Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum
Abstract
1. Introduction
2. Results
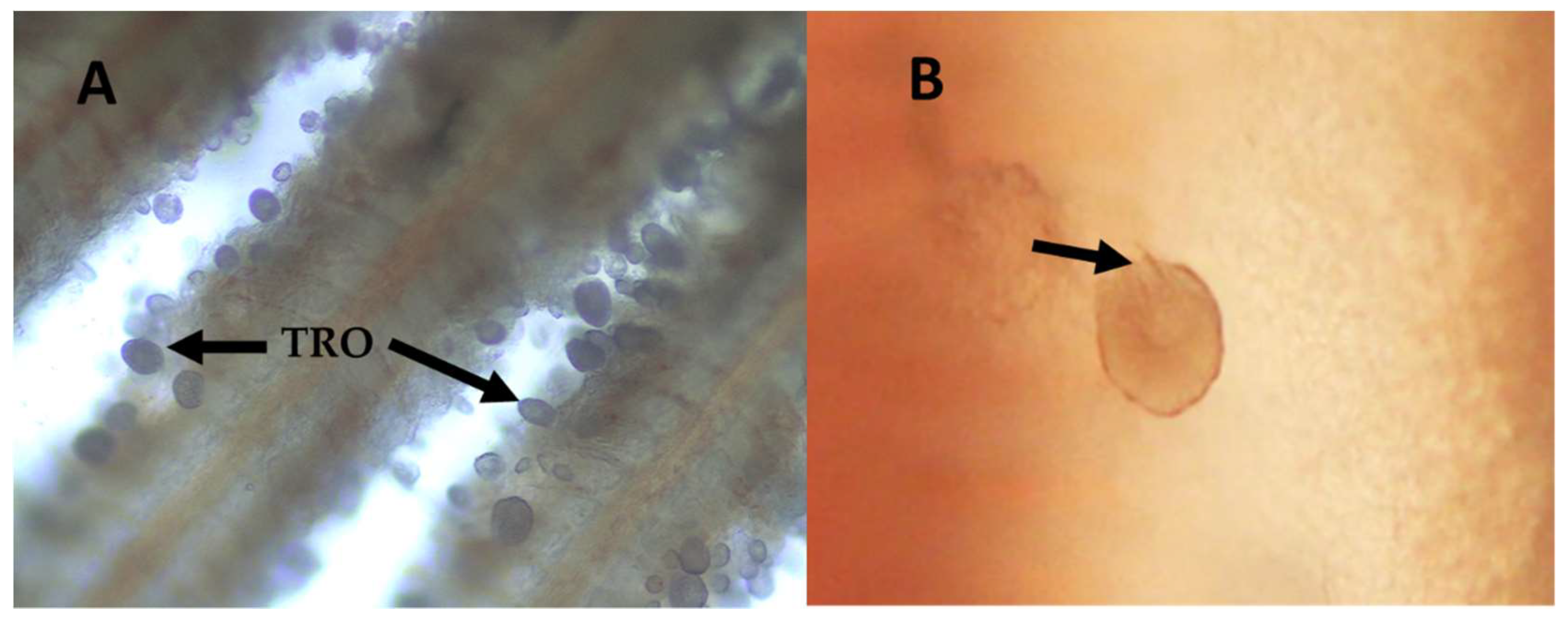
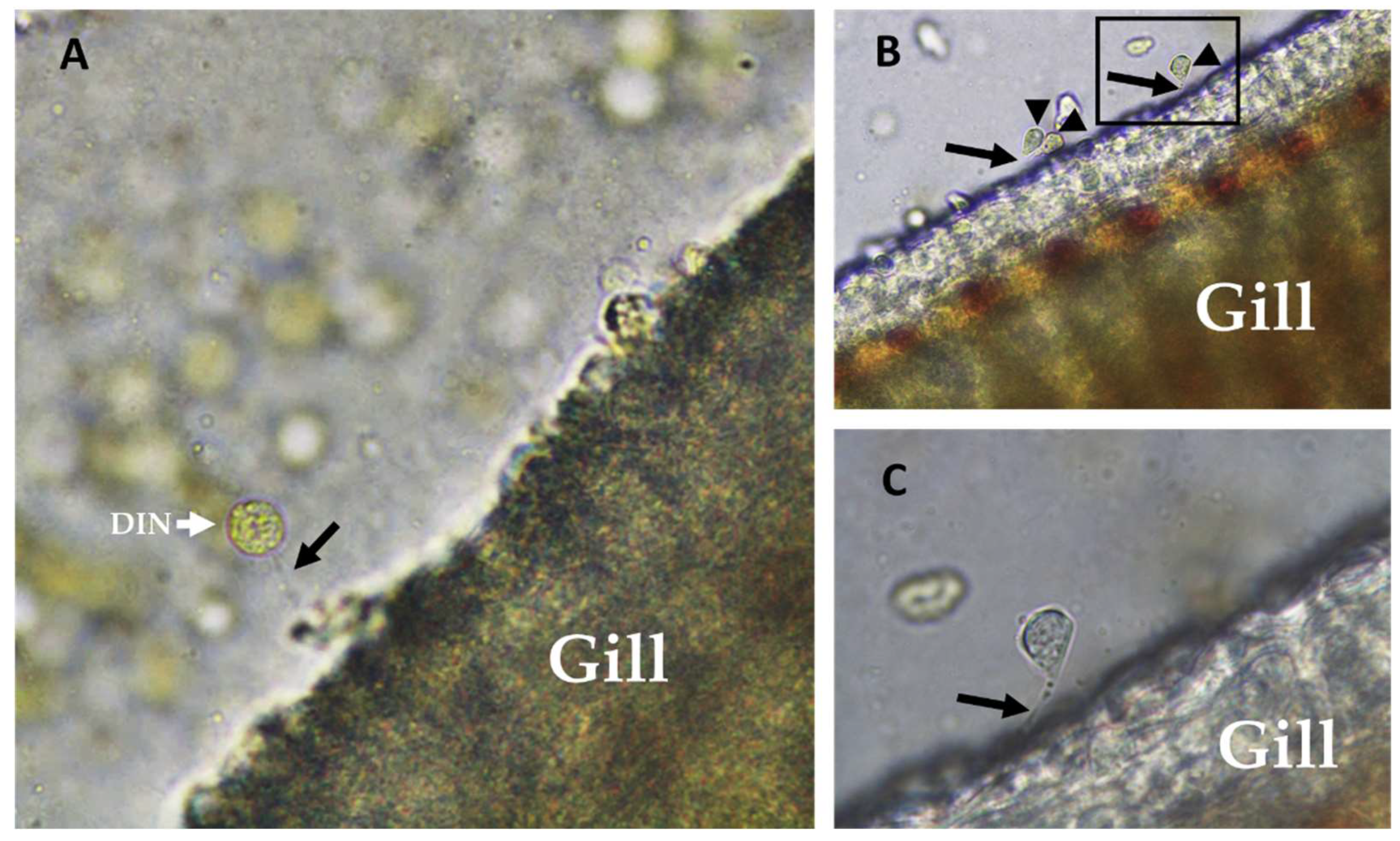
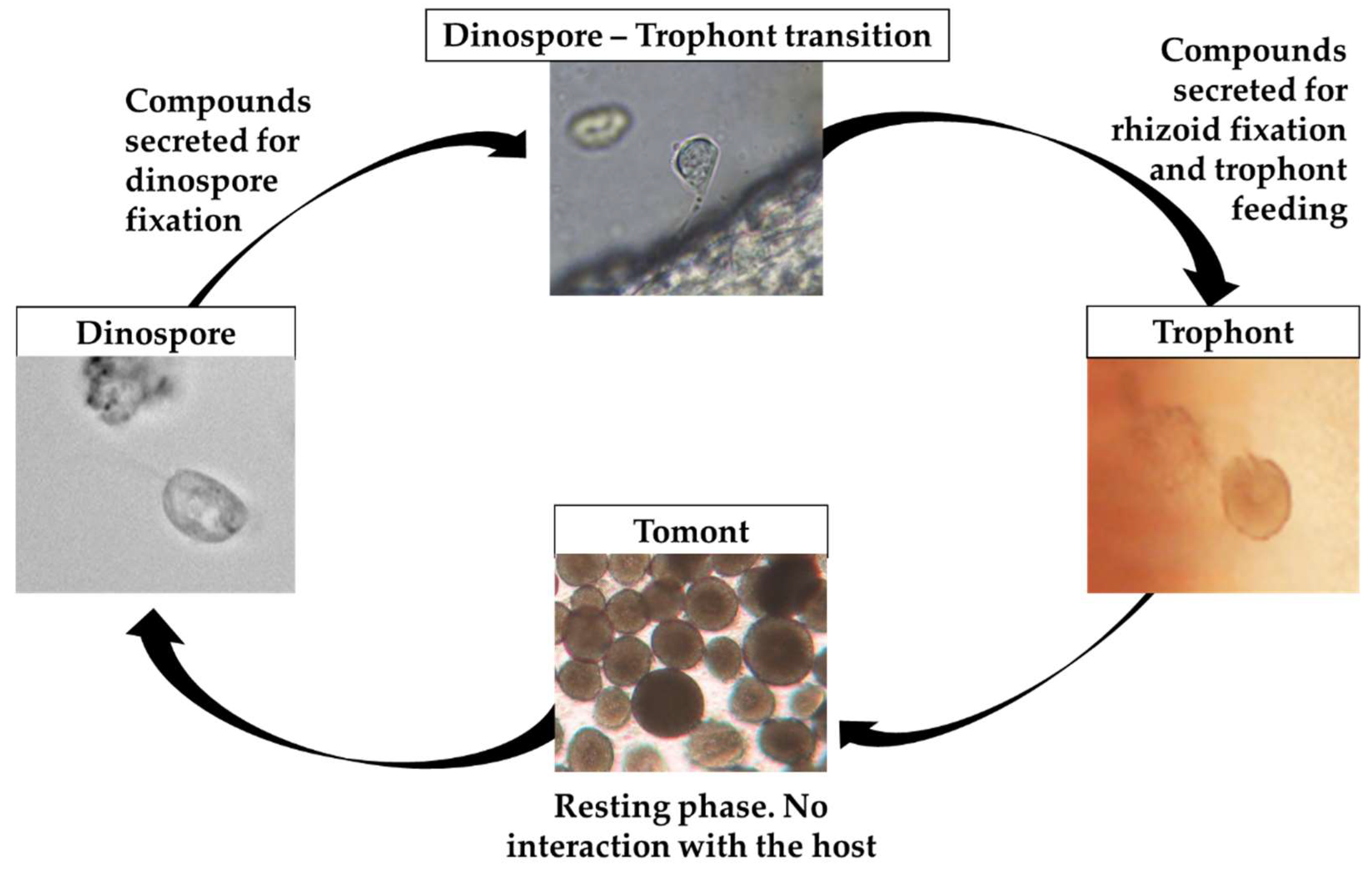
2.1. Amyloodinium ocellatum Life Stages Collection
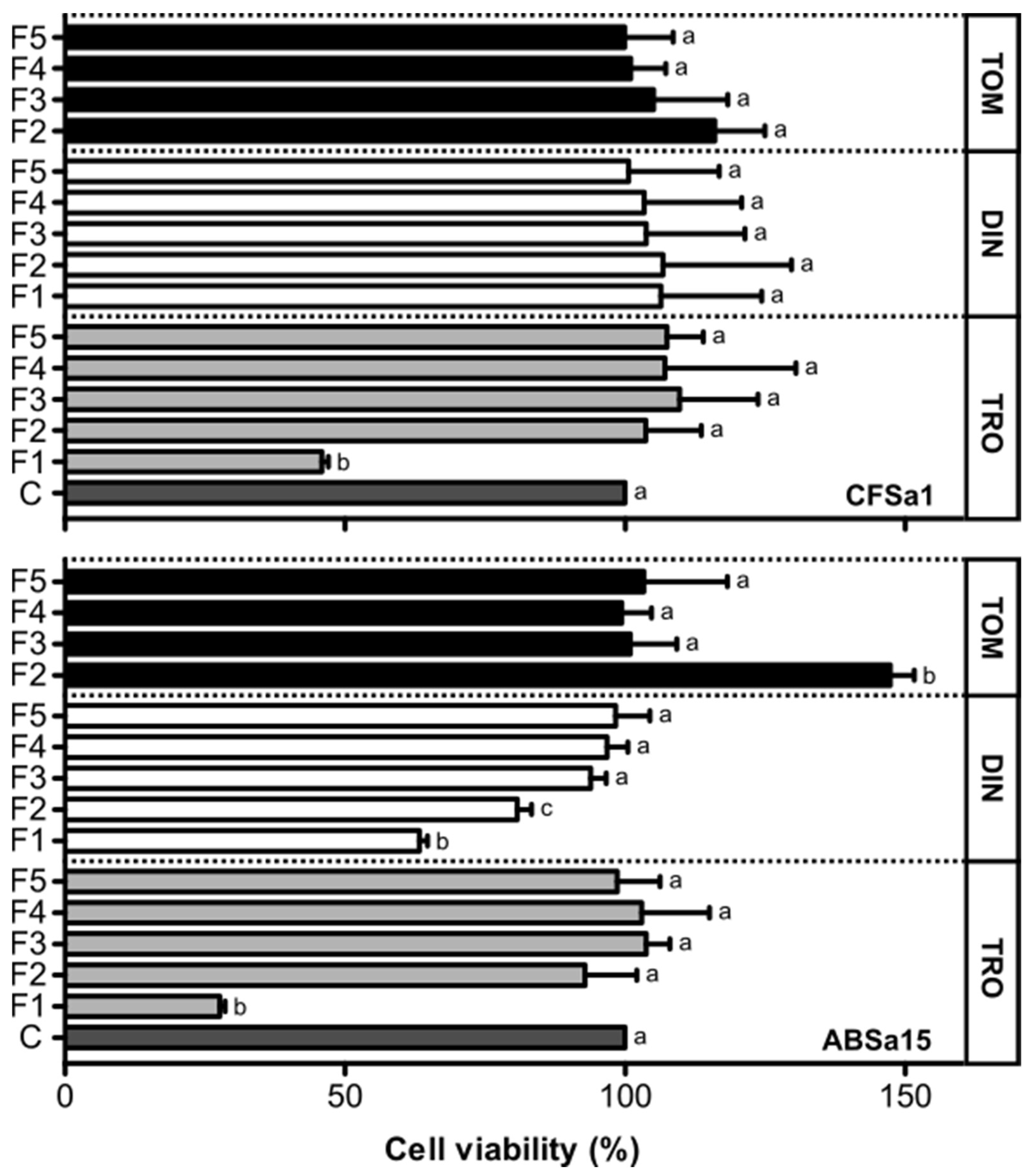
2.2. Viability of Fish Cells Exposed to Fractions of Amyloodinium ocellatum Life Stages Crude Extract
2.3. Hemolytic Effect of Amyloodinium ocellatum Crude Extract Fractions on Gilthead Seabream Erythrocytes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fish Culture Conditions
5.2. Establishment of Infestation Tanks for Parasite Production
5.3. Amyloodinium ocellatum Life Stages Collection
5.3.1. Tomont Collection
5.3.2. Dinospore Collection
5.3.3. Trophont Collection
5.4. Production of Amyloodinium ocellatum Extracts by Solid-Phase Extraction Fractioning
5.5. Impact of Amyloodinium ocellatum Extracts on Fish Cell Viability
5.6. Hemolytic Assays
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fensome, R.A.; Saldarriaga, J.F.; Taylor, M.F. Dinoflagellate phylogeny revisited: Reconciling morphological and molecular based phylogenies. Grana 1999, 38, 66–80. [Google Scholar] [CrossRef]
- Drebes, G. Life cycle and host specificity of marine parasitic dinophytes. Helgol. Meeresunters. 1984, 37, 603–622. [Google Scholar]
- Buckland-Nicks, J.; Reimchen, T. A novel association between an endemic stickleback and a parasitic dinoflagellate. 3. Details of the life cycle. Arch. Protistenkd. 1995, 145, 165–175. [Google Scholar] [CrossRef]
- Francis-Floyd, R.; Floyd, M.R. Amyloodinium Ocellatum, an Important Parasite of Cultured Marine Fish; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2011. [Google Scholar]
- Aravindan, N.; Chaganti, K.; Aravindan, S. Protozoan parasites in commercially important shrimp species from northeast coast of Andhra Pradesh, India. J. Exp. Zool. 2007, 10, 9–20. [Google Scholar]
- Shinn, A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G.; Brooker, E.E.; Brooker, A.J. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 2015, 142, 196–270. [Google Scholar] [CrossRef]
- Bessat, M.; Fadel, A. Amyloodiniosis in cultured Dicentrarchus labrax: Parasitological and molecular diagnosis, and an improved treatment protocol. Dis. Aquat. Org. 2018, 129, 41–51. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Blakesley, B.A.; Reese, R.O.; Mcrae, G.; Forstchen, P.R. Parasites of Fish as Indicators of Environmental Stress. Environ. Monit. Assess. 1998, 51, 211–232. [Google Scholar] [CrossRef]
- Kuperman, B.I.; Matey, V.E. Massive infestation by Amyloodinium ocellatum (Dinoflagellida) of fish in a highly saline lake, Salton Sea, California, USA. Dis. Aquat. Org. 1999, 39, 65–73. [Google Scholar] [CrossRef]
- Woo, P.T. Protective immunity in fish against protozoan diseases. Parassitologia 2007, 49, 185–191. [Google Scholar]
- Brown, E.M.; Hovasse, R. Amyloodinium ocellatum (Brown), a Peridinian Parasitic on Marine Fishes. A Complementary Study. Proc. Zool. Soc. Lond. 1946, 116, 33–46. [Google Scholar] [CrossRef]
- Masson, I.; Lotz, J.M.; Blaylock, R.B. Population model for Amyloodinium ocellatum infecting the spotted seatrout Cynoscion nebulosus and the red snapper Lutjanus campechanus. Dis. Aquat. Org. 2013, 106, 139–148. [Google Scholar] [CrossRef]
- Soares, F.; Quental Ferreira, H.; Cunha, E.; Pousão-Ferreira, P. Occurrence of Amyloodinium ocellatum in aquaculture fish production: A serious problem in semi-intensive earthen ponds. Aquacult. Eur. 2011, 36, 13–16. [Google Scholar]
- Soares, F.; Quental-Ferreira, H.; Moreira, M.; Cunha, E.; Ribeiro, L.; Pousao-Ferreira, P. First report of Amyloodinium ocellatum in farmed meagre (Argyrosomus regius). Bull. Eur. Assoc. Fish Pathol. 2012, 32, 30–33. [Google Scholar]
- Paperna, I.; Ross, B.; Colorni, A.; Colorni, B. Diseases of Marine Fish Cultured in Eilat Mariculture Project Based at the Gulf of Aqaba, Red Sea; 92-5-000964-X; FAO: Rome, Italy, 1980; pp. 29–32. [Google Scholar]
- Lawler, A.R. Studies on Amyloodinium ocellatum (Dinoflagellata) in Mississippi Sound—Natural and Experimental Hosts. Gulf Res. Rep. 1980, 6, 403–413. [Google Scholar] [CrossRef]
- Noga, E.J. Amyloodinium ocellatum. In Fish Parasites: Pathobiology and Protection; Woo, P.T.K., Buchmann, K., Eds.; CABI Publishers: Preston, UK, 2012; pp. 19–29. [Google Scholar]
- Ragab, R.H.; Elgendy, M.Y.; Sabry, N.M.; Sharaf, M.S.; Attia, M.M.; Korany, R.M.S.; Abdelsalam, M.; Eltahan, A.S.; Eldessouki, E.A.; El-Demerdash, G.O.; et al. Mass kills in hatchery-reared European seabass (Dicentrarchus labrax) triggered by concomitant infections of Amyloodinium ocellatum and Vibrio alginolyticus. Int. J. Vet. Sci. Med. 2022, 10, 33–45. [Google Scholar] [CrossRef]
- Moreira, M.; Schrama, D.; Soares, F.; Wulff, T.; Pousão-Ferreira, P.; Rodrigues, P. Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. J. Fish Dis. 2017, 40, 1545–1560. [Google Scholar] [CrossRef]
- Moreira, M.; Herrera, M.; Pousão-Ferreira, P.; Navas Triano, J.I.; Soares, F. Stress effects of amyloodiniosis in gilthead sea bream Sparus aurata. Dis. Aquat. Org. 2018, 127, 201–211. [Google Scholar] [CrossRef]
- Moreira, M.; Cordeiro-Silva, A.; Barata, M.; Pousão-Ferreira, P.; Soares, F. Influence of Age on Stress Responses of White Seabream to Amyloodiniosis. Fishes 2019, 4, 26. [Google Scholar] [CrossRef]
- Vivanco-Aranda, M.; Del Río-Zaragoza, O.B.; Lechuga-Sandoval, C.E.; Viana, M.T.; Rombenso, A.N. Health response in yellowtail Seriola dorsalis exposed to an Amyloodinium ocellatum outbreak. Cienc. Mar. 2018, 44, 267–277. [Google Scholar] [CrossRef]
- Lom, J. Fish invading dinoflagellates: A synopsis of existing and newly proposed genera. Folia Parasitol. 1981, 28, 3–11. [Google Scholar]
- Seaborn, D.W.; Tengs, T.; Cerbin, S.; Kokocinski, M.; Marshall, H.G. A group of dinoflagellates similar to Pfiesteria as defined by morphology and genetic analysis. Harmful Algae 2006, 5, 1–8. [Google Scholar] [CrossRef]
- Rensel, J.; Whyte, J. Finfish mariculture and harmful algal blooms. Man. Harmful Mar. Microalgae. Monogr. Oceanogr. Methodol. 2003, 11, 693–722. [Google Scholar]
- Caillaud, A.; Cañete, E.; de la Iglesia, P.; Giménez, G.; Diogène, J. Cell-based assay coupled with chromatographic fractioning: A strategy for marine toxins detection in natural samples. Toxicol. Vitr. 2009, 23, 1591–1596. [Google Scholar] [CrossRef]
- Moeller, P.; Morton, S.L.; Mitchell, B.A.; Sivertsen, S.K.; Fairey, E.R.; Mikulski, T.M.; Glasgow, H.; Deamer-Melia, N.J.; Burkholder, J.M.; Ramsdell, J.S. Current progress in isolation and characterization of toxins isolated from Pfiesteria piscicida. Environ. Health Perspect. 2001, 109, 739–743. [Google Scholar]
- Estevez, P.; Castro, D.; Leão-Martins, J.M.; Sibat, M.; Tudó, A.; Dickey, R.; Diogene, J.; Hess, P.; Gago-Martinez, A. Toxicity Screening of a Gambierdiscus australes Strain from the Western Mediterranean Sea and Identification of a Novel Maitotoxin Analogue. Mar. Drugs 2021, 19, 460. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Pardal, M.A.; Nascimento, S.M.; Oliveira, P.J.; Rodrigues, E.T. Screening-level evaluation of marine benthic dinoflagellates toxicity using mammalian cell lines. Ecotoxicol. Environ. Saf. 2020, 195, 110465. [Google Scholar] [CrossRef]
- Souid-Mensi, G.; Moukha, S.; Mobio, T.A.; Maaroufi, K.; Creppy, E.E. The cytotoxicity and genotoxicity of okadaic acid are cell-line dependent. Toxicon 2008, 51, 1338–1344. [Google Scholar] [CrossRef]
- Soliño, L.; Sureda, F.X.; Diogène, J. Evaluation of okadaic acid, dinophysistoxin-1 and dinophysistoxin-2 toxicity on Neuro-2a, NG108-15 and MCF-7 cell lines. Toxicol. Vitr. 2015, 29, 59–62. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Caillaud, A.; Yasumoto, T.; Diogène, J. Detection and quantification of maitotoxin-like compounds using a neuroblastoma (Neuro-2a) cell based assay. Application to the screening of maitotoxin-like compounds in Gambierdiscus spp. Toxicon 2010, 56, 36–44. [Google Scholar] [CrossRef]
- Ledreux, A.; Krys, S.; Bernard, C. Suitability of the Neuro-2a cell line for the detection of palytoxin and analogues (neurotoxic phycotoxins). Toxicon 2009, 53, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Viallon, J.; Chinain, M.; Darius, H.T. Revisiting the Neuroblastoma Cell-Based Assay (CBA-N2a) for the Improved Detection of Marine Toxins Active on Voltage Gated Sodium Channels (VGSCs). Toxins 2020, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.C.; Litaker, R.W.; Tomas, C.R.; Kibler, S.R.; Place, A.R.; Davenport, E.D.; Tester, P.A. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Riobó, P.; Paz, B.; Franco, J.M.; Vázquez, J.A.; Murado, M.A. Proposal for a simple and sensitive haemolytic assay for palytoxin: Toxicological dynamics, kinetics, ouabain inhibition and thermal stability. Harmful Algae 2008, 7, 415–429. [Google Scholar] [CrossRef]
- Brovedani, V.; Sosa, S.; Poli, M.; Forino, M.; Varello, K.; Tubaro, A.; Pelin, M. A revisited hemolytic assay for palytoxin detection: Limitations for its quantitation in mussels. Toxicon 2016, 119, 225–233. [Google Scholar] [CrossRef]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative methods for the detection of emerging marine toxins: Biosensors, biochemical assays and cell-based assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef]
- Alves-Ferreira, M.; da Silva, E.C.C.; Ferreira-Pereira, A.; Scofano, H.M. Regulatory differences between Ca2+-ATPase in plasma membranes from chicken (nucleated) and pig (anucleated) erythrocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 405–415. [Google Scholar] [CrossRef]
- Thomas, S.; Egée, S. Fish Red Blood Cells: Characteristics and Physiological Role of the Membrane Ion Transporters. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 79–86. [Google Scholar] [CrossRef]
- Yan, M.; Leung, P.T.Y.; Gu, J.; Lam, V.T.T.; Murray, J.S.; Harwood, D.T.; Wai, T.-C.; Lam, P.K.S. Hemolysis associated toxicities of benthic dinoflagellates from Hong Kong waters. Mar. Pollut. Bull. 2020, 155, 111114. [Google Scholar] [CrossRef]
- Tatters, A.O.; Muhlstein, H.I.; Tomas, C.R. The hemolytic activity of Karenia selliformis and two clones of Karenia brevis throughout a growth cycle. J. Appl. Phycol. 2010, 22, 435–442. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.J.; Nigrelli, R.F.; Ruggieri, G.D. Development of Oodinium ocellatum (Dinoflagellida): A Scanning Electron Microscopic Study. Trans. Am. Microsc. Soc. 1981, 100, 415–420. [Google Scholar] [CrossRef]
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Hamuro, K.; Suetake, H.; Saha, N.R.; Kikuchi, K.; Suzuki, Y. A Teleost Polymeric Ig Receptor Exhibiting Two Ig-like Domains Transports Tetrameric IgM into the Skin. J. Immunol. 2007, 178, 5682–5689. [Google Scholar] [CrossRef]
- Puri, S.; Aegerter-Wilmsen, T.; Jaźwińska, A.; Aegerter, C.M. In vivo quantification of mechanical properties of caudal fins in adult zebrafish. J. Exp. Biol. 2018, 221, jeb171777. [Google Scholar] [CrossRef]
- Igarashi, T.; Aritake, S.; Yasumoto, T. Mechanisms underlying the hemolytic and ichthyotoxic activities of maitotoxin. Nat. Toxins 1999, 7, 71–79. [Google Scholar] [CrossRef]
- Bellocci, M.; Sala, G.L.; Prandi, S. The cytolytic and cytotoxic activities of palytoxin. Toxicon 2011, 57, 449–459. [Google Scholar] [CrossRef]
- Lom, J.; Lawler, A. An ultrastructural study on the mode of attachment in dinoflagellates invading gills of Cyprinodontidae. Protistologica 1973, 9, 293–309. [Google Scholar]
- Brown, E.M. On Oodinium ocellatum Brown, a Parasitic Dinoflagellate causing Epidemic Disease in Marine Fish. Proc. Zool. Soc. Lond. 1934, 104, 583–607. [Google Scholar] [CrossRef]
- Nigrelli, R.F. The Morphology, Cytology and Life-History of Oodinium Ocellatum Brown: A Dinoflagellate Parasitic on Marine Fishes; New York University: New York, NY, USA, 1936. [Google Scholar]
- Lom, J.; Dyková, I. Protozoan Parasites of Fishes; Elsevier Science Publishers: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Deeds, J.R.; Hoesch, R.E.; Place, A.R.; Kao, J.P.Y. The cytotoxic mechanism of karlotoxin 2 (KmTx 2) from Karlodinium veneficum (Dinophyceae). Aquat. Toxicol. 2015, 159, 148–155. [Google Scholar] [CrossRef]
- Pousão-Ferreira, P.; Gonçalves, C.; Dores, E. Larval rearing of four sparidae species. In Larvi 2005: Proceedings of the 4th Fish and Shellfish Larviculture Symposium, Gent, Belgium, September 2005; Hendry, C.I., Van Stappen, G., Wille, M., Sorgeloos, P., Eds.; European Aquaculture Society: Oostende, Belgium, 2005. [Google Scholar]
- UNESCO. Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis, 1st ed.; Karlson, B., Cusack, C., Bresnan, E., Eds.; UNESCO: Paris, France, 2010; p. 110. [Google Scholar]
- Paperna, I. Chemical control of Amyloodinium ocellatum (Brown 1931) (Dinoflagellida) infections: In vitro tests and treatment trials with infected fishes. Aquaculture 1984, 38, 1–18. [Google Scholar] [CrossRef]
- Marques, C.L.; Rafael, M.S.; Cancela, M.L.; Laizé, V. Establishment of primary cell cultures from fish calcified tissues. Cytotechnology 2007, 55, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiago, D.M.; Marques, C.L.; Roberto, V.P.; Cancela, M.L.; Laizé, V. Mir-20a regulates in vitro mineralization and BMP signaling pathway by targeting BMP-2 transcript in fish. Arch. Biochem. Biophys. 2014, 543, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Costabile, M. Measuring the 50% haemolytic complement (CH50) activity of serum. J. Vis. Exp. JoVE 2010, 37, 1923. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, M.; Soliño, L.; Marques, C.L.; Laizé, V.; Pousão-Ferreira, P.; Costa, P.R.; Soares, F. Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum. Toxins 2022, 14, 467. https://doi.org/10.3390/toxins14070467
Moreira M, Soliño L, Marques CL, Laizé V, Pousão-Ferreira P, Costa PR, Soares F. Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum. Toxins. 2022; 14(7):467. https://doi.org/10.3390/toxins14070467
Chicago/Turabian StyleMoreira, Márcio, Lucía Soliño, Cátia L. Marques, Vincent Laizé, Pedro Pousão-Ferreira, Pedro Reis Costa, and Florbela Soares. 2022. "Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum" Toxins 14, no. 7: 467. https://doi.org/10.3390/toxins14070467
APA StyleMoreira, M., Soliño, L., Marques, C. L., Laizé, V., Pousão-Ferreira, P., Costa, P. R., & Soares, F. (2022). Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum. Toxins, 14(7), 467. https://doi.org/10.3390/toxins14070467







