Botulinum Toxin Type A for the Treatment of Skin Ulcers: A Review Article
Abstract
1. Introduction
2. Methods
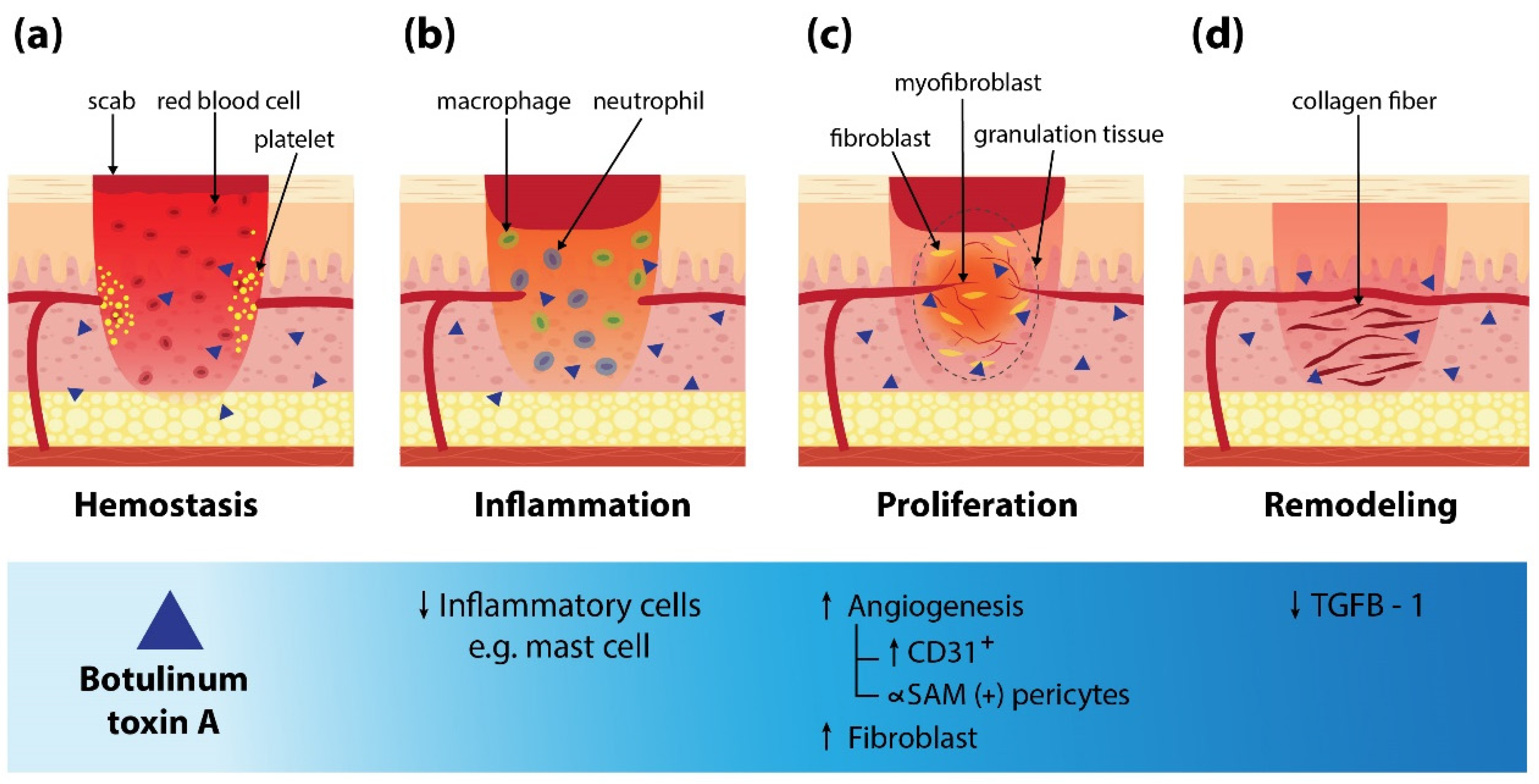
3. Mechanism of BoNT-A on Wound Healing
4. BoNT-A for Various Types of Skin Ulcers
4.1. Ischemic Ulcers Secondary to Raynaud’s Phenomenon (RP)
4.2. BoNT-A for Pressure Ulcers
4.3. BoNT-A for Traumatic Ulcers
4.4. BoNT-A for Other Types of Chronic Ulcers
5. Practical Guidelines for Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT-A | botulinum neurotoxin A |
| eNOS | endothelial nitric oxide synthase |
| MCP | Metacarpophalangeal |
| MRA | magnetic resonance angiography |
| NR | not reported |
| NSS | normal saline |
| PSV | peak systolic velocity |
| RCT | randomized controlled trial study |
| RP | Raynaud’s phenomenon |
| SU | Speywood unit |
| SD | standard deviation |
| VAS | visual analog scale |
References
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Goldman, R. Growth factors and chronic wound healing: Past, present, and future. Adv. Skin Wound Care 2004, 17, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Halder, S.; Menon, G.R.; Chumber, S.; Misra, M.C.; Sharma, L.K.; Srivastava, A. Meta-analysis of randomized controlled trials on hydrocolloid occlusive dressing versus conventional gauze dressing in the healing of chronic wounds. Asian J. Surg. 2004, 27, 326–332. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Nelzen, O.; Bergqvist, D.; Lindhagen, A. Venous and non-venous leg ulcers: Clinical history and appearance in a population study. Br. J. Surg. 1994, 81, 182–187. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef]
- Brem, H.; Sheehan, P.; Rosenberg, H.J.; Schneider, J.S.; Boulton, A.J. Evidence-based protocol for diabetic foot ulcers. Plast. Reconstr. Surg. 2006, 117, 193S–209S. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Erbguth, F.J. From poison to remedy: The chequered history of botulinum toxin. J. Neural Transm. (Vienna) 2008, 115, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Alster, T.S.; Harrison, I.S. Alternative Clinical Indications of Botulinum Toxin. Am. J. Clin. Dermatol. 2020, 21, 855–880. [Google Scholar] [CrossRef]
- Campanati, A.; Martina, E.; Giuliodori, K.; Consales, V.; Bobyr, I.; Offidani, A. Botulinum Toxin Off-Label Use in Dermatology: A Review. Skin Appendage Disord. 2017, 3, 39–56. [Google Scholar] [CrossRef]
- Lewandowski, M.; Świerczewska, Z.; Barańska-Rybak, W. Off-Label Use of Botulinum Toxin in Dermatology-Current State of the Art. Molecules 2022, 27, 3143. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Werbel, T.; Wang, Z.; Wu, C.C.; Yaksh, T.L.; Di Nardo, A. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J. Dermatol. Sci. 2019, 93, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Jobling, P.; Gibbins, I.L. Botulinum neurotoxin A attenuates release of norepinephrine but not NPY from vasoconstrictor neurons. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2627–H2635. [Google Scholar] [CrossRef]
- Morris, J.L.; Jobling, P.; Gibbins, I.L. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2124–H2132. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lora, V.R.; Clemente-Napimoga, J.T.; Abdalla, H.B.; Macedo, C.G.; Canales, G.T.; Barbosa, C.M. Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomadibular joint of rats. Toxicon 2017, 129, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, G.; Dobrota, R.; Mihai, C.; Distler, O.; Calcagni, M.; Maurer, B. Evaluation of botulinum toxin A injections for the treatment of refractory chronic digital ulcers in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2020, 38, 154–160. [Google Scholar]
- Al-Qattan, M.M.; Al-Shanawani, B.N.; Alshomer, F. Botulinum toxin type A: Implications in wound healing, facial cutaneous scarring, and cleft lip repair. Ann. Saudi Med. 2013, 33, 482–488. [Google Scholar] [CrossRef]
- Chang, C.S.; Wallace, C.G.; Hsiao, Y.C.; Chang, C.J.; Chen, P.K. Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 2014, 9, e115690. [Google Scholar] [CrossRef]
- Gassner, H.G.; Sherris, D.A.; Otley, C.C. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast. Reconstr. Surg. 2000, 105, 1948–1953. [Google Scholar] [CrossRef]
- Park, T.H. The effects of botulinum toxin A on mast cell activity: Preliminary results. Burns 2013, 39, 816–817. [Google Scholar] [CrossRef]
- Lee, B.J.; Jeong, J.H.; Wang, S.G.; Lee, J.C.; Goh, E.K.; Kim, H.W. Effect of botulinum toxin type a on a rat surgical wound model. Clin. Exp. Otorhinolaryngol. 2009, 2, 20–27. [Google Scholar] [CrossRef]
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S. Protective effect of botulinum toxin A after cutaneous ischemia-reperfusion injury. Sci. Rep. 2015, 5, 9072. [Google Scholar] [CrossRef]
- Kim, Y.S.; Roh, T.S.; Lee, W.J.; Yoo, W.M.; Tark, K.C. The effect of botulinum toxin A on skin flap survival in rats. Wound Repair Regen. 2009, 17, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Z.; Wang, J.; Zhang, X.; Lei, L.; Zhang, Y.; Su, Y.; Ma, X. Effects of Botulinum Toxin A on the Blood Flow in Expanded Rat Skin. J. Investig. Surg. 2022, 35, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Rah, D.K.; Chong, Y.; Kim, J.K. The effects of botulinum toxin A on survival of rat TRAM flap with vertical midline scar. Ann. Plast. Surg. 2015, 74, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, D.F.; Schweizer, R.; Zhang, S.; Kamat, P.; Contaldo, C.; Rieben, R.; Eberli, D.; Giovanoli, P.; Erni, D.; Plock, J.A. Botulinum toxin A and B raise blood flow and increase survival of critically ischemic skin flaps. J. Surg. Res. 2013, 184, 1205–1213. [Google Scholar] [CrossRef]
- Swartling, C.; Karlqvist, M.; Hymnelius, K.; Weis, J.; Vahlquist, A. Botulinum toxin in the treatment of sweat-worsened foot problems in patients with epidermolysis bullosa simplex and pachyonychia congenita. Br. J. Dermatol. 2010, 163, 1072–1076. [Google Scholar] [CrossRef]
- Segreto, F.; Marangi, G.F.; Cerbone, V.; Persichetti, P. The Role of Botulinum Toxin A in the Treatment of Raynaud Phenomenon. Ann. Plast. Surg. 2016, 77, 318–323. [Google Scholar] [CrossRef]
- Shwe, S.; Sharma, A.A.; Chahal, H.S.; Doan, L.T.; Rojek, N.W. Botulinum Toxin for the Treatment of Intractable Raynaud Phenomenon. Cutis 2021, 108, E11–E14. [Google Scholar] [CrossRef]
- Ennis, D.; Ahmad, Z.; Anderson, M.A.; Johnson, S.R. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101684. [Google Scholar] [CrossRef]
- Bello, R.J.; Cooney, C.M.; Melamed, E.; Follmar, K.; Yenokyan, G.; Leatherman, G.; Shah, A.A.; Wigley, F.M.; Hummers, L.K.; Lifchez, S.D. The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud’s Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017, 69, 1661–1669. [Google Scholar] [CrossRef]
- Uppal, L.; Dhaliwal, K.; Butler, P.E. A prospective study of the use of botulinum toxin injections in the treatment of Raynaud’s syndrome associated with scleroderma. J. Hand Surg. Eur. Vol. 2014, 39, 876–880. [Google Scholar] [CrossRef]
- Smith, L.; Polsky, D.; Franks, A.G., Jr. Botulinum toxin-A for the treatment of Raynaud syndrome. Arch. Dermatol. 2012, 148, 426–428. [Google Scholar] [PubMed]
- Zhang, X.; Hu, Y.; Nie, Z.; Song, Y.; Pan, Y.; Liu, Y.; Jin, L. Treatment of Raynaud’s phenomenon with botulinum toxin type A. Neurol. Sci. 2015, 36, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Fregene, A.; Ditmars, D.; Siddiqui, A. Botulinum toxin type A: A treatment option for digital ischemia in patients with Raynaud’s phenomenon. J. Hand Surg. Am. 2009, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Castanedo, L.Q.; Rodríguez, M.F.; Pedrero, R.M.; Fernández, C.C.; Laguna, R.d.L. Ischemic ulcers of the toes secondary to Raynaud’s phenomenon in a child successfully treated with botulinum toxin. Pediatr. Dermatol. 2020, 37, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ríos, A.A.; González-Olivares, M.; Navarro-Vidal, B.; Martínez-Morán, C.; Borbujo, J. Ischaemic ulcers on the toes secondary to Raynaud phenomenon in a patient with systemic sclerosis successfully treated with botulinum toxin. Clin. Exp. Dermatol. 2018, 43, 503–505. [Google Scholar] [CrossRef]
- Motegi, S.; Yamada, K.; Toki, S.; Uchiyama, A.; Kubota, Y.; Nakamura, T.; Ishikawa, O. Beneficial effect of botulinum toxin A on Raynaud’s phenomenon in Japanese patients with systemic sclerosis: A prospective, case series study. J. Dermatol. 2016, 43, 56–62. [Google Scholar] [CrossRef]
- Blaise, S.; Roustit, M.; Forli, A.; Imbert, B.; Cracowski, J.L. Non-healing ischaemic digital ulcer in a systemic sclerosis patient: A challenging clinical case. Int. Wound J. 2017, 14, 978–981. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, H.R.; Lee, S.H.; Park, S.H.; Oh, J.; Choi, K. Refractory Digital Ulcers Treated by Botulinum Toxin and Endothelin Receptor-1 Antagonist in Anti-MDA5-Antibody-Positive Dermatomyositis. J. Clin. Neurol. 2020, 16, 160–162. [Google Scholar] [CrossRef]
- Medina, S.; Gómez-Zubiaur, A.; Valdeolivas-Casillas, N.; Polo-Rodríguez, I.; Ruíz, L.; Izquierdo, C.; Guirado, C.; Cabrera, A.; Trasobares, L. Botulinum toxin type A in the treatment of Raynaud’s phenomenon: A three-year follow-up study. Eur. J. Rheumatol. 2018, 5, 224–229. [Google Scholar] [CrossRef]
- Neumeister, M.W. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J. Hand Surg. Am. 2010, 35, 2085–2092. [Google Scholar] [CrossRef]
- Habib, S.M.; Brenninkmeijer, E.E.A.; Vermeer, M.H.; de Vries-Bouwstra, J.K.; Velthuis, P.J. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. Dermatol. Ther. 2020, 33, e14182. [Google Scholar] [CrossRef] [PubMed]
- Souk, J.W.; Kim, H.S. Effects of botulinum toxin injection on systemic sclerosis-related digital ulcers. Korean J. Intern. Med. 2019, 34, 1169–1170. [Google Scholar] [CrossRef]
- Zhong, J.; Lan, Y.; Fu, S.; Zhang, J.; Lu, S.; He, Y.; Zhang, J.M. Botulinum Toxin A Injection for Treatment of Chronic Skin Ulcer: A Case Series and Literature Review. Int. J. Low. Extrem. Wounds 2019, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Herlyn, K.; Martin, R.W.; Anderson, J.J.; Mayes, M.D.; Bell, P.; Korn, J.H.; Simms, R.W.; Csuka, M.E.; Medsger, T.A., Jr.; et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002, 46, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Wilson, D.H. Use of botulinum toxin to heal atypical pressure ulcers in the palm. Med. J. Aust. 2020, 212, 55–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.; Basciani, M. Botulinum toxin type A in the healing of a chronic buttock ulcer in a patient with spastic paraplegia after spinal cord injury. J. Rehabil. Med. 2009, 41, 1100–1102. [Google Scholar] [CrossRef]
- Intiso, D.; Basciani, M.; Di Rienzo, F.; Tolfa, M.; Grimaldi, G.; Fiore, P. Botulinum toxin type A in the healing of ulcer following oro-mandibular dyskinesia in a patient in a vegetative state. J. Rehabil. Med. 2008, 40, 315–316. [Google Scholar] [CrossRef][Green Version]
- Sillitoe, A.T.; Bains, R.D.; Stanley, P.R.W. Botulinum toxin as a treatment for leg ulcers. Plast. Reconstr. Surg. 2007, 119, 1633. [Google Scholar] [CrossRef]
- Turkel, C.C.; Bowen, B.; Liu, J.; Brin, M.F. Pooled analysis of the safety of botulinum toxin type A in the treatment of poststroke spasticity. Arch. Phys. Med. Rehabil. 2006, 87, 786–792. [Google Scholar] [CrossRef]
- Bjornson, K.; Hays, R.; Graubert, C.; Price, R.; Won, F.; McLaughlin, J.F.; Cohen, M. Botulinum toxin for spasticity in children with cerebral palsy: A comprehensive evaluation. Pediatrics 2007, 120, 49–58. [Google Scholar] [CrossRef]
- Laarakker, A.S.; Borah, G. Botulinum Toxin A Salvage of Ischemic Hand Trauma. Plast. Reconstr. Surg. 2020, 145, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.; Garcia, J.; Liao, E. Botox to the rescue. Plast. Reconstr. Surg. 2009, 123, 38e. [Google Scholar] [CrossRef] [PubMed]
- Alsharqi, A.; Curley, R.; Winhoven, S. Botulinum toxin type A in the management of a neuropathic foot ulcer. Clin. Exp. Dermatol. 2011, 36, 915–916. [Google Scholar] [CrossRef] [PubMed]


| Authors, Year | Study Type | N (Gender) | Age, Years (Mean or Range) | Type of BoNT-A | BoNT-A Dilution with 0.9% (NSS) | BoNT-A Dose/Location | Follow-Up Period | Results | Reinjection (Interval) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Quinatana Castanedo et al., 2020 [44] | Case report | 1 (female) | 15 | NR | NR | 32 units/Foot | 4 weeks |
| Yes (every 12 months) | |
| Habib et al., 2020 [51] | Case series | 3 (female) | 23–50 | NR | NR | 32 units/Hand | 1 week |
| No | |
| Min et al., 2020 [48] | Case report | 1 (male) | 48 | Medytoxin® (Medytox, Seoul, Korea) | 10 units/0.1 mL | 10 units/Hand | 12 weeks |
| Yes (weekly for 3 weeks) | |
| Souk and Kim, 2019 [52] | Case report | 2 (female) | 50 and 62 | Medytoxin® (Medytox, Seoul, Korea) | 10 units/0.1 mL | 10 units/Hand | 8 weeks |
| Yes 2/2 (4 and 5 weeks, 7 and 8 weeks) | |
| Garrido-Rios et al., 2018 [45] | Case report | 1 (female) | 30 | NR | 8–10 units/0.4 mL | 80–100 units/Hand | 2 months |
| No | |
| Medina et al., 2018 [49] | Retrospective case series | 15 (female 14/male 1) | 35–71 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/5 mL | Average 45 units/Hand | 3 years |
| Yes 6/15 (annually) | 4/15 temporary decrease intrinsic muscle strength |
| Blaise et al., 2017 [47] | Case report | 1 (female) | 55 | NR | NR | 100 units/Hand | 4 months |
| No | |
| Motegi et al., 2016 [46] | Prospective, case series | 10 (NR) | 62.5 (±3.5) | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 20 units/0.1 mL | 10 units/Hand | 16 weeks |
| No | |
| Zhang et al., 2015 [42] | Retrospective case series | 10 (female 5/male 5) | 48–91 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/5 mL | 60 units/Hand | 6 months (average) |
| No | |
| Smith et al., 2012 [41] | Case report | 1 (female) | 52 | NR | 5 units/0.1 mL | 100 units/Hand | 3 months |
| No | Mild, nonlimiting thenar muscle weakness |
| Neumeister. 2010 [50] | Retrospective case series | 33 (female 19/male 14) | 18–72 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/20 mL | 50 units/Hand | 6 years |
| Yes 7/33 (not reported) | - 3 patients had temporary intrinsic muscle weakness that lasted 2 months |
| Fregene et al., 2009 [43] | Retrospective case series | 26 (female 14/male 12) | 60.7 (±1.9) | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | 100 units/2 mL | Average 77 units/Hand | 18 months (average) |
| No | - Some reported intrinsic muscle weakness and 1 dysesthesia digit which resolved completely by 5 months |
| Authors, Year | Study Type | N (Gender) | Age, Years (Mean or Range) | Type of BoNT-A | BoNT-A Dilution with 0.9% NSS | BoNT-A Dose/Location | Follow-Up Period | Results | Reinjection (Interval) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Gupta and Wilson, 2020 [55] | Case report | 1 (female) | 59 | NR | NR | 150 units for pectoralis major, 150 for elbow flexors, 100 for flexor digitorum superficialis | 5 months | Completely healed ulcer | Yes (5 months) | Pressure ulcer |
| Insito and Basciani, 2009 [56] | Case report | 1 (male) | 27 | Dysport®, Ipsen Limited, Slough, UK | NR | 660 Speywood units (left Gluteus maximus) | 6 months | Weaken muscle contraction Healed ulcer | Yes (3 months) | Pressure ulcer |
| Insito et al., 2008 [57] | Case report | 1 (male) | 73 | Dysport®, Ipsen Limited, Slough, UK | NR | 200 Speywood units for Orbicularis oris, 120 for Masseter | 3 months | Improved dyskinetic disorder Completely healed ulcer | Yes (2 months) | Pressure ulcer |
| Sillitoe et al., 2007 [58] | Letter to editors | 1 (male) | 58 | NR | NR | NR (adductor muscle bellies lower limbs) | 16 weeks | Marked reduction in spasticity Ulcers showed signs of healing Ulcers show significant improvement Ulcers fully healed | No | Pressure ulcer |
| Laarakker and Borah, 2020 [61] | Retrospective cohort, case series | 5 (NR) | 31–71 | NR | NR | 80–100 units (palm and wrist) | NR | All Digits were preserved | No | Traumatic ulcer |
| Upton et al., 2009 [62] | Letter to editors | 1 (NR) | 4 | NR | NR | 10 units (palm) | NR | The digits were rescued | No | Traumatic ulcer |
| Zhong et al., 2019 [53] | Case series | 4 (female 1/male 3) | 16–78 | NR | NR | 32–48 units (face, leg, foot) | 50 days | Ulcers healed | No | Chronic skin ulcer |
| Alsharqi et al., 2011 [63] | Correspondence | 1 (male) | 51 | Botox® (Allergan Pharmaceuticals Ltd., Westport, Ireland) | NR | 70 units (right foot) | 3 months | Completely healed ulcer | Yes (3 months) | Neuropathic ulcer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winayanuwattikun, W.; Vachiramon, V. Botulinum Toxin Type A for the Treatment of Skin Ulcers: A Review Article. Toxins 2022, 14, 406. https://doi.org/10.3390/toxins14060406
Winayanuwattikun W, Vachiramon V. Botulinum Toxin Type A for the Treatment of Skin Ulcers: A Review Article. Toxins. 2022; 14(6):406. https://doi.org/10.3390/toxins14060406
Chicago/Turabian StyleWinayanuwattikun, Waranaree, and Vasanop Vachiramon. 2022. "Botulinum Toxin Type A for the Treatment of Skin Ulcers: A Review Article" Toxins 14, no. 6: 406. https://doi.org/10.3390/toxins14060406
APA StyleWinayanuwattikun, W., & Vachiramon, V. (2022). Botulinum Toxin Type A for the Treatment of Skin Ulcers: A Review Article. Toxins, 14(6), 406. https://doi.org/10.3390/toxins14060406






