configuration of fungal dihydropyranpyran-4-5-diones phytotoxins, potential herbicides for buffelgrass (Cenchrus Ciliaris) biocontrol. Molecules 2019, 24, 3022. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, P.; Verekar, S.A.; Gohil, A.R.; Mishra, P.D.; Khanna, A.; Deshmukh, S.K. Antiproliferative activity of hamigerone and radicinol isolated from Bipolaris papendorfii. Biomed. Res. Int. 2014, 2014, 890904. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, M.-K.; Yang, X.; Xu, C.-X.; Cheng, J.-T.; Zhao, Q.-W.; Wu, R.; Sheng, R.; Mao, X.-M. A target and efficient synthetic strategy for structural and bioactivity optimization of a fungal natural product. Eur. J. Med. Chem. 2022, 229, 114067. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and cytotoxic activities of ten commercially available essential oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef]
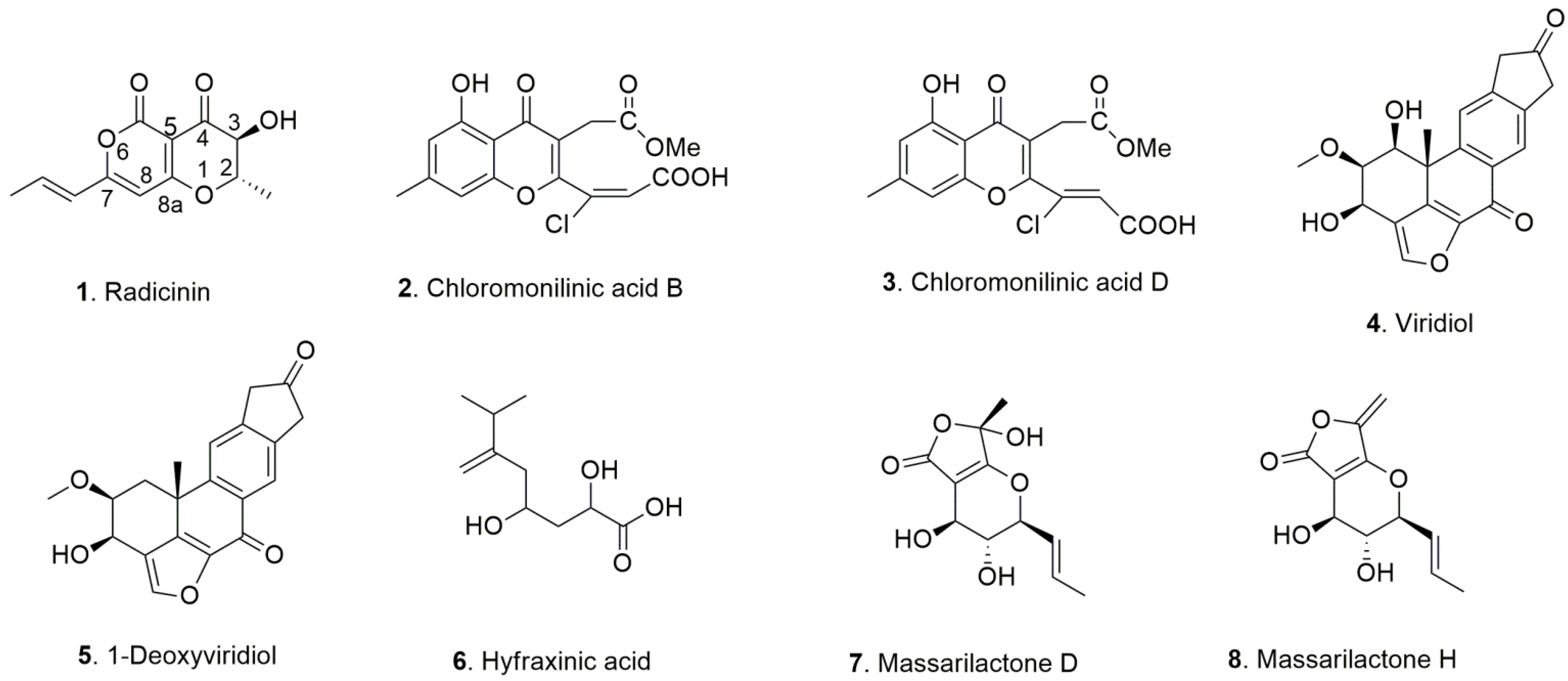
| Fungus | Host Plant | Disease | Metabolite | Ref |
|---|---|---|---|---|
| Cochliobolus australiensis | Buffelgrass (Cenchrus ciliaris L.) | Leaf Spots | Radicinin | [13] |
| Radicinol | ||||
| 3-epi-Radicinin | ||||
| Cochliotoxin | ||||
| Chloromonilinic acid B | [14] | |||
| Chloromonilinic acid C | ||||
| Chloromonilinic acid D | ||||
| Hymenoscyphus fraxineus | Ash (Fraxinus excelsior L.) | Dieback | Viridiol | [15] |
| 1-Deoxyviridiol | ||||
| Demethoxyviridiol | ||||
| Nodulisporiviridin M | ||||
| Hyfraxinic acid | ||||
| Kalmusia variispora | Grapevine (Vitis vinifera L.) | Trunk disease | Massarilactone D | [16] |
| Massarilactone H |
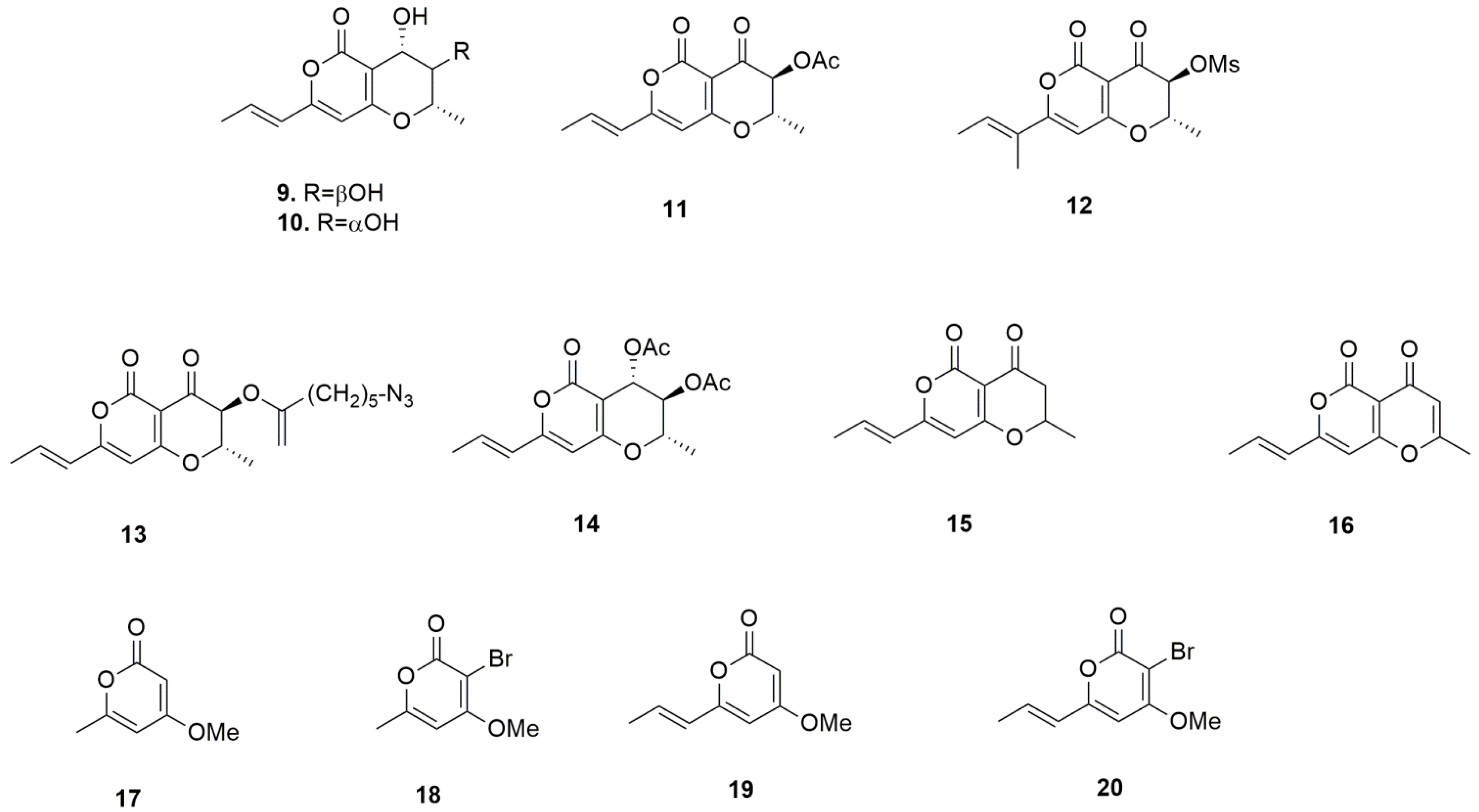
| Compound | A549 | Hs683 | SKMEL-28 | Mean |
|---|---|---|---|---|
| cisplatin | 6.3 ± 1.4 | 8.8 ± 0.8 | 10.0 ± 2.8 | 8.4 |
| 1. Radicinin | 7.7 ± 0.6 | 8.7 ± 0.4 | 8.2 ± 0.2 | 8.2 |
| 2. Chloromonilinic acid B | >100 | >100 | >100 | >100 |
| 3. Chloromonilinic acid D | >100 | >100 | >100 | >100 |
| 4. Viridiol | 65.5 ± 7.6 | 51.7 ± 5.3 | 62.4 ± 7.6 | 59.9 |
| 5. 1-deoxyviridiol | 74.0 ± 6.6 | 64.9 ± 12.9 | 91.2 ± 18.8 | 76.7 |
| 6. Hyfraxinic acid | >100 | >100 | >100 | >100 |
| 7. Massarilactone D | >100 | >100 | >100 | >100 |
| 8. Massarilactone H | 32.9 ± 3.5 | 31.6 ± 2.5 | 35.2 ± 2.8 | 33.2 |
| Compound | A549 | Hs683 | SKMEL-28 | Mean |
|---|---|---|---|---|
| 1. radicinin | 7.7 ± 0.6 | 8.7 ± 0.4 | 8.2 ± 0.2 | 8.2 |
| 9. radicinol | >100 | >100 | >100 | >100 |
| 10. 3-epi-radicinol | >100 | >100 | >100 | >100 |
| 11. 3-O-acetylradicinin | 7.0 ± 0.7 | 18.8 ± 2.5 | 7.9 ± 3.1 | 11.2 |
| 12. 3-O-mesylradicinin | 8.5 ± 0.5 | 24.0 ± 0.2 | 6.9 ± 0.3 | 13.1 |
| 13. 3-O-(5-azidopentanoyl)radicinin | 20.0 ± 2.1 | 23.9 ± 4.3 | 31.7 ± 1.5 | 25.2 |
| 14. 3,4-O,O’-diacetylradicinol | >100 | >100 | >100 | >100 |
| 15. 3-deoxyradicinin | 12.0 ±2.8 | 22.4 ± 2.0 | 20.4 ± 4.7 | 18.3 |
| 16. 2,3-dehydroradicinin | 28.3 ± 1.6 | 30.5 ± 0.8 | 28.8 ± 2.1 | 29.2 |
| 17. 4-methoxy-6-methyl-2H-pyran-2-one | >100 | >100 | >100 | >100 |
| 18. 3-bromo-4-methoxy-6-methyl-2H-pyran-2-one | 59.7 ± 5.5 | 53.1 ± 2.8 | 73.2 ± 3.8 | 62.0 |
| 19. (E)-4-methoxy-6-(propen-1-yl)-2H-pyran-2-one | >100 | >100 | >100 | >100 |
| 20. (E)-3-bromo-4-methoxy-6-(propen-1-yl)-2H-pyran-2-one | 96.9 ± 8.6 | 99.4 ± 6.5 | >100 | >98.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu, V.; Superchi, S.; Masi, M.; Scafato, P.; Kornienko, A.; Evidente, A. In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin. Toxins 2022, 14, 517. https://doi.org/10.3390/toxins14080517
Mathieu V, Superchi S, Masi M, Scafato P, Kornienko A, Evidente A. In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin. Toxins. 2022; 14(8):517. https://doi.org/10.3390/toxins14080517
Chicago/Turabian StyleMathieu, Veronique, Stefano Superchi, Marco Masi, Patrizia Scafato, Alexander Kornienko, and Antonio Evidente. 2022. "In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin" Toxins 14, no. 8: 517. https://doi.org/10.3390/toxins14080517
APA StyleMathieu, V., Superchi, S., Masi, M., Scafato, P., Kornienko, A., & Evidente, A. (2022). In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin. Toxins, 14(8), 517. https://doi.org/10.3390/toxins14080517










