Characterization of Four Medically Important Toxins from Centruroides huichol Scorpion Venom and Its Neutralization by a Single Recombinant Antibody Fragment
Abstract
:1. Introduction
2. Results
2.1. Preliminary Neutralization Assays of C. huichol Venom with scFvs 10FG2 and LR
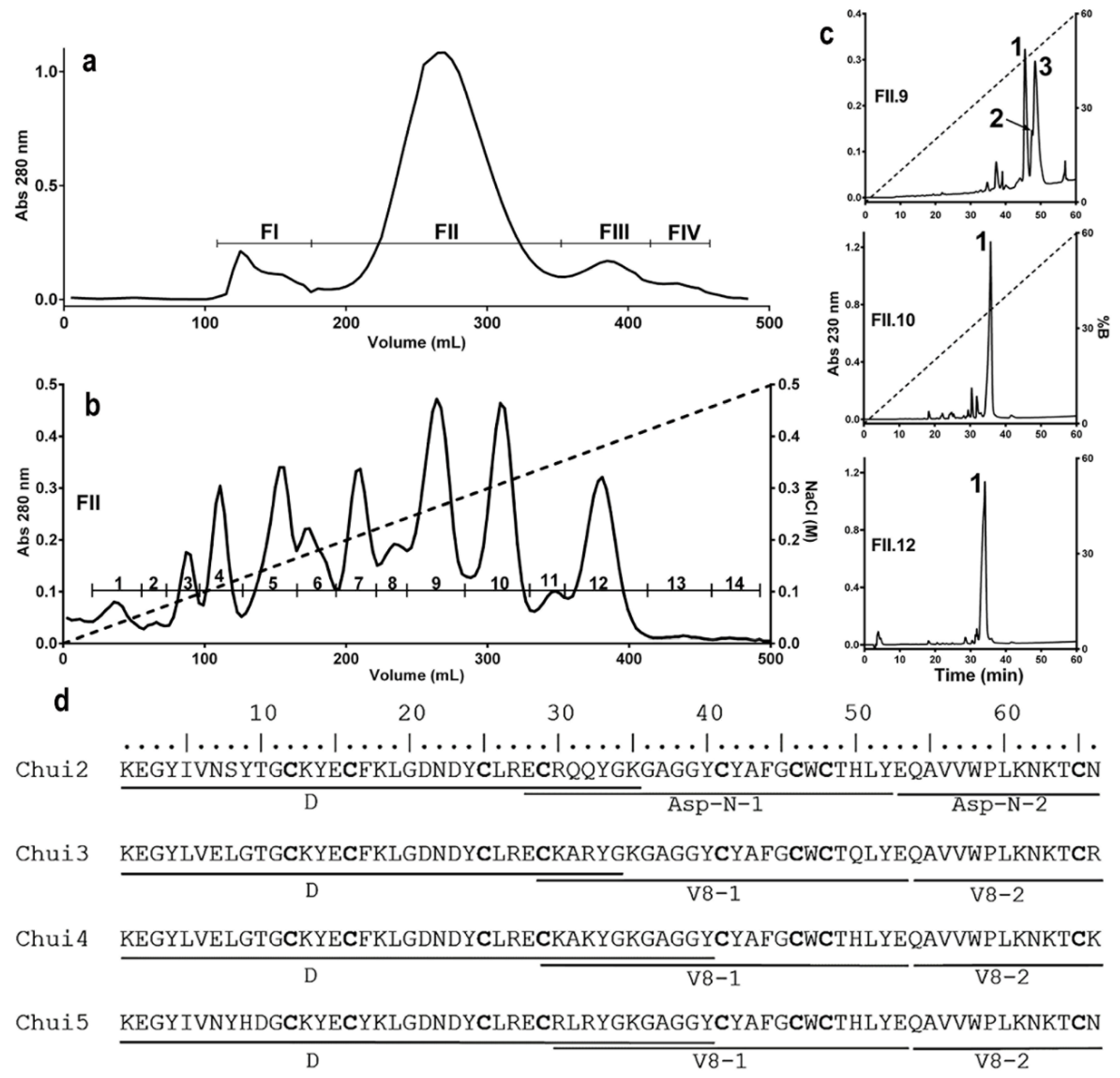
2.2. Characterization of Toxic Components
2.3. Evaluation of the Recognition of scFvs LR and 10FG2 toward C. huichol Toxins
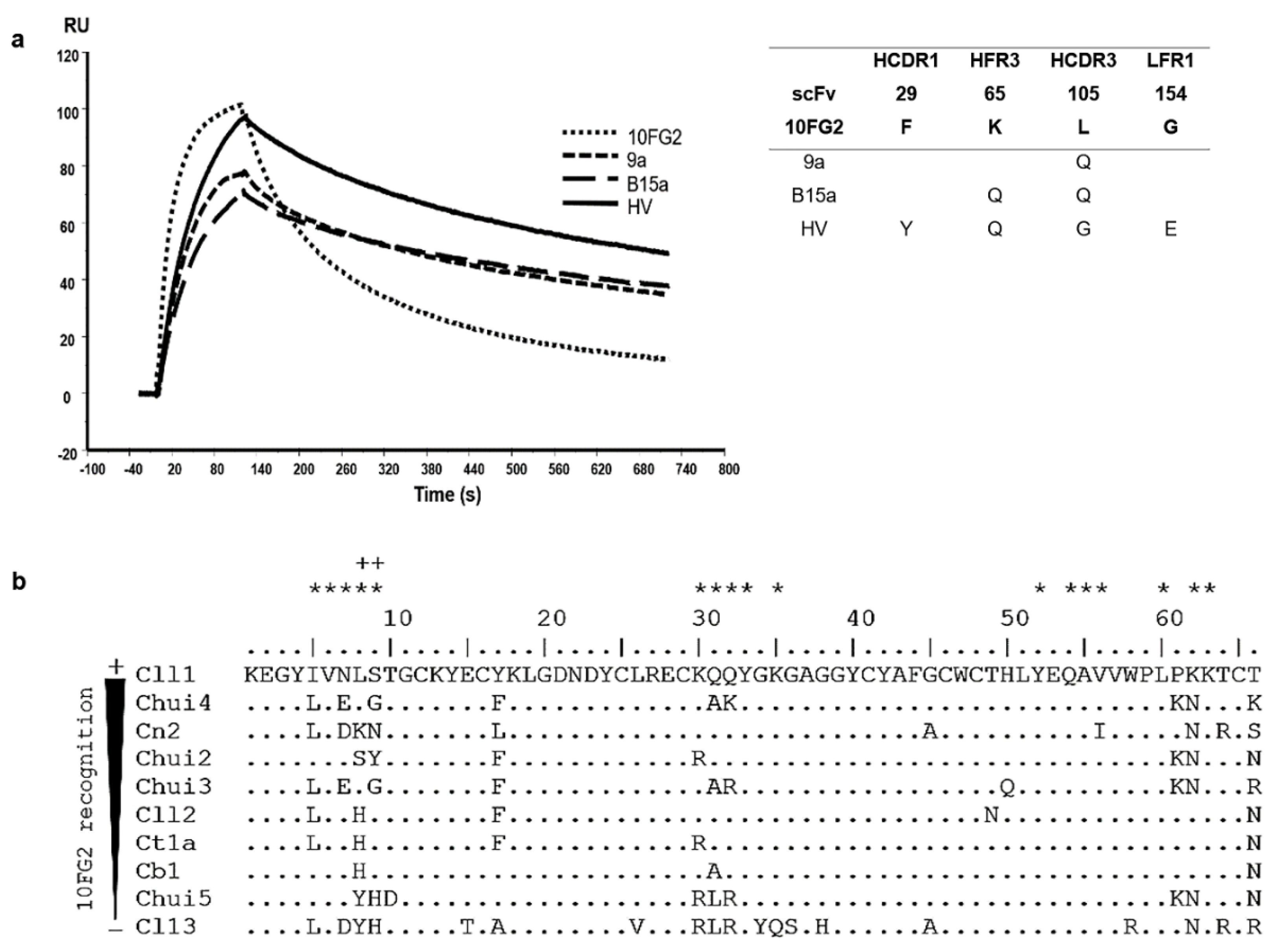
2.4. Affinity Maturation of scFv 10FG2 against Chui5 Toxin
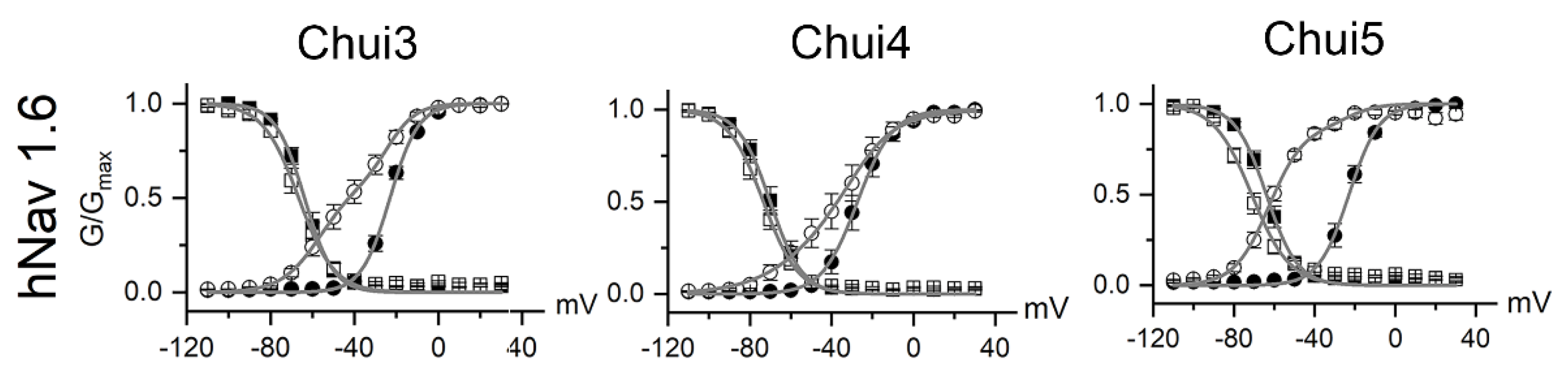
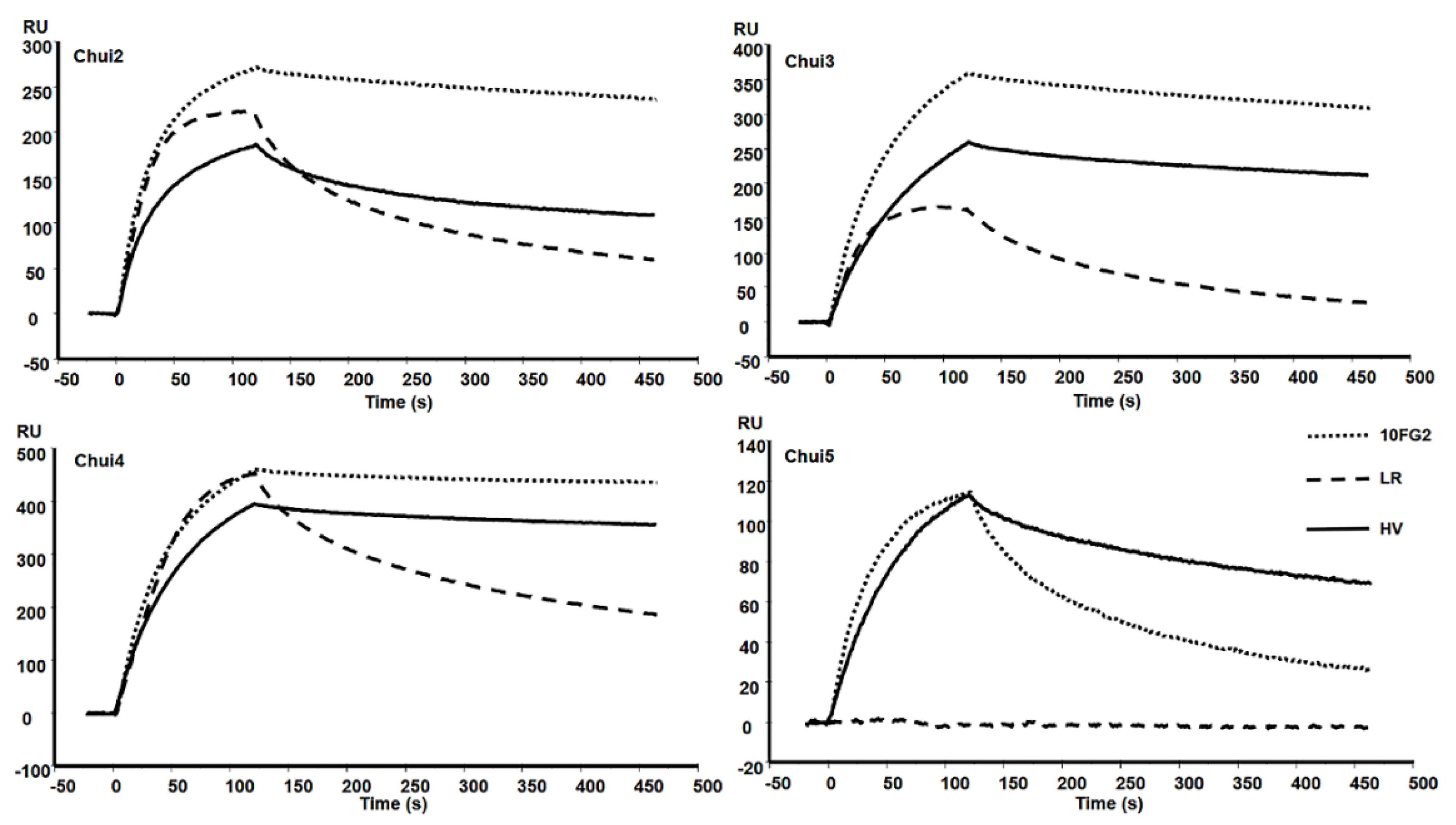
2.5. Determination of the Kinetic Constants of scFv HV against C. huichol Toxins
2.6. Neutralization of C. huichol Venom with scFv HV
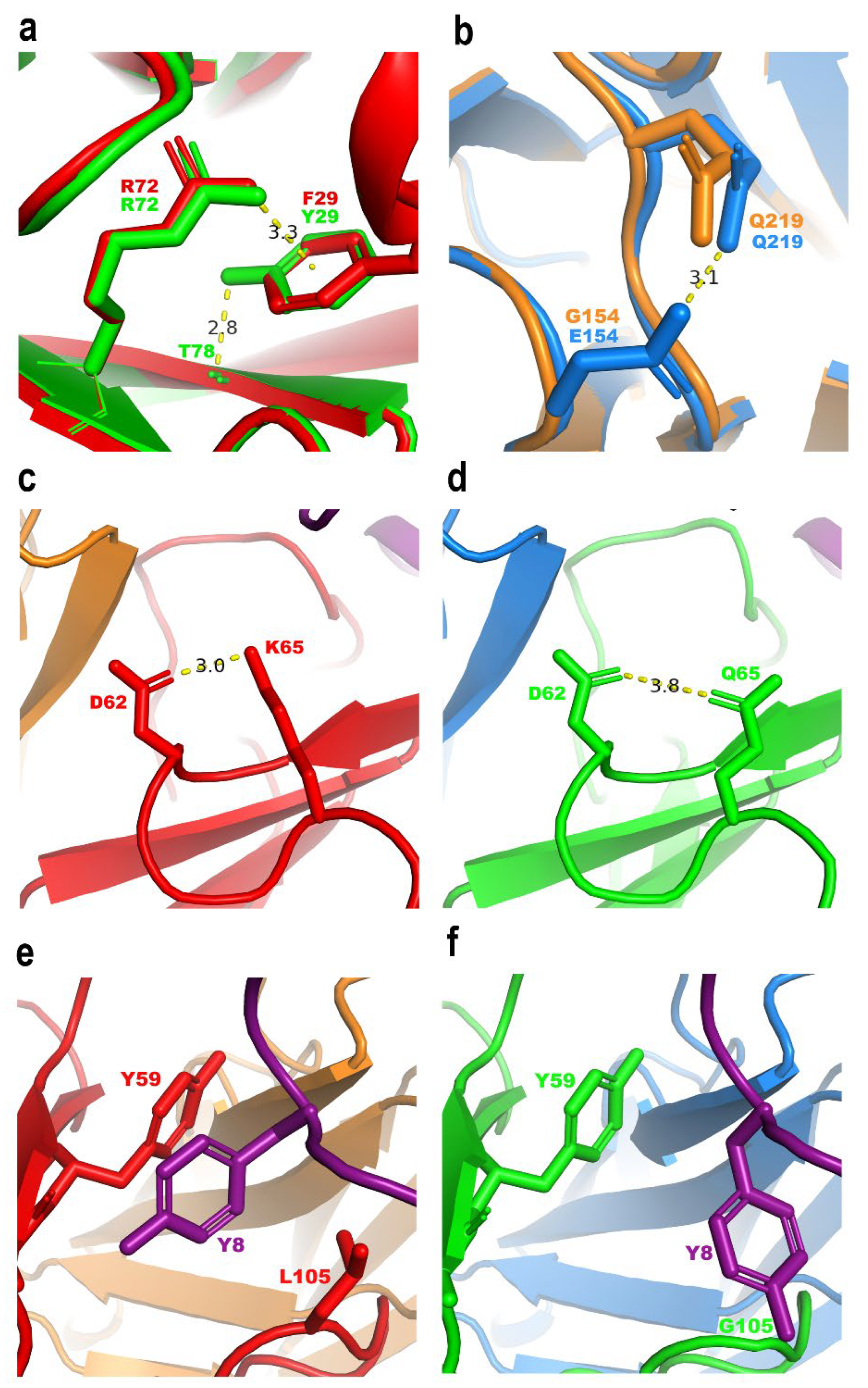
2.7. Thermodynamic and Structural Analysis of the Improvement of scFv HV
2.7.1. Thermodynamic Analysis
2.7.2. In Silico Models of Chui5 Toxin with scFvs 10FG2 and HV
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venom and Reagents
5.2. Enzymes
5.3. Purification, Analysis by Mass Spectrometry, and Sequencing of Toxins
5.4. Alkylation and Enzymatic Digestion of Toxins
5.5. Electro Physiology Assays
5.6. scFv Expression
5.7. Directed Evolution and Screening
5.7.1. First Library with Site-Directed Mutagenesis
5.7.2. Library Construction with Random Mutagenesis
5.7.3. Construction of the Second Library with Site Directed Mutagenesis
5.8. Surface Plasmon Resonance Measurements
5.9. Toxin Neutralization Assays
5.10. Neutralization Assays with Whole Fresh Venom of C. huichol
5.10.1. Classic Protection (Mix-Type Assays)
5.10.2. Rescue Assays
5.11. Calculation of Thermodynamic Parameters
5.12. In Silico Mutagenesis and Modeling
5.13. Access Numbers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| scFv | Single chain fragment variable |
| SPR | Surface plasmon resonance |
| LD | Lethal dose |
| HCDR | Heavy chain Complementary-determining regions |
| HFR | Heavy chain Framework region |
| LFR | Light chain Framework region |
| CMC | Carboxymethylcellulose |
| nM | nanomolar |
| KD | Affinity constant |
| TR | Residence time |
References
- Casos Nuevos de Intoxicación por Picadura de Alacrán (T63.2, X22) por Fuente de Notificación. 2020. Available online: https://epidemiologia.salud.gob.mx/anuario/2020/casos/fuente/094.pdf (accessed on 8 March 2022).
- Riano-Umbarila, L.; Rodriguez-Rodriguez, E.R.; Santibanez-Lopez, C.E.; Guereca, L.; Uribe-Romero, S.J.; Gomez-Ramirez, I.V.; Carcamo-Noriega, E.N.; Possani, L.D.; Becerril, B. Updating knowledge on new medically important scorpion species in Mexico. Toxicon Off. J. Int. Soc. Toxinol. 2017, 138, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santillan, E.; Possani, L.D. North American scorpion species of public health importance with a reappraisal of historical epidemiology. Acta Trop. 2018, 187, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Teruel, R.; Ponce-Saavedra, J.; Quijano-Ravell, A.F. Redescription of Centruroides noxius and description of a closely related new species from western Mexico (Scorpiones: Buthidae). Rev. Mex. Biodivers. 2015, 86, 896–911. [Google Scholar] [CrossRef] [Green Version]
- Pucca, M.B.; Zoccal, K.F.; Roncolato, E.C.; Bertolini, T.B.; Campos, L.B.; Cologna, C.T.; Faccioli, L.H.; Arantes, E.C.; Barbosa, J.E. Serrumab: A human monoclonal antibody that counters the biochemical and immunological effects of Tityus serrulatus venom. J. Immunotoxicol. 2012, 9, 173–183. [Google Scholar] [CrossRef]
- Gomes, M.; Alvarez, M.A.; Quellis, L.R.; Becher, M.L.; Castro, J.M.A.; Gameiro, J.; Caporrino, M.C.; Moura-da-Silva, A.M.; de Oliveira Santos, M. Expression of an scFv antibody fragment in Nicotiana benthamiana and in vitro assessment of its neutralizing potential against the snake venom metalloproteinase BaP1 from Bothrops asper. Toxicon Off. J. Int. Soc. Toxinol. 2019, 160, 38–46. [Google Scholar] [CrossRef]
- Khanongnoi, J.; Phanthong, S.; Reamtong, O.; Tungtronchitr, A.; Chaicumpa, W.; Sookrung, N. Human Monoclonal scFvs that Neutralize Fribrinogenolytic Activity of Kaouthiagin, a Zinc-Metalloproteinase in Cobra (Naja kaouthia) Venom. Toxins 2018, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Batra, S.K.; Jain, M.; Wittel, U.A.; Chauhan, S.C.; Colcher, D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr. Opin. Biotechnol. 2002, 13, 603–608. [Google Scholar] [CrossRef]
- Barderas, R.; Desmet, J.; Timmerman, P.; Meloen, R.; Casal, J.I. Affinity maturation of antibodies assisted by in silico modeling. Proc. Natl. Acad. Sci. USA 2008, 105, 9029–9034. [Google Scholar] [CrossRef] [Green Version]
- Riano-Umbarila, L.; Contreras-Ferrat, G.; Olamendi-Portugal, T.; Morelos-Juarez, C.; Corzo, G.; Possani, L.D.; Becerril, B. Exploiting cross-reactivity to neutralize two different scorpion venoms with one single chain antibody fragment. J. Biol. Chem. 2011, 286, 6143–6151. [Google Scholar] [CrossRef] [Green Version]
- Myszka, D.G. Kinetic, equilibrium, and thermodynamic analysis of macromolecular interactions with BIACORE. Methods Enzymol. 2000, 323, 325–340. [Google Scholar] [CrossRef]
- Riano-Umbarila, L.; Juarez-Gonzalez, V.R.; Olamendi-Portugal, T.; Ortiz-Leon, M.; Possani, L.D.; Becerril, B. A strategy for the generation of specific human antibodies by directed evolution and phage display. An example of a single-chain antibody fragment that neutralizes a major component of scorpion venom. FEBS J. 2005, 272, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Riano-Umbarila, L.; Ledezma-Candanoza, L.M.; Serrano-Posada, H.; Fernandez-Taboada, G.; Olamendi-Portugal, T.; Rojas-Trejo, S.; Gomez-Ramirez, I.V.; Rudino-Pinera, E.; Possani, L.D.; Becerril, B. Optimal Neutralization of Centruroides noxius Venom Is Understood through a Structural Complex between Two Antibody Fragments and the Cn2 Toxin. J. Biol. Chem. 2016, 291, 1619–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riano-Umbarila, L.; Olamendi-Portugal, T.; Morelos-Juarez, C.; Gurrola, G.B.; Possani, L.D.; Becerril, B. A novel human recombinant antibody fragment capable of neutralizing Mexican scorpion toxins. Toxicon Off. J. Int. Soc. Toxinol. 2013, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, E.R.; Olamendi-Portugal, T.; Serrano-Posada, H.; Arredondo-Lopez, J.N.; Gomez-Ramirez, I.; Fernandez-Taboada, G.; Possani, L.D.; Anguiano-Vega, G.A.; Riano-Umbarila, L.; Becerril, B. Broadening the neutralizing capacity of a family of antibody fragments against different toxins from Mexican scorpions. Toxicon Off. J. Int. Soc. Toxinol. 2016, 119, 52–63. [Google Scholar] [CrossRef]
- Riano-Umbarila, L.; Gomez-Ramirez, I.V.; Ledezma-Candanoza, L.M.; Olamendi-Portugal, T.; Rodriguez-Rodriguez, E.R.; Fernandez-Taboada, G.; Possani, L.D.; Becerril, B. Generation of a Broadly Cross-Neutralizing Antibody Fragment against Several Mexican Scorpion Venoms. Toxins 2019, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Olamendi-Portugal, T.; Restano-Cassulini, R.; Riano-Umbarila, L.; Becerril, B.; Possani, L.D. Functional and immuno-reactive characterization of a previously undescribed peptide from the venom of the scorpion Centruroides limpidus. Peptides 2017, 87, 34–40. [Google Scholar] [CrossRef]
- Fernandez-Taboada, G.; Riano-Umbarila, L.; Olvera-Rodriguez, A.; Gomez-Ramirez, I.V.; Losoya-Uribe, L.F.; Becerril, B. The venom of the scorpion Centruroides limpidus, which causes the highest number of stings in Mexico, is neutralized by two recombinant antibody fragments. Mol. Immunol. 2021, 137, 247–255. [Google Scholar] [CrossRef]
- Dixon, W.J.; Mood, A.M. A Method for Obtaining and Analyzing Sensitivity Data. J. Am. Stat. Assoc. 1948, 43, 109–126. [Google Scholar] [CrossRef]
- Riano-Umbarila, L.; Romero-Moreno, J.A.; Ledezma-Candanoza, L.M.; Olamendi-Portugal, T.; Possani, L.D.; Becerril, B. Full Neutralization of Centruroidessculpturatus Scorpion Venom by Combining Two Human Antibody Fragments. Toxins 2021, 13, 708. [Google Scholar] [CrossRef]
- Alagon, A.C.; Guzman, H.S.; Martin, B.M.; Ramirez, A.N.; Carbone, E.; Possani, L.D. Isolation and characterization of two toxins from the Mexican scorpion Centruroides limpidus limpidus Karsch. Comp. Biochem. Physiol. B 1988, 89, 153–161. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Bartok, A.; Zamudio-Zuniga, F.; Balajthy, A.; Becerril, B.; Panyi, G.; Possani, L.D. Isolation, chemical and functional characterization of several new K(+)-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon Off. J. Int. Soc. Toxinol. 2016, 115, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restano-Cassulini, R.; Olamendi-Portugal, T.; Zamudio, F.; Becerril, B.; Possani, L.D. Two novel ergtoxins, blockers of K+-channels, purified from the Mexican scorpion Centruroides elegans elegans. Neurochem. Res. 2008, 33, 1525–1533. [Google Scholar] [CrossRef]
- Gomez-Ramirez, I.V.; Riano-Umbarila, L.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Possani, L.D.; Becerril, B. Biochemical, electrophysiological and immunological characterization of the venom from Centruroides baergi, a new scorpion species of medical importance in Mexico. Toxicon Off. J. Int. Soc. Toxinol. 2020, 184, 10–18. [Google Scholar] [CrossRef]
- Deinum, J.; Gustavsson, L.; Gyzander, E.; Kullman-Magnusson, M.; Edstrom, A.; Karlsson, R. A thermodynamic characterization of the binding of thrombin inhibitors to human thrombin, combining biosensor technology, stopped-flow spectrophotometry, and microcalorimetry. Anal. Biochem. 2002, 300, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein-Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 114. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure-function relationships and evolution. Toxicon Off. J. Int. Soc. Toxinol. 2005, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Chames, P. Selection of Stable scFv Antibodies by Phage Display. In Antibody Engineering; Chames, P., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar]
- Rodriguez-Rodriguez, E.R.; Ledezma-Candanoza, L.M.; Contreras-Ferrat, L.G.; Olamendi-Portugal, T.; Possani, L.D.; Becerril, B.; Riano-Umbarila, L. A single mutation in framework 2 of the heavy variable domain improves the properties of a diabody and a related single-chain antibody. J. Mol. Biol. 2012, 423, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Roos, H.; Karlsson, R.; Nilshans, H.; Persson, A. Thermodynamic analysis of protein interactions with biosensor technology. J. Mol. Recognit. JMR 1998, 11, 204–210. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef] [Green Version]
| Venom | μg/20 g of Mouse | LD50 | Molar Ratio | Survivors (Alive/Total) | |
|---|---|---|---|---|---|
| Control | Mix-Type Assay | ||||
| C. huichol | 15.4 | 1 | 1:10:10 | 1/4 *** | 3/4 ** |
| Sub-Fraction | Name | Toxicity | Experimental MW (Da) | Theoretical MW (Da) | Relative Abundance (%) |
|---|---|---|---|---|---|
| FII.9-1 | Chui1 | No | 7405.8 | ND | 5.9 |
| FII.9-2 | Chui2 | Yes | 7633.5 | 7633.7 | 0.3 |
| FII.9-3 | Chui3/Cn3 | Yes | 7543.9 | 7544.7 | 1.8 |
| FII.10-1 | Chui4 | Yes | 7496.7 | 7497.6 | 6.2 |
| FII.12-1 | Chui5 | Yes | 7725.6 | 7726.8 | 5.7 |
| scFv | Toxin | kon (×105 M−1 s−1) | koff (×10−4 s−1) | TR (min) | KD (nM) |
|---|---|---|---|---|---|
| 10FG2 | Chui2 | 2.8 | 2.8 | 60.4 | 1.1 |
| Chui3 | 1.8 | 3.1 | 53.1 | 1.8 | |
| Chui4 | 2.1 | 0.7 | 241.5 | 0.3 | |
| Chui5 | 2.6 | 34.1 | 4.9 | 12.2 | |
| HV | Chui2 | 1.6 | 4.9 | 34.2 | 3.1 |
| Chui3 | 0.9 | 3.4 | 48.8 | 3.8 | |
| Chui4 | 1.8 | 1.6 | 104.8 | 0.9 | |
| Chui5 | 1.6 | 10.8 | 15.5 | 6.9 |
| Toxin | 1 LD100 (μg/20 g of Mouse) | Molar Ratio | Survivors (Alive/Total) | |
|---|---|---|---|---|
| Control | Mix-Type Assay | |||
| Chui3 | 2 | 1:10 | 0/6 *** | 6/6 |
| Chui4 | 2.5 | 1:10 | 0/6 *** | 6/6 |
| Chui5 | 2 | 1:10 | 0/6 *** | 2/6 *** |
| scFv | kon (×105 M−1 s−1) | koff (×10−4 s−1) | TR (min) | KD (nM) | Yield (mg/L) |
|---|---|---|---|---|---|
| 10FG2 | 2.6 | 34.1 | 4.9 | 12.2 | 3.1 |
| 9a | 1.5 | 11.9 | 14.0 | 8.2 | 2.3 |
| B15a | 1.3 | 9.7 | 17.1 | 7.3 | 1.5 |
| HV | 1.6 | 10.8 | 15.5 | 6.9 | 1.7 |
| scFv | LD50 | μg/20 g of Mouse | Molar Ratio | Appearance of Signs (min) | Survivors (Alive/Total) | |
|---|---|---|---|---|---|---|
| Control | Mix-Type Assay | |||||
| - | 2 | 2.2 | - | 8 | 0/6 *** | - |
| 10FG2 | 2 | 2.2 | 1:10 | 18 | 0/6 *** | 1/6 *** |
| 9a | 2 | 2.2 | 1:10 | 99 | 0/4 *** | 3/5 ** |
| B15a | 2 | 2.2 | 1:10 | 125 | 0/6 *** | 2/6 ** |
| HV | 2 | 2.2 | 1:10 | 138 | 0/4 *** | 3/4 * |
| HV | 1 | 1.1 | 1:20 | NS | 3/6 *** | 6/6 |
| Assay | Group | LD50 | Molar Ratio | Survivors (Alive/Total) |
|---|---|---|---|---|
| Control | 2 | - | 0/6 *** | |
| HV | 1 | 1:10 | 6/6 * | |
| Mix-type | 2 | 1:5 | 4/6 * | |
| 3 | 1:3.3 | 2/6 ** | ||
| 10FG2 + HV | 1 | 1:10:10 | 6/6 | |
| 5 | 1:5:5 | 6/6 ** | ||
| Rescue | Control | 3 | - | 0/6 *** |
| HV | 3 | 1:10 | 6/6 ** |
| scFv | ΔG (kcal·mol−1) | ΔH (kcal·mol−1) | ΔS (cal·mol−1·K−1) |
|---|---|---|---|
| 10FG2 | −10.8 | −2.6 | 27.3 |
| B15a | −11.1 | −2.6 | 28.6 |
| HV | −11.1 | −1.6 | 31.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Martínez, H.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Serrano-Posada, H.; Zamudio, F.; Possani, L.D.; Riaño-Umbarila, L.; Becerril, B. Characterization of Four Medically Important Toxins from Centruroides huichol Scorpion Venom and Its Neutralization by a Single Recombinant Antibody Fragment. Toxins 2022, 14, 369. https://doi.org/10.3390/toxins14060369
Valencia-Martínez H, Olamendi-Portugal T, Restano-Cassulini R, Serrano-Posada H, Zamudio F, Possani LD, Riaño-Umbarila L, Becerril B. Characterization of Four Medically Important Toxins from Centruroides huichol Scorpion Venom and Its Neutralization by a Single Recombinant Antibody Fragment. Toxins. 2022; 14(6):369. https://doi.org/10.3390/toxins14060369
Chicago/Turabian StyleValencia-Martínez, Hugo, Timoteo Olamendi-Portugal, Rita Restano-Cassulini, Hugo Serrano-Posada, Fernando Zamudio, Lourival D. Possani, Lidia Riaño-Umbarila, and Baltazar Becerril. 2022. "Characterization of Four Medically Important Toxins from Centruroides huichol Scorpion Venom and Its Neutralization by a Single Recombinant Antibody Fragment" Toxins 14, no. 6: 369. https://doi.org/10.3390/toxins14060369
APA StyleValencia-Martínez, H., Olamendi-Portugal, T., Restano-Cassulini, R., Serrano-Posada, H., Zamudio, F., Possani, L. D., Riaño-Umbarila, L., & Becerril, B. (2022). Characterization of Four Medically Important Toxins from Centruroides huichol Scorpion Venom and Its Neutralization by a Single Recombinant Antibody Fragment. Toxins, 14(6), 369. https://doi.org/10.3390/toxins14060369







