Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Selection and Sampling
2.2. Chemicals
2.3. Sample Preparation
2.4. HPLC Conditions
2.5. Exposure to AFB1 and OTA
3. Results and Discussion
3.1. Occurrence of AFB1
3.2. Occurrence of OTA
3.3. Risk Characterization and Dietary Exposure to AFB1
3.4. Risk Characterization and Dietary Exposure to OTA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thanushree, M.P.; Sailendri, D.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019, 93, 69–80. [Google Scholar] [CrossRef]
- Costa, J.; Rodríguez, R.; Garcia-Cela, E.; Medina, A.; Magan, N.; Lima, N.; Santos, C. Overview of fungi and mycotoxin contamination in Capsicum pepper and in its derivatives. Toxins 2019, 11, 27. [Google Scholar] [CrossRef]
- Hassan, H.F.; Kordahi, R.; Dimassi, H.; Khoury, A.; Daou, R.; Alwan, N.; Merhi, S.; Haddad, J.; Karam, L. Aflatoxin B1 in rice: Effects of storage duration, grain type and size, production site and season. J. Food Prot. 2022. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Alvito, P.; Oliveira, C.A. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019, 128, 21–34. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.; Hintzsche, H.; Oliveira, C.A. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Raad, F.; Nasreddine, L.; Hilan, C.; Bartosik, M.; Parent-Massin, D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem. Toxicol. 2014, 73, 35–43. [Google Scholar] [CrossRef]
- Al-Jaal, B.; Salama, S.; Al-Qasmi, N.; Jaganjac, M. Mycotoxin contamination of food and feed in the Gulf Cooperation Council countries and its detection. Toxicon 2019, 171, 43–50. [Google Scholar] [CrossRef]
- World Health Organization; International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2013. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Bignami, M. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D. Mycotoxins in spices and herbs–An update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.; Marquez, S.; de Mejia, E.G. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K. Thyme: History, applications, and overview of potential health benefits. Nutr. Today 2016, 51, 40–49. [Google Scholar] [CrossRef]
- Batal, M.; Hunter, E. Traditional Lebanese recipes based on wild plants: An answer to diet simplification? Food Nutr. Bull. 2007, 28 (Suppl. 2), S303–S311. [Google Scholar] [CrossRef] [PubMed]
- Hamade, K. Non-Wood Forest Product, Value Chains in Lebanon; Food and Agriculture Organization: Beirut, Lebanon, 2016. [Google Scholar]
- Mandeel, Q.A. Fungal contamination of some imported spices. Mycopathologia 2005, 159, 291–298. [Google Scholar] [CrossRef]
- Pallarés, N.; Tolosa, J.; Mañes, J.; Ferrer, E. Occurrence of mycotoxins in botanical dietary supplement infusion beverages. J. Nat. Prod. 2019, 82, 403–406. [Google Scholar] [CrossRef]
- Reinholds, I.; Pugajeva, I.; Bavrins, K.; Kuckovska, G.; Bartkevics, V. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit. Contam. Part B 2017, 10, 5–14. [Google Scholar] [CrossRef]
- Gambacorta, L.; El Darra, N.; Fakhoury, R.; Logrieco, A.F.; Solfrizzo, M. Incidence and levels of Alternaria mycotoxins in spices and herbs produced worldwide and commercialized in Lebanon. Food Control 2019, 106, 106724. [Google Scholar] [CrossRef]
- NL 677:2017; Thyme and Thyme Mixes. Lebanese Standards Institution (LIBNOR): Dekwaneh, Lebanon, 2017.
- European Commission. Commission Regulation (EC) No 401/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Forty-Ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA); World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives; Geneva, Switzerland, 2007; Volume 68. [Google Scholar]
- Daou, R.; Joubrane, K.; Khabbaz, L.R.; Maroun, R.G.; Ismail, A.; El Khoury, A. Aflatoxin B1 and ochratoxin A in imported and Lebanese wheat and-products. Food Addit. Contam. Part B 2021, 14, 227–235. [Google Scholar] [CrossRef]
- Al Ayoubi, M.; Salman, M.; Gambacorta, L.; El Darra, N.; Solfrizzo, M. Assessment of Dietary Exposure to Ochratoxin A in Lebanese Students and Its Urinary Biomarker Analysis. Toxins 2021, 13, 795. [Google Scholar] [CrossRef]
- Al Ayoubi, M.; Solfrizzo, M.; Gambacorta, L.; Watson, I.; El Darra, N. Risk of exposure to aflatoxin B1, ochratoxin A, and fumonisin B1 from spices used routinely in Lebanese cooking. Food Chem. Toxicol. 2021, 147, 111895. [Google Scholar] [CrossRef] [PubMed]
- El Darra, N.; Gambacorta, L.; Solfrizzo, M. Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control. 2019, 95, 63–70. [Google Scholar] [CrossRef]
- Zareshahrabadi, Z.; Bahmyari, R.; Nouraei, H.; Khodadadi, H.; Mehryar, P.; Asadian, F.; Zomorodian, K. Detection of Aflatoxin and Ochratoxin A in Spices by High-Performance Liquid Chromatography. J. Food Qual. 2020, 2020, 8858889. [Google Scholar] [CrossRef]
- Bircan, C. The determination of aflatoxins in spices by immunoaffinity column extraction using HPLC. Int. J. Food Sci. Technol. 2005, 40, 929–934. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Wallace, H. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5. [Google Scholar]
- Siruguri, V.; Bhat, R.V. Assessing intake of spices by pattern of spice use, frequency of consumption and portion size of spices consumed from routinely prepared dishes in southern India. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Jeambey, Z.; Johns, T.; Talhouk, S.; Batal, M. Perceived health and medicinal properties of six species of wild edible plants in north-east Lebanon. Public Health Nutr. 2009, 12, 1902–1911. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and spices-biomarkers of intake based on human intervention studies—A systematic review. Genes Nutr. 2019, 14, 1–27. [Google Scholar] [CrossRef]
- NL 240:2011; Arabic Lebanese Bread. Lebanese Standards Institution (LIBNOR): Dekwaneh, Lebanon, 2011.
- Kabak, B. Aflatoxins in foodstuffs: Occurrence and risk assessment in Turkey. J. Food Compos. Anal. 2021, 96, 103734. [Google Scholar] [CrossRef]
- Serdar, S.A.; El Tawila, M.M.; Madkour, M.H.; Alrasheedi, A.A. Determination of aflatoxins (AFs) in different food samples: A case study from Jeddah, Saudi Arabia. JKAU Met. Env. Arid Land Agric. Sci. 2020, 29, 23–34. [Google Scholar]
- Raiola, A.; Meca, G.; Mañes, J.; Ritieni, A. Bioaccessibility of deoxynivalenol and its natural co-occurrence with ochratoxin A and aflatoxin B1 in Italian commercial pasta. Food Chem. Toxicol. 2012, 50, 280–287. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2015/1137 of 13 July 2015 amending Regulation (EC) No 1881–2006 as regards the maximum level of Ochratoxin A in Capsicum spp. spices. Off. J. Eur. Union 2015, 185, 12. [Google Scholar]
- Mannani, N.; Tabarani, A.; Zinedine, A. Assessment of aflatoxin levels in herbal green tea available on the Moroccan market. Food Control 2020, 108, 106882. [Google Scholar] [CrossRef]
- Ismail, A.; Akhtar, S.; Riaz, M.; Gong, Y.Y.; Routledge, M.N.; Naeem, I. Prevalence and exposure assessment of aflatoxins through black tea consumption in the Multan City of Pakistan and the impact of tea making process on aflatoxins. Front. Microbiol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Reinholds, I.; Bogdanova, E.; Pugajeva, I.; Bartkevics, V. Mycotoxins in herbal teas marketed in Latvia and dietary exposure assessment. Food Addit. Contam. Part B 2019, 12, 199–208. [Google Scholar] [CrossRef]
- Romagnoli, B.; Menna, V.; Gruppioni, N.; Bergamini, C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control 2007, 18, 697–701. [Google Scholar] [CrossRef]
- Škrinjar, M.M.; Vesković-Moračanin, S.M.; Janković, V.V.; Vukojević, J.B. Xerophilic moulds isolated from spices used in meat industry as potential producers of mycotoxins. Zb. Matice Srp. Prir. Nauk. 2012, 123, 7–16. [Google Scholar] [CrossRef]
- Migahed, F.F.; Abdel-Gwad, M.M.; Mohamed, S.R. Aflatoxigenic fungi associated with some medicinal plants. Annu. Res. Rev. Biol. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Koutsias, I.; Kollia, E.; Makri, K.; Markaki, P.; Proestos, C. Occurrence and Risk Assessment of Aflatoxin B1 in Spices Marketed in Greece. Anal. Lett. 2021, 54, 1995–2008. [Google Scholar] [CrossRef]
- Tosun, H.; Arslan, R. Determination of aflatoxin B1 levels in organic spices and herbs. Sci. World J. 2013, 2013, 874093. [Google Scholar] [CrossRef]
- Khazaeli, P.; Mehrabani, M.; Heidari, M.R.; Asadikaram, G.; Najafi, M.L. Prevalence of aflatoxin contamination in herbs and spices in different regions of Iran. Iran. J. Public Health 2017, 46, 1540. [Google Scholar] [PubMed]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control 2014, 39, 192–197. [Google Scholar] [CrossRef]
- Karam, L.; Salloum, T.; El Hage, R.; Hassan, H. How can packaging, source and food safety management system affect the microbiological quality of spices and dried herbs? The case of a developing country. Int. J. Food Microbiol. 2021, 353, 109295. [Google Scholar] [CrossRef] [PubMed]
- Akpo-Djènontin, D.O.O.; Gbaguidi, F.; Soumanou, M.M.; Anihouvi, V.B. Mold infestation and aflatoxins production in traditionally processed spices and aromatic herbs powder mostly used in West Africa. Food Sci. Nutr. 2018, 6, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Joubrane, K.; Khoury, A.E.; Lteif, R.; Rizk, T.; Kallassy, M.; Hilan, C.; Maroun, R. Occurrence of aflatoxin B1 and ochratoxin A in Lebanese cultivated wheat. Mycotoxin Res. 2011, 27, 249–257. [Google Scholar] [CrossRef]
- Mokhtarian, M.; Tavakolipour, H.; Bagheri, F.; Oliveira, C.A.F.; Corassin, C.H.; Khaneghah, A.M. Aflatoxin B1 in the Iranian pistachio nut and decontamination methods: A systematic review. Qual. Assur. Saf. Crops Foods 2020, 12, 15–25. [Google Scholar] [CrossRef]
- Eneroth, H.; Wallin, S.; Leander, K.; Nilsson Sommar, J.; Åkesson, A. Risks and benefits of increased nut consumption: Cardiovascular health benefits outweigh the burden of carcinogenic effects attributed to aflatoxin b1 exposure. Nutrients 2017, 9, 1355. [Google Scholar] [CrossRef]
- Emadi, A.; Jayedi, A.; Mirmohammadkhani, M.; Abdolshahi, A. Aflatoxin reduction in nuts by roasting, irradiation and fumigation: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Kollia, E.; Tsourouflis, K.; Markaki, P. Aflatoxin B1 in sesame seeds and sesame products from the Greek market. Food Addit. Contam. Part B 2016, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Khorshidi, M.; Khaneghah, A.M. The prevalence and risk assessment of aflatoxin in sesame based products. Ital. J. Food Sci. 2021, 33 (Suppl. 1), 92–102. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Significance of ochratoxin A in breakfast cereals from the United States. J. Agric. Food Chem. 2015, 63, 9404–9409. [Google Scholar] [CrossRef] [PubMed]
- Araguás, C.; González-Peñas, E.; De Cerain, A.L. Study on ochratoxin A in cereal-derived products from Spain. Food Chem. 2015, 92, 459–464. [Google Scholar] [CrossRef]
- Kulahi, A.; Kabak, B. A preliminary assessment of dietary exposure of ochratoxin A in Central Anatolia Region, Turkey. Mycotoxin Res. 2020, 36, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Dofkova, M.; Skarkova, J.; Pfohl-Leszkowicz, A.; Ruprich, J. Ochratoxin A dietary exposure of ten population groups in the Czech Republic: Comparison with data over the world. Toxins 2015, 7, 3608–3635. [Google Scholar] [CrossRef] [PubMed]
- Tabarani, A.; Zinedine, A.; Bouchriti, N. Exposure assessment to ochratoxin A through the intake of three cereal derivatives from the Moroccan market. Food Res. Int. 2020, 137, 109464. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Beszterda, M.; Bocianowski, J.; Goliński, P. Natural occurrence of fumonisins and ochratoxin A in some herbs and spices commercialized in Poland analyzed by UPLC–MS/MS method. Food Microbiol. 2013, 36, 426–431. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; Andjelkovic, M.; De Boevre, M.; Tangni, E.K.; Huybrechts, B.; De Saeger, S. Dietary exposure assessment and risk characterization of citrinin and ochratoxin A in Belgium. Food Chem. Toxicol. 2021, 147, 111914. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef]
- Ozden, S.; Akdeniz, A.S.; Alpertunga, B. Occurrence of ochratoxin A in cereal-derived food products commonly consumed in Turkey. Food Control 2012, 25, 69–74. [Google Scholar] [CrossRef]
- Ali, N.; Watt, J. Risk assessment of dietary exposure to Aflatoxin contamination in spices. Adv. Clin. Toxicol. 2019, 4, 1–16. [Google Scholar]
- Ibáñez-Vea, M.; Martínez, R.; González-Peñas, E.; Lizarraga, E.; de Cerain, A.L. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from Spanish market. Food Control 2011, 22, 1949–1955. [Google Scholar] [CrossRef]
- Jahanbakhsh, M.; Afshar, A.; Momeni Feeli, S.; Pabast, M.; Ebrahimi, T.; Mirzaei, M.; Arabameri, M. Probabilistic health risk assessment (Monte Carlo simulation method) and prevalence of aflatoxin B1 in wheat flours of Iran. Int. J. Environ. Anal. Chem. 2021, 101, 1074–1085. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liang, B.; Zhang, Y.; Zhong, X.; Luo, X.; Chen, K. Probabilistic risk assessment of dietary exposure to aflatoxin B 1 in Guangzhou, China. Sci. Rep. 2020, 10, 7973. [Google Scholar] [CrossRef]
- Cressey, P.J.; Reeve, J. Dietary exposure and risk assessment for aflatoxins in New Zealand. World Mycotoxin J. 2013, 6, 427–437. [Google Scholar] [CrossRef]
- Park, J.W.; Chung, S.H.; Kim, Y.B. Ochratoxin A in Korean food commodities: Occurrence and safety evaluation. J. Agric. Food Chem. 2005, 53, 4637–4642. [Google Scholar] [CrossRef]
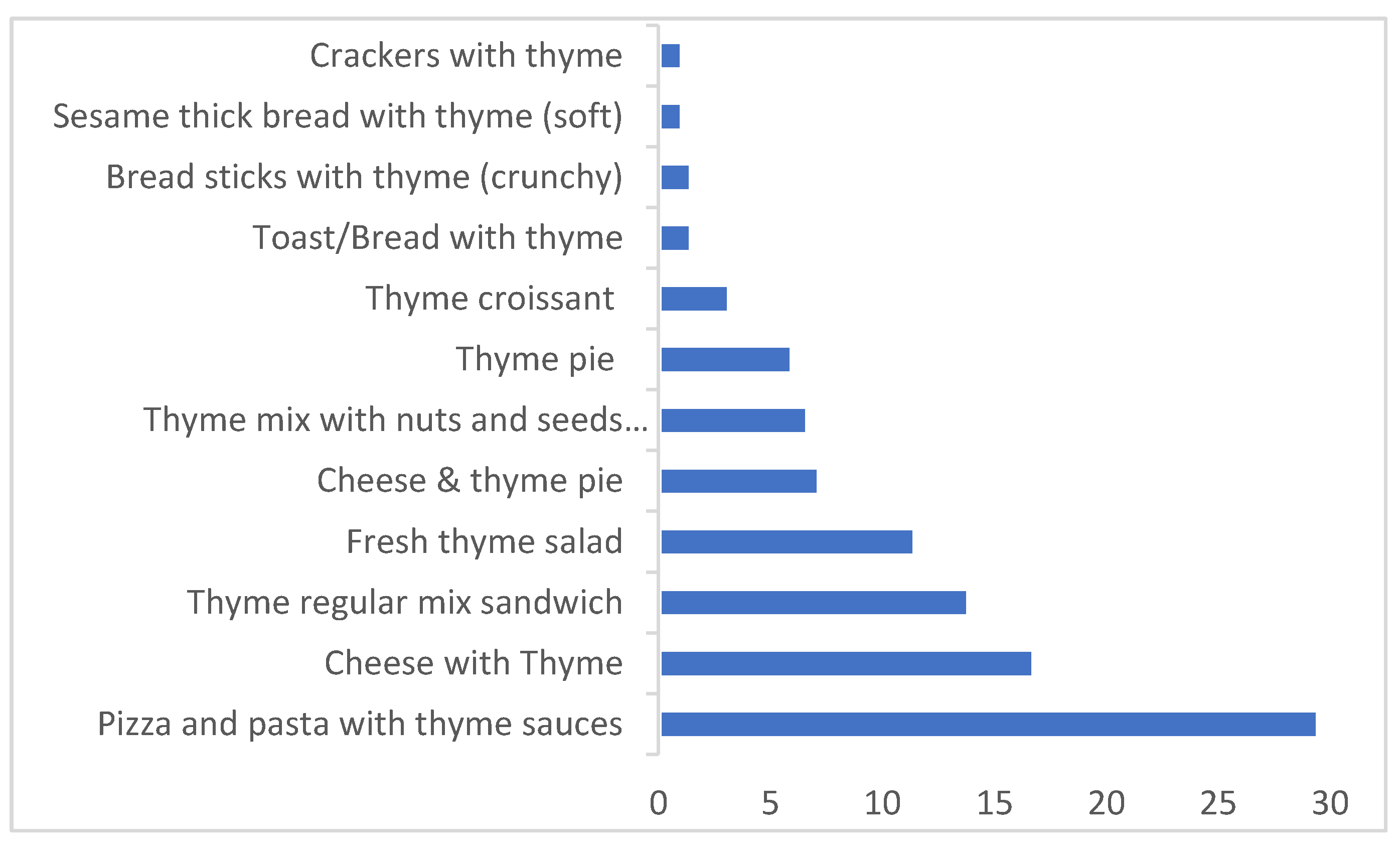
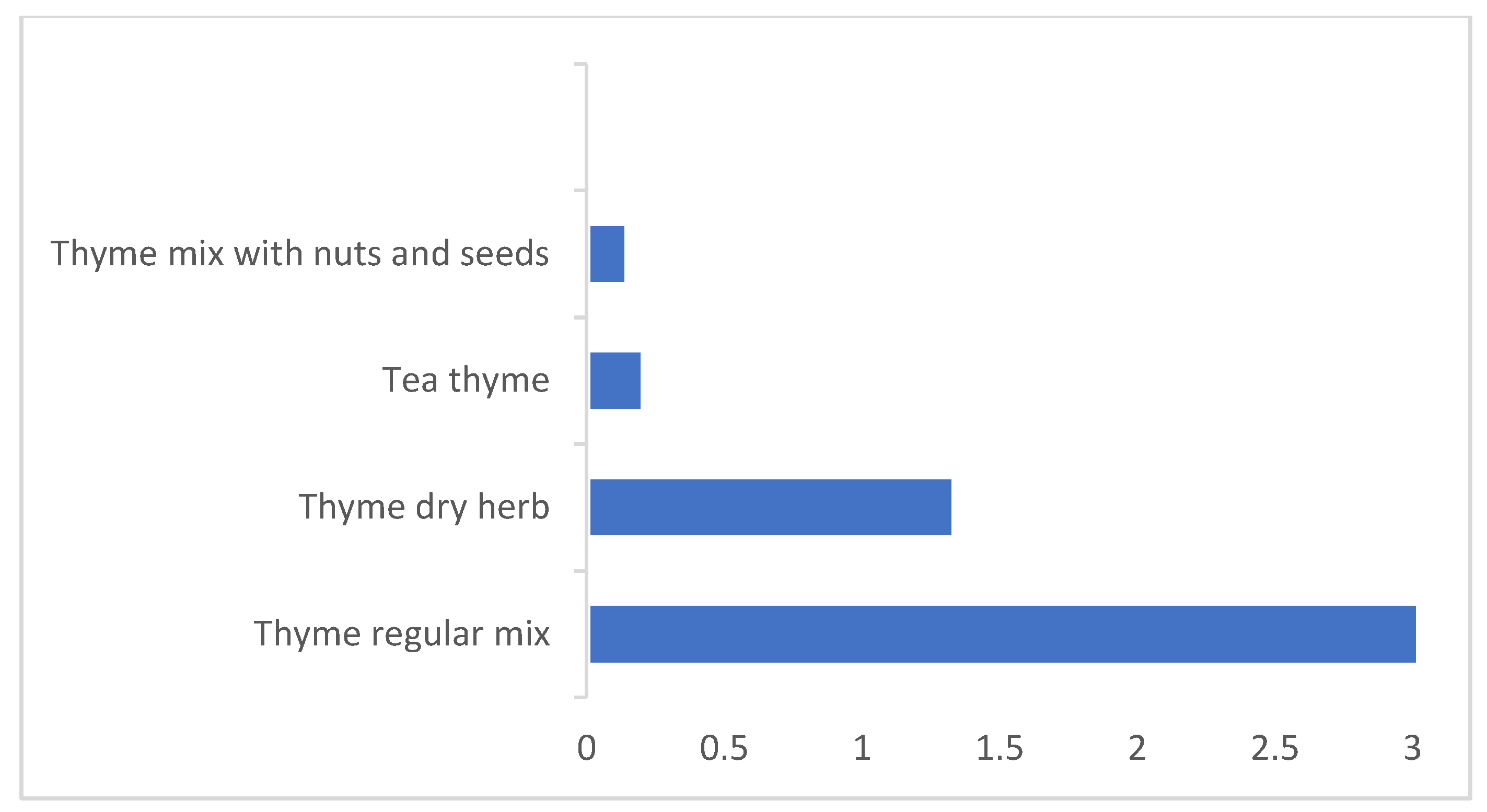
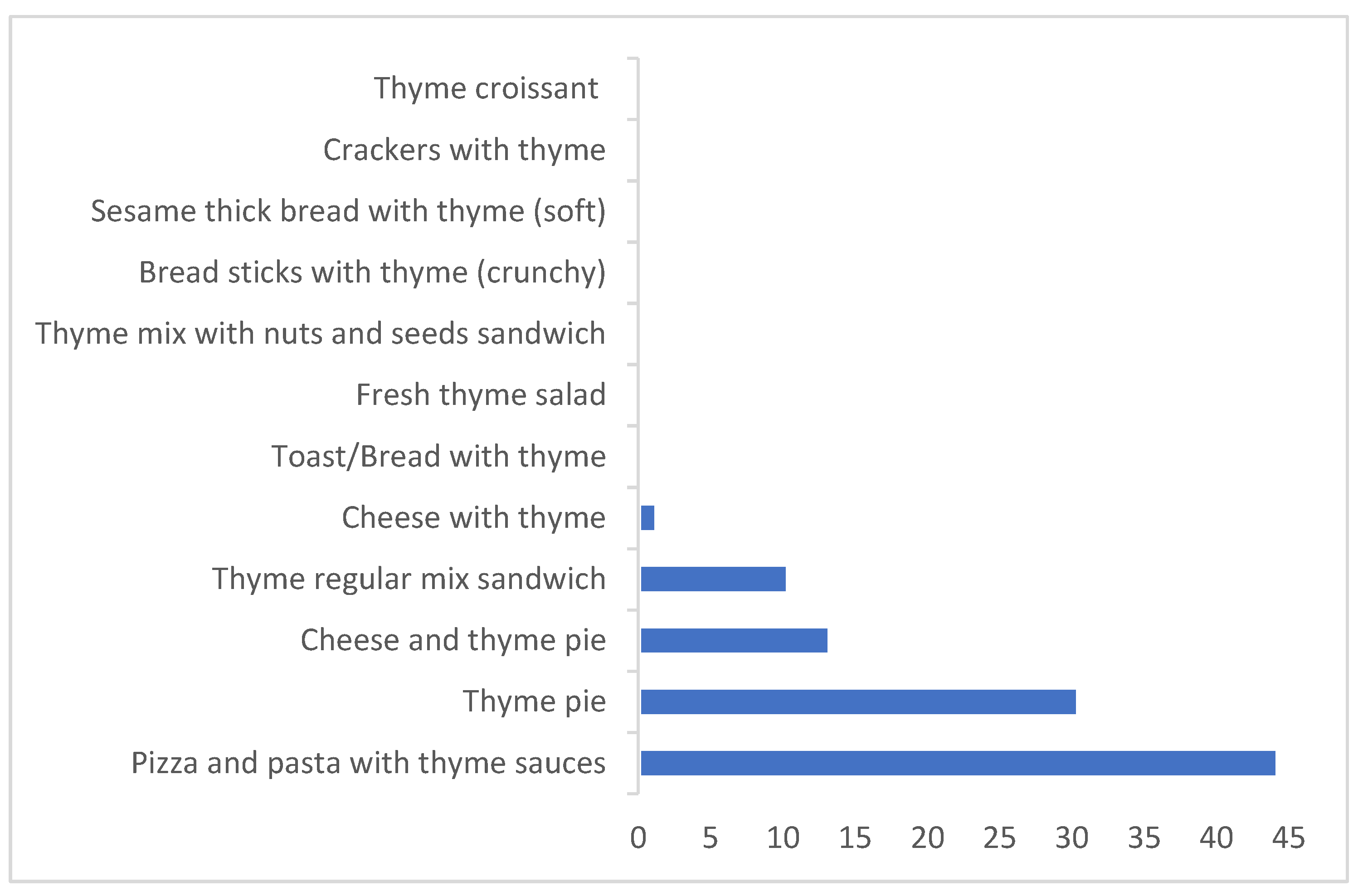
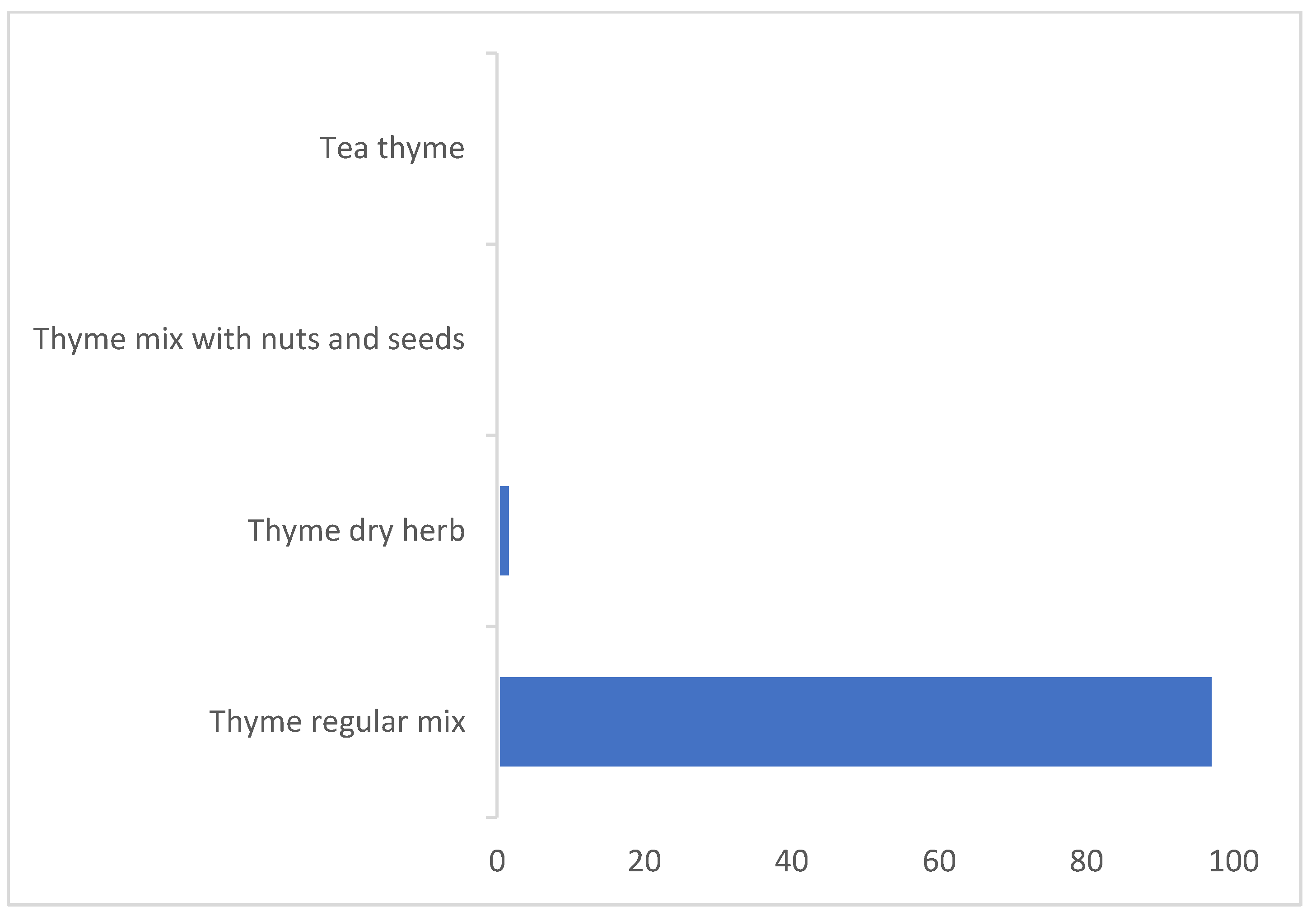
| Daily Consumption (g/day) | Thyme Content % (w/w) | |||
|---|---|---|---|---|
| Food Items | Regular | High | ||
| Thyme based products | Thyme pie | 72.5 | 141.9 | 14 |
| Cheese and thyme pie | 55.2 | 167.3 | 6 | |
| Fresh thyme salad | 68.2 | 115.8 | 4 | |
| Thyme regular mix sandwich | 16.8 | 34.0 | 31 | |
| Thyme mix with nuts and seeds sandwich | 11.2 | 29.2 | 35 | |
| Pizza and pasta with thyme sauces | 27.3 | 28.8 | 1 | |
| Bread sticks with thyme (crunchy) | 5.0 | 13.4 | 12 | |
| Sesame thick bread with thyme (soft) | 13.9 | 40.8 | 6 | |
| Crackers with thyme | 3.7 | 7.7 | 5 | |
| Toast/bread with thyme | 6.6 | 9.8 | 3 | |
| Thyme croissant | 9.6 | 23.0 | 9 | |
| Cheese with thyme | 20.9 | 38.8 | 1 | |
| Dried thyme herbs a | ||||
| Thyme regular mix a | 20.5 | 45.1 | ||
| Thyme mix with nuts and seeds b | 3.9 | 7.1 | ||
| Thyme herbs c | 3.9 | 10.1 | ||
| Tea thyme | 0.9 | 2 | ||
| Mycotoxin Type | Classification | Contamination Status N (%) |
|---|---|---|
| AFB1 | No. of contaminated samples (%) | 27 (84) |
| 0.08–2 µg/kg | 14 (44) | |
| 2–5 µg/kg | 7 (22) | |
| >5 µg/kg | 6 (19) | |
| Mean ± SD | 4.6 ± 6.5 | |
| Range | 0.08–25.8 | |
| No. of unacceptable samples (%) | ||
| Total | 13/32 (41) | |
| Thyme-based products | 5/24 (21) b | |
| Dried thyme herbs | 8/8 (100) b/6 (75) c | |
| OTA | No. of contaminated samples (%) | 12 (38) |
| 0.04–3 µg/kg | 10 (31) | |
| 3–15 µg/kg | 2 (6) | |
| >15 µg/kg | 0 (0) | |
| Mean ± SD | 1.4 ± 2.3 | |
| Range | 0.04–8.0 | |
| No. of unacceptable samples (%) | ||
| Total | 2/32 (6) | |
| Thyme-based products | 1/24 (4) d | |
| Dried thyme herbs | 1/8 (13) d |
| Food Items | AFB1 | OTA | |
|---|---|---|---|
| Thyme based products | Thyme pie | 0.26 ± 0.36 b | 0.41 ± 0.58 d |
| Cheese and thyme pie | 0.40 ± 0.57 b | 0.23 ± 0.33 d | |
| Fresh thyme salad | 0.52 ± 0.74 c | 0.00 ± 0.00 e | |
| Thyme regular mix sandwich | 2.55 ± 2.08 b | 0.60 ± 0.85 d | |
| Thyme mix with nuts and seeds sandwich | 1.85 ± 0.07 b | 0.00 ± 0.00 d | |
| Pizza and pasta with thyme sauces | 3.35 ± 0.99 b | 1.58 ± 2.03 d | |
| Bread sticks with thyme (crunchy) | 0.92 ± 1.30 b | 0.00 ± 0.00 d | |
| Sesame thick bread with thyme (soft) | 0.25 ± 0.35 b | 0.00 ± 0.00 d | |
| Crackers with thyme | 0.90 ± 0.79 b | 0.00 ± 0.00 d | |
| Toast/Bread with thyme | 0.69 ± 0.87 b | 0.02 ± 0.03 d | |
| Thyme croissant | 1.03 ± 0.33 b | 0.00 ± 0.00 d | |
| Cheese with thyme | 2.49 ± 0.50 c | 0.06 ± 0.09 e | |
| Total | 1.27 ± 0.75 | 0.24 ± 0.33 | |
| Dried thyme herbs | |||
| Thyme regular mix | 11.58 a ± 5.92 c | 4.83 ± 4.44 e | |
| Thyme mix with nuts and seeds | 2.78 ± 0.06 c | 0.09 ± 0.13 e | |
| Thyme dry herb | 15.38 a ± 0.30 c | 0.35 ± 0.49 e | |
| Thyme tea | 16.79 a ± 12.80 c | 0.07 ± 0.10 e | |
| Total | 11.63 ± 7.02 | 1.34 ± 1.29 |
| Regular Consumption | High Consumption | |||
|---|---|---|---|---|
| Thyme-Based Products | Exposure (ng/kg bw/day) a | MOE b | Exposure (ng/kg bw/day) a | MOE b |
| Thyme pie | 0.257 | 1554 | 0.504 | 794 |
| Cheese and thyme pie | 0.306 | 1308 | 0.927 | 432 |
| Fresh thyme salad | 0.490 | 817 | 0.832 | 481 |
| Thyme regular mix sandwich | 0.592 | 675 | 1.198 | 334 |
| Thyme mix with nuts and seeds sandwich | 0.287 | 1395 | 0.748 | 535 |
| Pizza and pasta with thyme sauces | 1.262 | 317 | 1.332 | 300 |
| Bread sticks with thyme (crunchy) | 0.063 | 6322 | 0.170 | 2359 |
| Sesame thick bread with thyme (soft) | 0.047 | 8474 | 0.139 | 2887 |
| Crackers with thyme | 0.046 | 8663 | 0.096 | 4163 |
| Toast/bread with thyme | 0.063 | 6352 | 0.094 | 4278 |
| Thyme croissant | 0.136 | 2932 | 0.327 | 1224 |
| Cheese with thyme | 0.720 | 556 | 1.336 | 299 |
| Total | 4.270 | 94 | 7.701 | 52 |
| Dried thyme herbs | ||||
| Thyme regular mix | 3.280 | 122 | 7.216 | 55 |
| Thyme mix with nuts and seeds | 0.150 | 2675 | 0.272 | 1469 |
| Thyme dry herb | 1.339 | 299 | 2.146 | 186 |
| Thyme tea | 0.209 | 1917 | 0.464 | 863 |
| Total | 4.977 | 80 | 10.980 | 36 |
| Thyme-Based Products | Exposure (ng/kg bw/day) | Exposure (ng/kg bw/week) | Dietary Exposure Expressed as %TRV JECFA a | MOE Non-Neoplastic Effect b | MOE Neoplastic Effect b |
|---|---|---|---|---|---|
| Thyme pie | 0.410 | 2.870 | 3 | 11,536 | 35,364 |
| Cheese and thyme pie | 0.179 | 1.250 | 1 | 26,478 | 81,170 |
| Fresh thyme salad | 0.000 | 0.000 | 0 | - | - |
| Thyme regular mix sandwich | 0.140 | 0.981 | 1 | 33,752 | 103,467 |
| Thyme mix with nuts and seeds sandwich | 0.000 | 0.000 | 0 | - | - |
| Pizza and pasta with thyme sauces | 0.596 | 4.171 | 4 | 7938 | 24,333 |
| Bread sticks with thyme (crunchy) | 0.000 | 0.000 | 0 | - | - |
| Sesame thick bread with thyme (soft) | 0.000 | 0.000 | 0 | - | - |
| Crackers with thyme | 0.000 | 0.000 | 0 | - | - |
| Toast/bread with thyme | 0.002 | 0.013 | 0 | 2,607,340 | 7,992,902 |
| Thyme croissant | 0.000 | 0.000 | 0 | - | - |
| Cheese with thyme | 0.018 | 0.127 | 0 | 260,685 | 799,140 |
| Total | 1.345 | 9.415 | 9 | 3517 | 10,781 |
| Dried thyme herbs | |||||
| Thyme regular mix | 1.368 | 9.575 | 10 | 3458 | 10,601 |
| Thyme mix with nuts and seeds | 0.005 | 0.035 | 0 | 958,357 | 2,937,880 |
| Thyme dry herb | 0.02 | 0.131 | 0 | 252,264 | 773,324 |
| Thyme tea | 0.001 | 0.006 | 0 | 5,637,070 | 17,280,658 |
| Total | 1.392 | 9.747 | 10 | 3398 | 10,417 |
| Thyme-Based Products | Exposure (ng/kg bw/day) a | Exposure (ng/kg bw/week) | Dietary Exposure Expressed as %TRV JECFA b | MOE Non-Neoplastic Effect | MOE Neoplastic Effect |
|---|---|---|---|---|---|
| Thyme pie | 0.803 | 5.618 | 6 | 5894 | 18,068 |
| Cheese and thyme pie | 0.541 | 3.790 | 4 | 8736 | 26,782 |
| Fresh thyme salad | 0.000 | 0.000 | 0 | - | - |
| Thyme regular mix sandwich | 0.284 | 1.985 | 2 | 16,677 | 51,125 |
| Thyme mix with nuts and seeds sandwich | 0.000 | 0.000 | 0 | - | - |
| Pizza and pasta with thyme sauces | 0.629 | 4.400 | 4 | 7524 | 23,066 |
| Bread sticks with thyme (crunchy) | 0.000 | 0.000 | 0 | - | - |
| Sesame thick bread with thyme (soft) | 0.000 | 0.000 | 0 | - | - |
| Crackers with thyme | 0.000 | 0.000 | 0 | - | - |
| Toast/bread with thyme | 0.003 | 0.019 | 0 | 1,755,964 | 5,382,975 |
| Thyme croissant | 0.000 | 0.000 | 0 | - | - |
| Cheese with thyme | 0.034 | 0.236 | 0 | 140,420 | 43,046 |
| Total | 2.293 | 16.048 | 16 | 2063 | 6323 |
| Dried thyme herbs | |||||
| Thyme regular mix | 3.009 | 21.065 | 21 | 1572 | 4818 |
| Thyme mix with nuts and seeds | 0.009 | 0.063 | 0 | 526,421 | 1,613,765 |
| Thyme dry herb | 0.049 | 0.340 | 97,409 | 298,610 | |
| Thyme tea | 0.002 | 0.013 | 0 | 2,536,681 | 7,776,296 |
| Total | 3.069 | 21.481 | 21 | 1541 | 4725 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, H.F.; Koaik, L.; Khoury, A.E.; Atoui, A.; El Obeid, T.; Karam, L. Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins 2022, 14, 331. https://doi.org/10.3390/toxins14050331
Hassan HF, Koaik L, Khoury AE, Atoui A, El Obeid T, Karam L. Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins. 2022; 14(5):331. https://doi.org/10.3390/toxins14050331
Chicago/Turabian StyleHassan, Hussein F., Lara Koaik, André El Khoury, Ali Atoui, Tahra El Obeid, and Layal Karam. 2022. "Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon" Toxins 14, no. 5: 331. https://doi.org/10.3390/toxins14050331
APA StyleHassan, H. F., Koaik, L., Khoury, A. E., Atoui, A., El Obeid, T., & Karam, L. (2022). Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins, 14(5), 331. https://doi.org/10.3390/toxins14050331







