Multi-Omics Reveal Additive Cytotoxicity Effects of Aflatoxin B1 and Aflatoxin M1 toward Intestinal NCM460 Cells
Abstract
:1. Introduction
2. Results
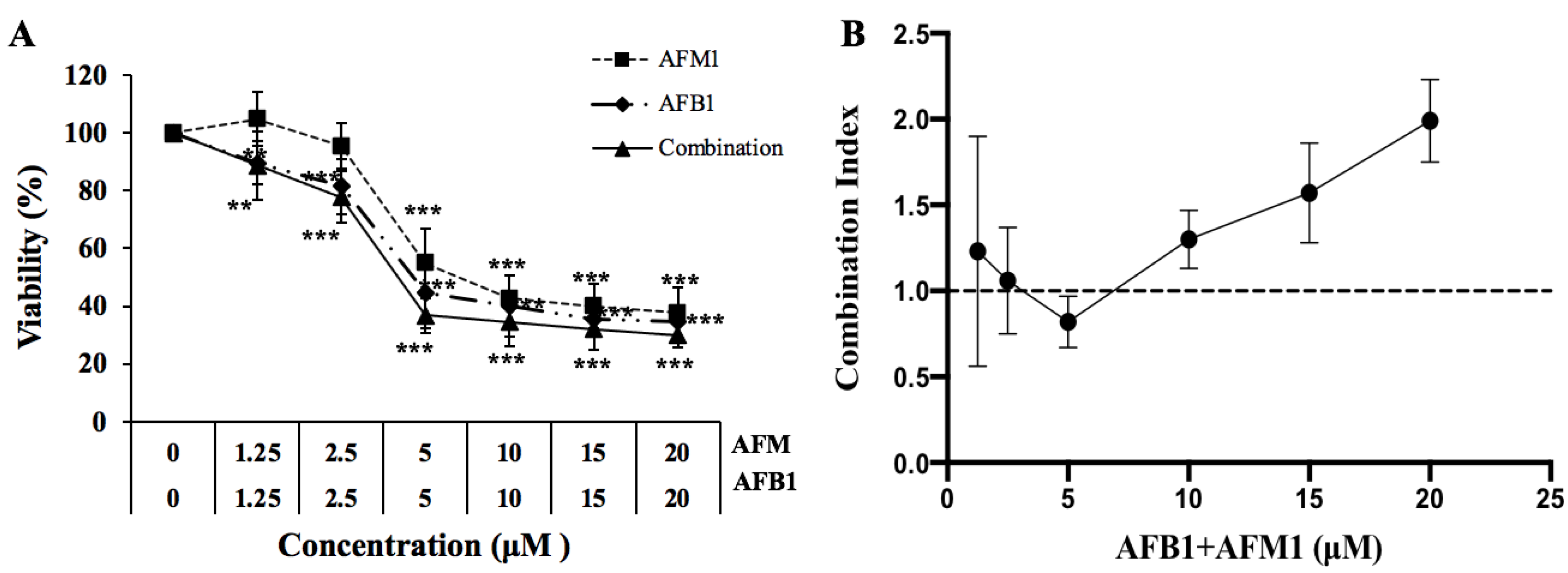
2.1. NCM460 Cytotoxicity Is Induced by AFB1 and AFM1
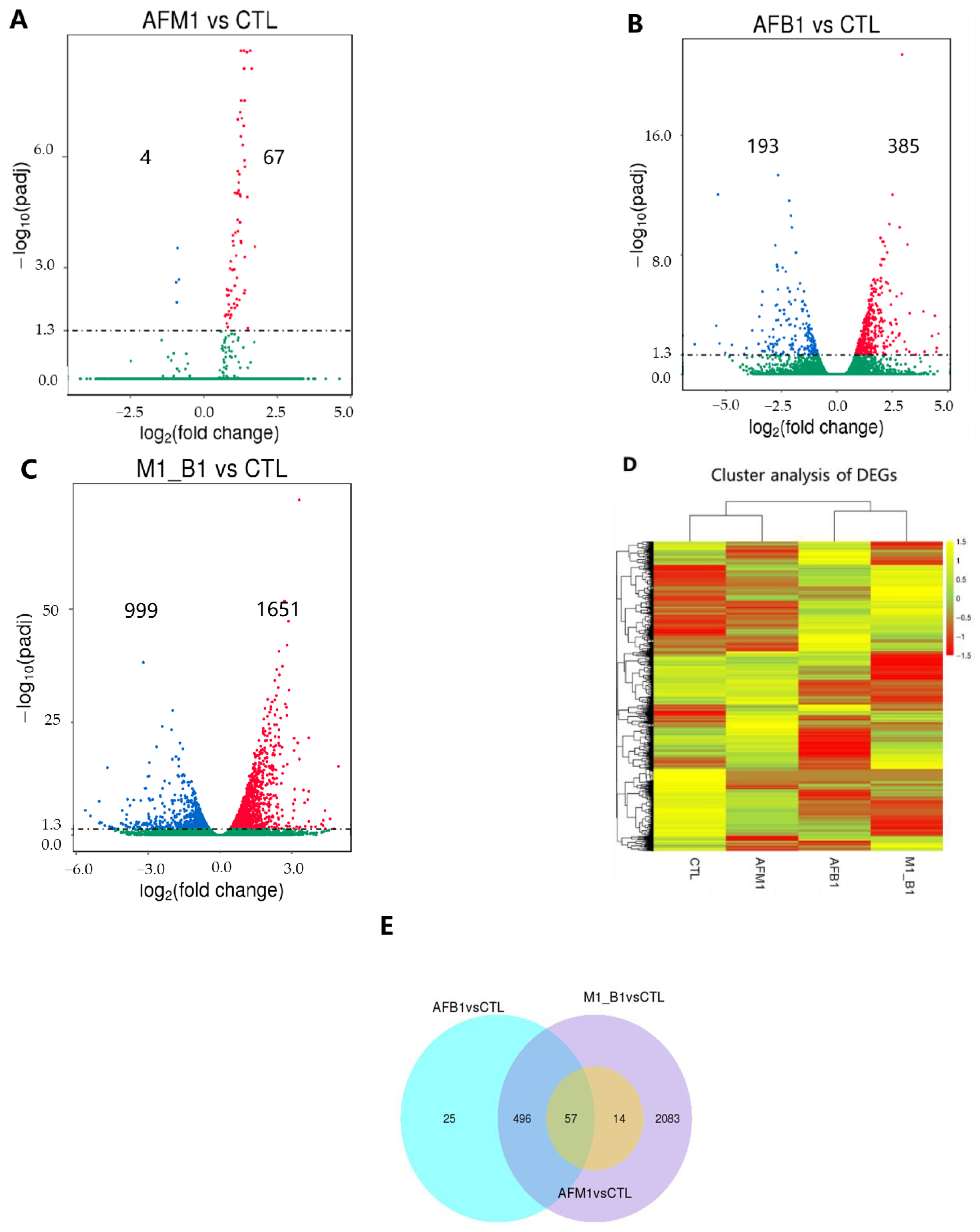
2.2. Transcriptomic Analysis of NCM460 Cells
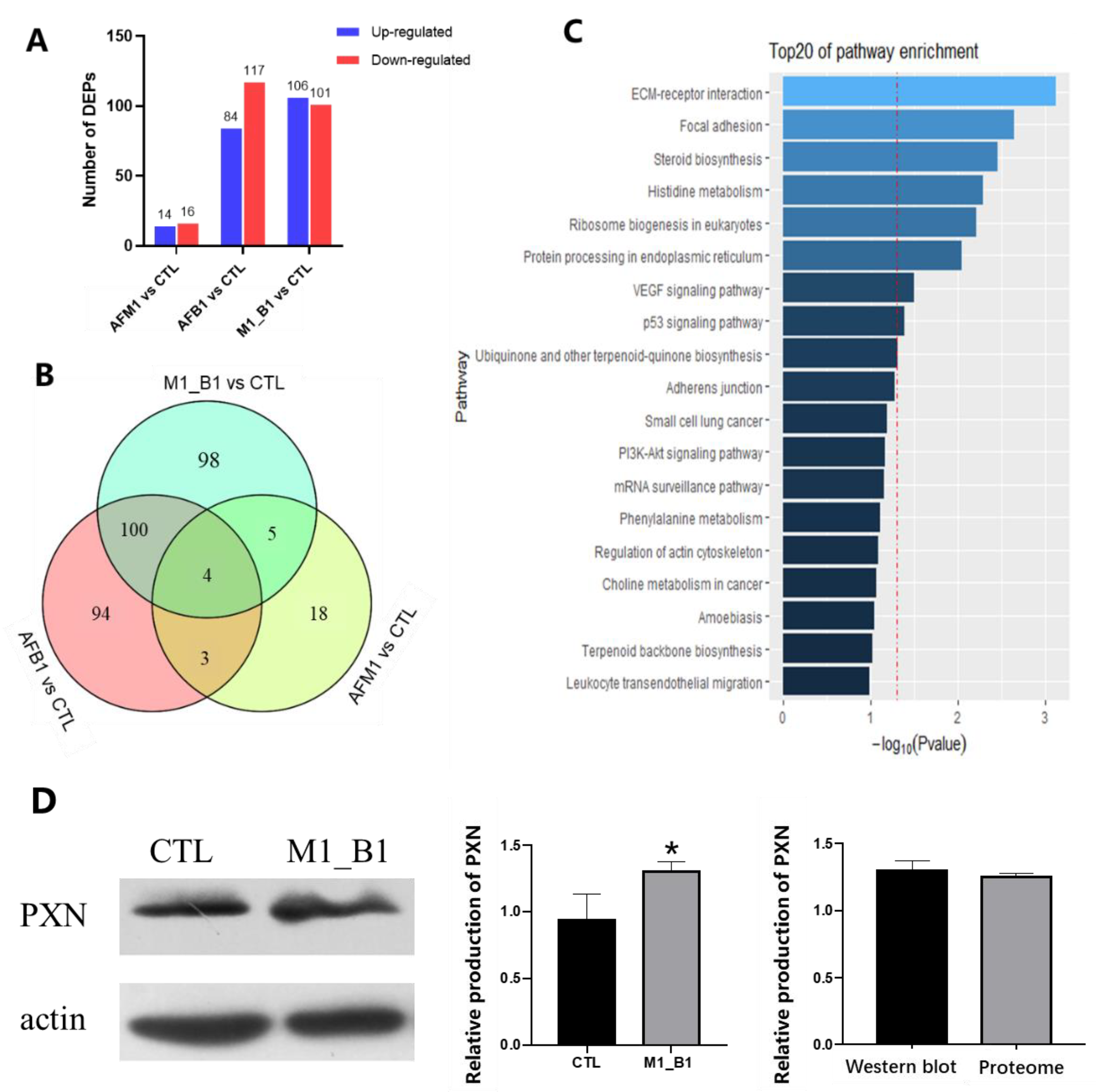
2.3. Proteomic Analysis of NCM460 Cells
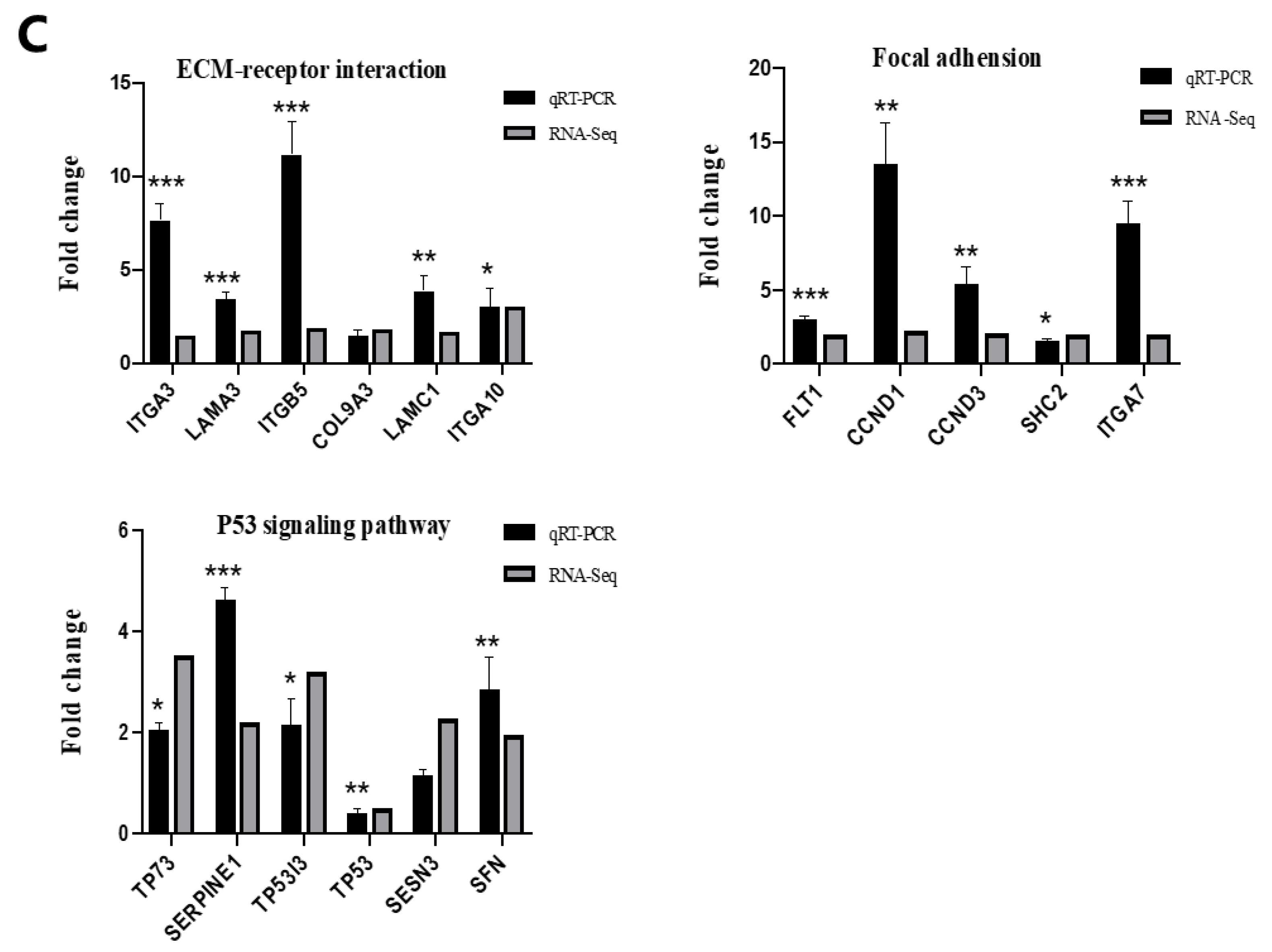
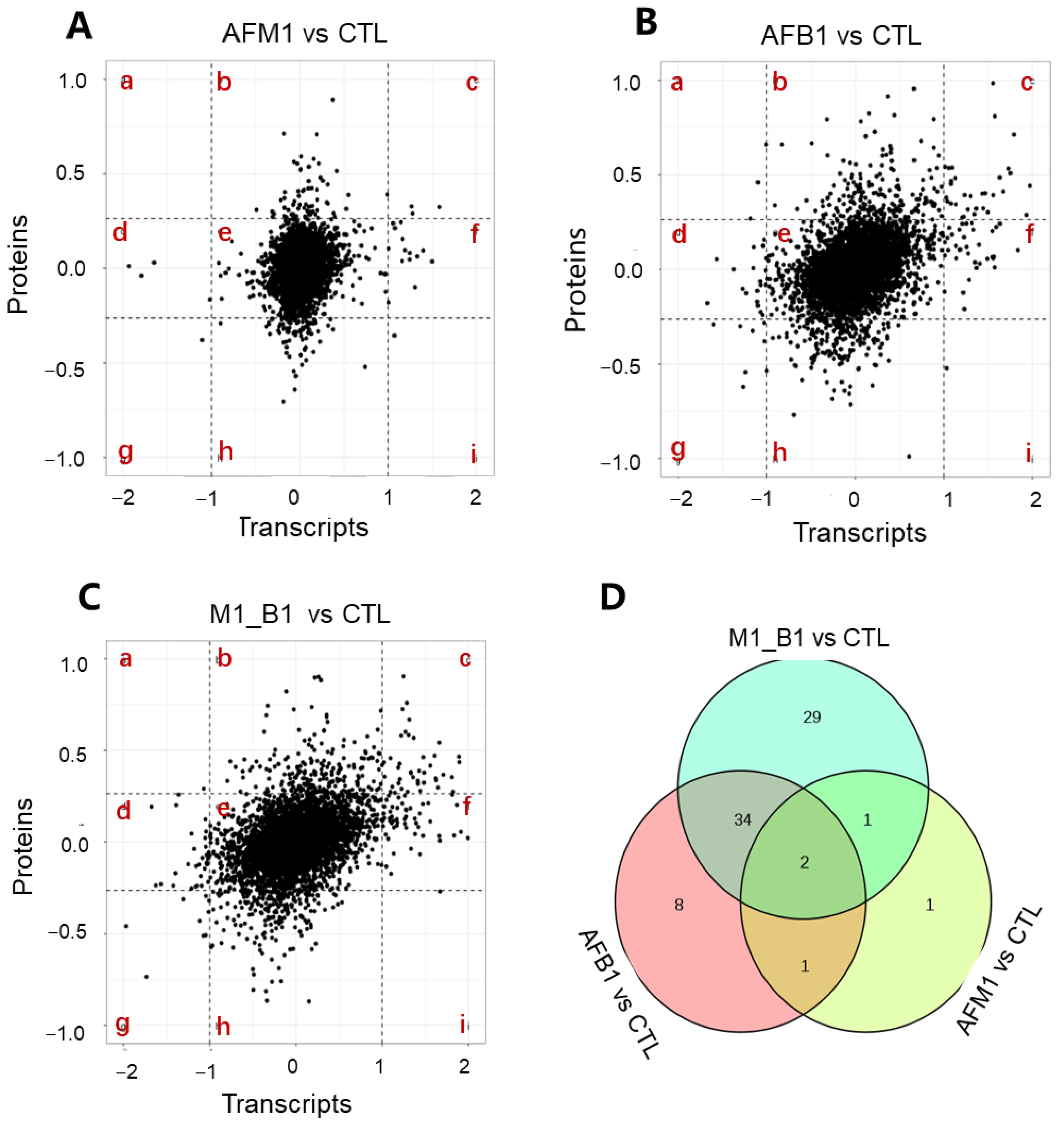
2.4. Cross-Omics Analysis of the Transcriptome and Proteome Induced by AFB1 and AFM1
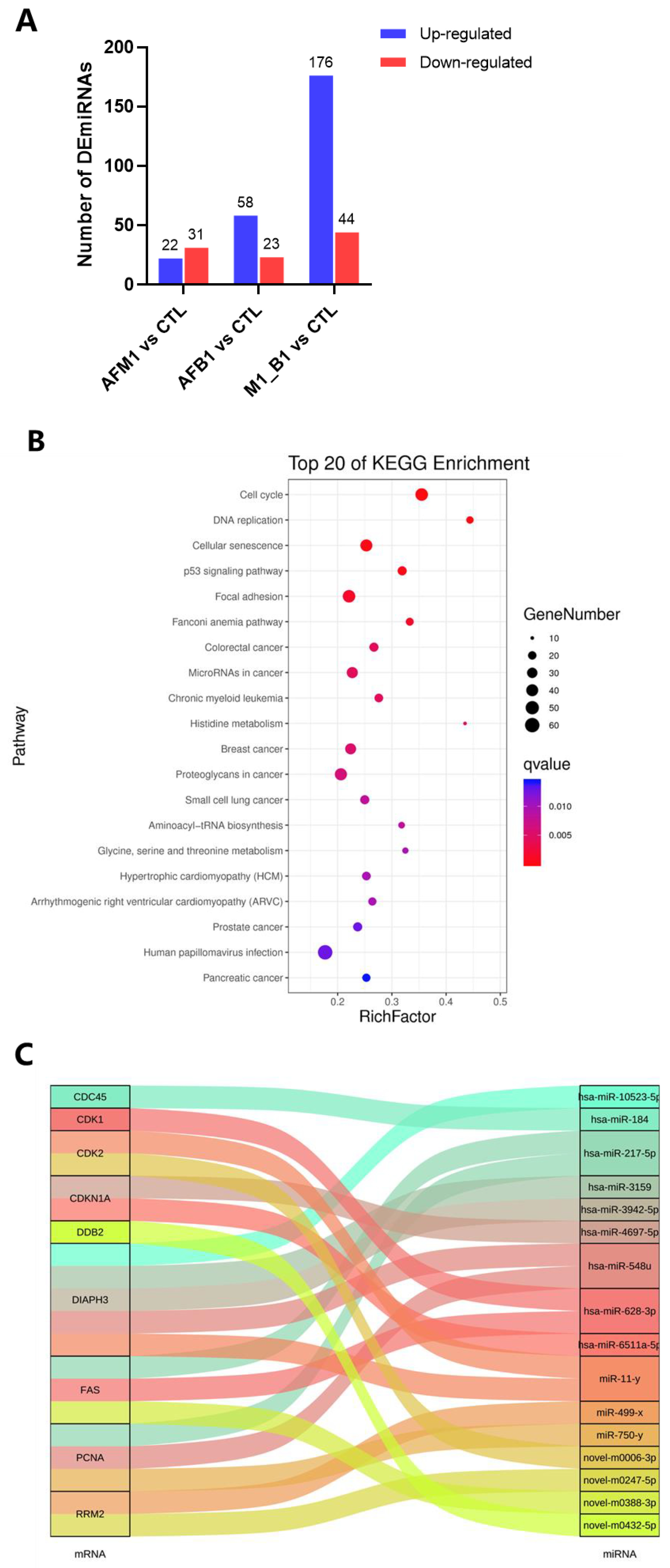
2.5. miRNA Analysis of NCM460 Cytotoxicity Induced by AFB1 and AFM1
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Treatment
4.3. Cytotoxicity Assay
4.4. Isobologram Analysis
4.5. RNA Sequencing and Validation
4.6. Proteome Analysis and Validation
4.7. miRNA Sequencing
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, R.; Reddy, K.R. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, L.L.; Wang, J.S. Aflatoxin B1 Induces Gut-Inflammation-Associated Fecal Lipidome Changes in F344 Rats. Toxicol. Sci. 2021, 183, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021, 268, 11571333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.J.; Liu, X.D.; Chen, L.Y.; Dai, J.F.; Karrow, N.A.; Sun, L.H. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Gong, G.X.; Zhang, L.; Shi, L.; Dai, J.F.; Han, Y.M.; Wu, Y.Y.; Khalil, M.M.; Sun, L.H. Invited review: Remediation strategies for mycotoxin control in feed. Anim. Feed. Sci. Technol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, J.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Simal-Gandara, J.; Xiao, J.B.; Ali, S.; Huh, Y.S.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Advances 2016, 6, 47844–47857. [Google Scholar] [CrossRef] [Green Version]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Habrowska-Gorczynska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Bao, X.; Luo, C.; Yang, H.; Wang, J.; Zhao, S.; Zheng, N. Transcriptional and Proteomic Analysis Revealed a Synergistic Effect of Aflatoxin M1 and Ochratoxin A Mycotoxins on the Intestinal Epithelial Integrity of Differentiated Human Caco-2 Cells. J. Proteome Res. 2018, 17, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, Y.; Yan, Q.; Bao, X.; Zhao, S.; Wang, J.; Zheng, N. Transcriptome Analysis of Ochratoxin A-Induced Apoptosis in Differentiated Caco-2 Cells. Toxins 2019, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Gao, J.; Huang, K.; Luo, Y.; Zhang, B.; Xu, W. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci. Rep. 2015, 5, 16732. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tang, D.; Wang, B.; Wang, Z.; Liu, M. Analysis of miRNA-mRNA regulatory network revealed key genes induced by aflatoxin B1 exposure in primary human hepatocytes. Mol. Genet. Genom. Med. 2019, 7, e971. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Szeto, C.H.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Li, S.; Zhang, Y.; Zheng, N. Aflatoxin M1 cytotoxicity against human intestinal Caco-2 cells is enhanced in the presence of other mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef]
- Clarke, R.; Connolly, L.; Frizzell, C.; Elliott, C.T. Cytotoxic assessment of the regulated, co-existing mycotoxins aflatoxin B1, fumonisin B1 and ochratoxin, in single, binary and tertiary mixtures. Toxicon 2014, 90, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, C.; Muller, Y.; Thalmann, B.; Hollert, H.; Seiler, T.B. p53 induction and cell viability modulation by genotoxic individual chemicals and mixtures. Environ. Sci. Pollut. Res. Int. 2018, 25, 4012–4022. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, S.; Stewart, M.J.; Ledermann, B.; Zilberman, F.; Terracciano, L.; Montini, E.; Grompe, M.; Kozma, S.C.; Thomas, G. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science 2000, 288, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L.; Gu, W. Ubiquitination, phosphorylation and acetylation: The molecular basis for p53 regulation. Curr. Opin. Cell Biol. 2003, 15, 164–171. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, R.; Kohli, S.; Das, S. p53 regulation upon genotoxic stress: Intricacies and complexities. Mol. Cell Oncol. 2014, 1, e969653. [Google Scholar] [CrossRef] [Green Version]
- Engin, A.B.; Engin, A. DNA damage checkpoint response to aflatoxin B1. Environ. Toxicol. Pharmacol. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Kuroda, K.; Hibi, D.; Ishii, Y.; Yokoo, Y.; Takasu, S.; Kijima, A.; Matsushita, K.; Masumura, K.; Kodama, Y.; Yanai, T.; et al. Role of p53 in the progression from ochratoxin A-induced DNA damage to gene mutations in the kidneys of mice. Toxicol. Sci. 2015, 144, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Bury, M.; Le Calve, B.; Lessard, F.; Dal Maso, T.; Saliba, J.; Michiels, C.; Ferbeyre, G.; Blank, V. NFE2L3 Controls Colon Cancer Cell Growth through Regulation of DUX4, a CDK1 Inhibitor. Cell Rep. 2019, 29, 1469–1481. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.H.; Do, K.H.; Kim, D.; Moon, Y. Interference with mutagenic aflatoxin B1-induced checkpoints through antagonistic action of ochratoxin A in intestinal cancer cells: A molecular explanation on potential risk of crosstalk between carcinogens. Oncotarget 2016, 7, 39627–39639. [Google Scholar] [CrossRef] [Green Version]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.Y.; Li, S.L.; Gao, Y.N.; Wang, J.Q.; Zheng, N. Transcriptome analysis revealed that aflatoxin M1 could cause cell cycle arrest in differentiated Caco-2 cells. Toxicol. Vitr. 2019, 59, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.L.; Zhang, F.L.; Tian, Y.; Zhu, M.; Meng, L.Y.; Dyce, P.W.; Shen, W.; Li, L. Whole-transcriptome analysis of the toxic effects of zearalenone exposure on ceRNA networks in porcine granulosa cells. Environ. Pollut. 2020, 261, 114007. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Papismadov, N.; Gal, H.; Ovadya, Y.; Vadai, E.; Miller, S.; Porat, Z.; Ben-Dor, S.; Krizhanovsky, V. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J. 2017, 36, 2280–2295. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Yu, F.Y.; Wu, T.S.; Wang, L.T.; Liu, B.H. Mycotoxin citrinin induced cell cycle G2/M arrest and numerical chromosomal aberration associated with disruption of microtubule formation in human cells. Toxicol. Sci. 2011, 119, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.W.; Prodeus, A.; Malkin, D.; Gariepy, J. p53 oligomerization status modulates cell fate decisions between growth, arrest and apoptosis. Cell Cycle 2016, 15, 3210–3219. [Google Scholar] [CrossRef] [Green Version]
- Giannattasio, M.; Branzei, D. S-phase checkpoint regulations that preserve replication and chromosome integrity upon dNTP depletion. Cell Mol. Life Sci. 2017, 74, 2361–2380. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pang, J.; Liu, Y.; Zhang, J.; Zhang, C.; Shen, G.; Song, L. Suppression of RRM2 inhibits cell proliferation, causes cell cycle arrest and promotes the apoptosis of human neuroblastoma cells and in human neuroblastoma RRM2 is suppressed following chemotherapy. Oncol. Rep. 2018, 40, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Ueta, M.; Nishigaki, H.; Komai, S.; Sotozono, C.; Kinoshita, S. Difference in the plasma level of miR-628-3p in atopic dermatitis patients with/without atopic keratoconjunctivitis. Immun. Inflamm. Dis. 2021, 9, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Flum, M.; Kleemann, M.; Schneider, H.; Weis, B.; Fischer, S.; Handrick, R.; Otte, K. miR-217-5p induces apoptosis by directly targeting PRKCI, BAG3, ITGAV and MAPK1 in colorectal cancer cells. J. Cell Commun. Signal. 2018, 12, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Wu, X.Y.; Yang, R.B.; Chen, F.; Liu, J.H.; Hu, Y.Y.; Wu, Z.D.; Wang, L.F.; Sun, X. Recombinant protein Schistosoma japonicum-derived molecule attenuates dextran sulfate sodium-induced colitis by inhibiting miRNA-217-5p to alleviate apoptosis. World J. Gastroenterol. 2021, 27, 7982–7994. [Google Scholar] [CrossRef]
- Dahiya, N.; Sarachana, T.; Kulkarni, S.; Wood, W.H.; Zhang, Y.; Becker, K.G.; Wang, B.D.; Atreya, C.D. miR-570 interacts with mitochondrial ATPase subunit g (ATP5L) encoding mRNA in stored platelets. Platelets 2017, 28, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, F.; Lei, Z.; Luo, H. LncRNA SNHG11 Promotes Proliferation, Migration, Apoptosis, and Autophagy by Regulating hsa-miR-184/AGO2 in HCC. OncoTargets Ther. 2020, 13, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Gao, Y.; Huang, S.; Su, C.; Wang, J.; Zheng, N. Whole transcriptome-based ceRNA network analysis revealed ochratoxin A-induced compromised intestinal tight junction proteins through WNT/Ca(2+) signaling pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112637. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.-N.; Yang, X.; Wang, J.-Q.; Liu, H.-M.; Zheng, N. Multi-Omics Reveal Additive Cytotoxicity Effects of Aflatoxin B1 and Aflatoxin M1 toward Intestinal NCM460 Cells. Toxins 2022, 14, 368. https://doi.org/10.3390/toxins14060368
Gao Y-N, Yang X, Wang J-Q, Liu H-M, Zheng N. Multi-Omics Reveal Additive Cytotoxicity Effects of Aflatoxin B1 and Aflatoxin M1 toward Intestinal NCM460 Cells. Toxins. 2022; 14(6):368. https://doi.org/10.3390/toxins14060368
Chicago/Turabian StyleGao, Ya-Nan, Xue Yang, Jia-Qi Wang, Hui-Min Liu, and Nan Zheng. 2022. "Multi-Omics Reveal Additive Cytotoxicity Effects of Aflatoxin B1 and Aflatoxin M1 toward Intestinal NCM460 Cells" Toxins 14, no. 6: 368. https://doi.org/10.3390/toxins14060368
APA StyleGao, Y.-N., Yang, X., Wang, J.-Q., Liu, H.-M., & Zheng, N. (2022). Multi-Omics Reveal Additive Cytotoxicity Effects of Aflatoxin B1 and Aflatoxin M1 toward Intestinal NCM460 Cells. Toxins, 14(6), 368. https://doi.org/10.3390/toxins14060368





