Abstract
In the last decade, foodborne outbreaks and individual cases caused by bacterial toxins showed an increasing trend. The major contributors are enterotoxins and cereulide produced by Bacillus cereus, which can cause a diarrheal and emetic form of the disease, respectively. These diseases usually induce relatively mild symptoms; however, fatal cases have been reported. With the aim to detected potential toxin producers that are able to grow at refrigerator temperatures and subsequently produce cereulide, we screened the prevalence of enterotoxin and cereulide toxin gene carriers and the psychrotrophic capacity of presumptive B. cereus obtained from 250 food products (cereal products, including rice and seeds/pulses, dairy-based products, dried vegetables, mixed food, herbs, and spices). Of tested food products, 226/250 (90.4%) contained presumptive B. cereus, which communities were further tested for the presence of nheA, hblA, cytK-1, and ces genes. Food products were mainly contaminated with the nheA B. cereus carriers (77.9%), followed by hblA (64.8%), ces (23.2%), and cytK-1 (4.4%). Toxigenic B. cereus communities were further subjected to refrigerated (4 and 7 °C) and mild abuse temperatures (10 °C). Overall, 77% (94/121), 86% (104/121), and 100% (121/121) were able to grow at 4, 7, and 10 °C, respectively. Enterotoxin and cereulide potential producers were detected in 81% of psychrotrophic presumptive B. cereus. Toxin encoding genes nheA, hblA, and ces gene were found in 77.2, 55, and 11.7% of tested samples, respectively. None of the psychrotrophic presumptive B. cereus were carriers of the cytotoxin K-1 encoding gene (cytK-1). Nearly half of emetic psychrotrophic B. cereus were able to produce cereulide in optimal conditions. At 4 °C none of the examined psychrotrophs produced cereulide. The results of this research highlight the high prevalence of B. cereus and the omnipresence of toxin gene harboring presumptive B. cereus that can grow at refrigerator temperatures, with a focus on cereulide producers.
Keywords:
Bacillus cereus; enterotoxins; cereulide; psychrotrophic growth; refrigeration temperature Key Contribution:
Our results provide new insights into the pcychrotrophic capacity of potentially pathogenic B. cereus, focusing on cereulide production. Almost 80% of tested products were contaminated with toxigenic B. cereus, from which a significant number was able to grow at refrigerator temperatures. Among 11 ces-positive psychrotrophic presumptive B. cereus, five were able to produce cereulide.
1. Introduction
Bacillus cereus sensu lato (s.l) is a group of Gram-positive, rod-shaped, motile, facultative anaerobes, mostly beta-hemolytic, catalase-positive, spore formers composed of a growing list of novel species which are widely distributed in the environment [1,2,3,4,5,6,7]. Officially, Bacillus anthracis, Bacillus cereus sensu stricto (s.s.), Bacillus thuringiensis, Bacillus cytotoxicus, Bacillus weihenstephanensis, Bacillus mycoides, Bacillus pseudomycoides, and Bacillus toyonensis are validated, and 12 more species have been described [1,2,3,4,5,6,7]. Genetically, these species are highly related [8]; however, B. cereus group members show diverse phenotypical characteristics. B. anthracis is a well-known zoonotic agent due to extreme pathogenicity, B. toyonensis is used as a probiotic, B. thuringiensis is an entomopathogen characterized by the insecticidal crystals and used as a bio-pesticide. In contrast, human foodborne pathogens are not strictly related to one species. Toxigenic B. cereus strains are widespread among different species and are identified as the causal agents of gastrointestinal diseases [9].
Two types of gastrointestinal diseases are caused by B. cereus toxins: diarrheal and emetic disease. The former is associated with enterotoxin(s) production in the gastrointestinal tract by ingested enterotoxigenic B. cereus strains. The latter is caused by emetic B. cereus strains, which produce cereulide in the food and ingredients during production or storage [10]. Although enterotoxins can be produced in food before ingestion, they typically do not reach the small intestine in sufficient amounts due to sensitivity toward low pH and digestive enzymes (e.g., pepsin) present in the upper parts of the gastrointestinal tract [11]. To induce food poisoning, enterotoxigenic B. cereus vegetative cells or spores have to be ingested in high counts, presumably corresponding to levels of 105 CFU/g. However, concentrations as low as 103 CFU/g have been implicated in cases of both diarrheal and emetic diseases [1]. B. cereus enterotoxins of the highest relevance to food safety are non-hemolytic enterotoxin (Nhe), hemolysin BL (Hbl), and cytotoxin K (CytK). Nhe and Hbl are three-component proteinaceous toxins encoded on the nheABC and hblACD operons. Both Nhe and Hbl are cytotoxic only in the presence of all three components (NheA, NheB, NheC and Hbl-B, Hbl-L1, and Hbl-L, respectively). CytK is composed of a single protein that exists in two forms, encoded by cytK-1 and cytK-2 genes, showing 89% of sequence similarity [12,13]. CytK-1 has been associated with several reported death cases, while CytK-2 is considered less cytotoxic. The second type of B. cereus foodborne disease, known as the emetic form, is caused by the thermo- and acid-stable cyclic peptide cereulide, encoded by the ces gene locus [14]. Usually, after consumption of food contaminated with cereulide, the disease manifests with emesis for up to five hours [15]. In high doses, cereulide can enter systemic circulation, cross the blood-brain barrier, cause fulminant liver failure, and damage other organs [16,17]. The effects are dose-dependent, but sensitive individuals, such as children, can suffer fatal consequences [10,18,19].
Up to date, several fatal cases and many outbreaks have been attributed to B. cereus foodborne poisoning [1,18,20,21,22,23]. Fatal cases and family outbreaks were usually caused by the intake of cereulide via mixed food products that contained farinaceous ingredients [16,18,20,23]. However, mild outbreaks and individual cases were related to many food types, including cereals/cereal products, herbs, spices, dairy products, confectionery products, canned food, seafood, vegetables, egg products, drinks, and dried food products [24,25,26,27,28]. The reason for such broad prevalence is that B. cereus is widespread in the environment (in dust, ground, plant surfaces, or in the rhizosphere, enteric tract insects, and mammals) and concomitantly in ingredients and final food products. The ability to form spores under unfavorable conditions enables B. cereus to survive, spread, and resist mild food processing and preservation treatments [29]. Moreover, mixing food ingredients of different microbiological quality in which intrinsic conditions are favorable for spore germination and vegetative outgrowth can change the overall microbiological picture of the newly formed product. Therefore, it is not surprising that mixed food was the most common cause of B. cereus food poisoning in the past [24,30,31]. Contaminated ingredients were the most important contributory factor reported for the strong-evidence outbreaks, together with the storage time/temperature abuse along the food production chain in the Europe Union (EU) in 2014 [25].
In food safety, a particular concern is given to B. cereus members that do not show uniform temperature growth affinities. Most strains are characterized by typical mesophilic properties, whose growth can be controlled by refrigeration. However, a subset of these mesophiles display as cold-tolerant (psychrotrophic) bacteria that can multiply at a temperature lower than 10 °C (at 7 and 4 °C). In this regard, both enterotoxin and cereulide producing psychrotrophic strains represent a microbial hazard in refrigerated food. Although significant amounts of cereulide cannot be produced at temperatures lower than 8 °C, temperature abuse is observed in each part of the cold chain, particularly in households of consumers, where the average temperature often surpass 8 °C [18,20,21,32,33].
In the last decade, B. cereus food poisonings outbreaks and individual cases showed an increasing trend, and in 2020, as the most frequently reported cause of food poisoning outbreaks in the EU [24,25,26,27,28]. Considering that B. cereus might induce diseases with fatal consequences, understanding B. cereus prevalence in food products and its ability to grow at refrigerator temperatures is an essential prerequisite to assure food safety. Therefore, the aim of this study was to estimate the prevalence of toxigenic B. cereus organisms among selected food products and ingredients, as well as the growth properties of B. cereus grown at refrigerator (4 and 7 °C) and mild abuse temperatures (10 °C). Additionally, the ability of psychrotrophic ces-positive presumptive B. cereus to produce cereulide at 37 °C and 4 °C was analyzed.
2. Results and Discussion
2.1. Distribution of Toxin Genes among Presumptive B. cereus Organisms Obtained from Food Products
B. cereus is one of the most common causal agents of foodborne diseases among bacterial toxin producers. Reports published by the European Food Safety Agency (EFSA) and European Center for Disease Control (ECDC) demonstrated that many food types were vehicles in strong-evidenced foodborne outbreaks [1,26,27,34,35]. In this study, we collected 250 food products and ingredients from the Belgian, Dutch and Serbian retail markets with the aim to estimate the prevalence of contaminated food products by the toxigenic B. cereus, toxigenic profiles of psychrotrophically grown presumptive B. cereus communities, and their ability to produce cereulide. In total, 226/250 (90.4%) food products contained presumptive B. cereus, which we further tested for the presence of nheA, hblA, cytK-1, and ces genes. Overall, selected food products were mainly contaminated with the nheA positive B. cereus carriers, found in 77.9% of the samples (176/250). Typically, nhe positive strains are the most prevalent among B. cereus food isolates [36,37,38,39,40]. However, the prevalence of the nhe gene among B. cereus food isolates reported in literature depends on the food type, as some studies indicated that the hbl gene was the most prevalent [41,42]. In our study, data showed that hblA positive presumptive B. cereus were found in 64.8% (162/250) of collected food samples. Also, Wijnands et al. [37] reported that hbl was detected in approximately 66% of the food isolates. Something higher proportion of Hbl-producing strains (766/1022), was reported by Berthold-Pluta et al. [38] in products collected from the Polish market (74.9%). Biesta-Peters et al. [39] found that nearly half of the food isolates contained the hbl genes.
Further, in our study, 23.2% (58/250) of examined food products were contaminated with emetic B. cereus (Table 1). Similarly, around 17% of food isolates obtained from the Dutch market were classified as emetic strains [39]. However, not all emetic strains were able to produce cereulide, indicating that toxin encoding genes do not always express their pathogenic potential. In our study, we observed that among psychrotrophic ces-positive presumptive B. cereus producers 5 out of 11 were able to produce cereulide (Section 2.4). Further, 10 out of 250 products were contaminated with cytK-1 positive presumptive B. cereus (4.4%). Generally, cytK-1 positive isolates belong to B. cytotoxicus species, which is not highly prevalent. In France, 5% of isolates among presumptive B. cereus were identified as the carriers of the cytK-1 gene, which is in line with our results [43]. Initially, cytK-1 was detected in the strain that induced several death cases [12] and for a long time it was considered that all carriers of this gene are highly cytotoxic. However, discoveries of the novel strains indicate that cytotoxicity among B. cytotoxicity is very diverse, and therefore should be further examined [44,45].

Table 1.
Prevalence of Toxigenic B. cereus in Selected Food Products.
In the category of cereal products, including rice and seeds/pulses (n = 162), nheA carriers were found in 73.4%, hblA in 68.5%, while cytK-1 was found in 0.6% of examined products (129, 108, and 1/162, respectively). Potential emetic producers were detected in 20.37% (33/162) of tested products in this group. Specifically, in the subcategory of cereal grains, nheA was detected in almost 80% of presumptive B. cereus isolated from food (39/49). Buckwheat, millet, bulgur, quinoa, and rice were evidenced as a source of potentially toxin-producing B. cereus. Also, an experiment conducted on the flax grain produced in Canada showed that a large proportion of the B. cereus isolates carried the nheA (96.33%), hbID (94.5%), cytK-1 (12.8%), while only 2.8% strains carried ces gene [46], indicating that cereal grains are an important source of potentially toxin-producing B. cereus. Further, raw rice is usually highly contaminated with B. cereus. Samapundo et al. [47] reported that 100% of tested rice marketed in Belgium was contaminated by presumptive B. cereus. We noted that the majority of tested raw rice samples harbored at least one toxigenic B. cereus (27/29). nheA carriers were the most prevalent (26/29), followed by hblA (21/29) and emetic B. cereus (5/29). Berthod-Pluta et al. found that 81.4% of isolates obtained from rice were able to produce Nhe, 57.6% Hbl, and 6.8% were ces-positive [47]. In rice obtained from the U.S. market, 89.1% of B. cereus isolates possessed the nheAB genes [48], and almost 53.0% of tested isolates were able to produce both Nhe and Hbl enterotoxins. Overall, 93.9% of those isolates were determined as pathogenic [48], but emetic strains were not found.
Subcategory of cereal-based products and derivatives (n = 94) harbored nheA positive B. cereus in 70% (66/94) and hblA in 67% (63/94), respectively, while ces carriers were detected in 21.3% (20/94) of tested products. One sample in this group, namely couscous (n = 5), was contaminated with the all tested toxigenic B. cereus. Kone et al. [49] reported that only one cereal-based product (improved millet flour) out of 210 was contaminated with B. cytotoxicus, indicating that cereals do not represent the main source of B. cytotoxicus.
The high prevalence of toxigenic B. cereus among cereals is not surprising. Cereals and cereal-based products (including seeds and rice) were determined as common sources of B. cereus food poisonings [1]. Nevertheless, cereals and cereal-based products are usually used as ingredients in mixed food or ready-to-eat products [24,25,26,31], indicating that they might be a significant contributory source of B. cereus. Several death cases were linked with cereulide production in ready-to-eat pasta and rice-based products [16,18,20,21,27]. Therefore, in this study, we also examined ready-to-eat products that contain pasta and rice. Based on the results obtained in our study, neither the ready-to-eat pasta nor rice-based products (n = 12) were contaminated with emetic strains. hblA and nheA positive B. cereus were found in two and 4/12 tested products, respectively, from which 2/12 we concomitantly contaminated with both toxin gene carriers. Yu et al. [50] reported that 35% of ready-to-eat products were positive for B. cereus, from which 39 and 83% of the isolated strains contained enterotoxin-encoding hblACD and nheABC genes, respectively, while 7% harbored the cesB gene. Similarly, in India, ready-to-eat products were mainly contaminated with nheABC genes (57.6%), followed by 36.8% of hblC positive B. cereus [51]. Ready-to-eat products are usually mild heat-treated, which enables bacterial spores to survive. These mild treatments inactivate the vegetative flora; however, they may trigger the germination of present spores. Depending on temperature growth affinities, intrinsic and extrinsic environmental factors, a growing population can produce cereulide or enterotoxins during refrigeration [15,52]. Furthermore, we tested instant soups (n = 13) constituted from starch and spices. Results showed that these products are largely contaminated with presumptive B. cereus (11/13). nheA, hblA, were identified in samples obtained from 11 soups, and ces was also found in six products. Similar observations were reported by Messelhäusseret et al. [53]; however, instant soups were not extensively examined in the past.
Milk powder has been a known source of B. cereus for many years [54,55], as the processing of milk into a powder does not eliminate B. cereus spores [56]. In our study, the toxin gene harboring presumptive B. cereus were highly prevalent in dairy-based powders. In total, 22/26 products were contaminated by the nheA, followed by hblA positive B. cereus (22/26). Although ces positive B. cereus s.l. are mainly related to starch-rich products, in 10 products belonging to dairy-based powders, potential cereulide producers were detected. Ten products from this group (10/26) were positive on nheA, hblA, and ces B. cereus carriers together. Liu et al. [57] reported that 75% of isolates were nha positive, 21% hbl positive, while ces encoding genes were not detected. Also, our study showed that other milk powder analogs, such as coffee creamers and chantilly cream represent the source of potentially toxin-producing B. cereus. One out of seven coffee creamers analyzed in this study were contaminated with nheA, hblA, and ces positive B. cereus together. In five products, nheA and hblA positive B. cereus were found combined, and one product was contaminated with the nheA gene carrier. Also, three products of chantilly cream obtained from the Belgian market were tested. Two products were contaminated in the combination of nheA, hblA, and ces positive B. cereus. Generally, the microbiological safety of dried milk powders depends on the initial level of contamination of raw milk, processing parameters, and hygiene in the production area. The main concern is related to reconstructed milk powder-based infant formulas, which in case of temperature abuse might cause infection in infants and babies due to the lack of competitive intestinal flora.
Dehydrated vegetable products such as mashed potato flakes are often contaminated with B. cereus s.l. In our study, nheA positive B. cereus were the most dominant contaminants of mashed potato flakes (n = 17). hblA positive B. cereus was found in nine products, ces carriers in seven products, and cytK-1 carriers were found in six products. Mashed potatoes flakes represent the most significant reported source of cytK-1 positive strains, considering that it has been rarely found in other products. Kone et al. [49] reported that 20/20 potato flakes were contaminated with B. cytotoxicus. Heini et al. [58] also found that mashed potato powder is the most significant source of the cytK-1 positive isolates. More considerably, our study showed that this product could be concomitantly contaminated with nhe, hbl, ces, and cytK-1 positive B. cereus together (2/17).
Spices (n = 12) and herbal teas (n = 8) also harbored potentially toxin-producing B. cereus. Among spices, 6/12 were contaminated with hblA positive B. cereus, while nheA positive B. cereus was found in 2/12 products. Six, four, and one tea product were contaminated with nheA, hblA, and ces carriers, respectively. Dry herbs and spices are well known to be contaminated with B. cereus. Indirectly, as ingredients in mixed food products, they can contribute to B. cereus food poisoning outbreaks and cases.
2.2. Growth of Toxigenic Presumptive B. cereus at 4, 7, and 10 °C
Psychrotrophic capacity varies within and among B. cereus species, as numerous lineages have been shown to grow at low temperatures. The principal food safety issue related to spore formers, such as B. cereus is the ability to survive the processing and preservation treatments and multiply during refrigerator storage to a concentration that might be infectious. Therefore in this study, we exposed toxigenic presumptive B. cereus communities obtained from different food products and ingredients (n = 121) to refrigerated (4 and 7 °C) and mild abuse temperatures (10 °C) for one month. In total, 94, 104, and 121/121 of presumptive B. cereus communities isolated from food products were able to grow at 4, 7, and 10 °C, respectively (Table 2). As expected, at 4 °C growth detection time (GDT) was the longest. The GDT at 4 °C ranged from 1–23 days, with an average of 11 days and a median of 15 days. In the first five days, 16/94 of presumptive B. cereus were detected to grow. The GDT for 7/94 and 11/94 ranged between 6–10 days and 11–15 days, respectively. The majority of tested B. cereus (32/94) grew in the range of 16–20 days, while for 18 tested B. cereus, the GDT was between 20–25 days. Day 23 was the last day when B. cereus was detected at 4 °C.

Table 2.
Growth Detection Time for Presumptive B. cereus.
It was not possible to determine a correlation between specific food groups and the presence of psychrotrophic presumptive B. cereus as psychrotrophs were highly prevalent in all food categories. In the category of cereal products, including rice and seeds/pulses, B. cereus was detected in a total of 48/70 of tested samples. Food products typically linked with B. cereus severe food poisonings, such as pasta and rice were also a source of psychrotrophic B. cereus (6/11 and 6/9, respectively). B. cereus obtained from powdered dairy-based products could also grow at 4 °C (7/7). Psychrotrophic presumptive B. cereus communities were found in coffee creamer (6/8) and chantilly cream (3/3), indicating that milk powder analogs are also a great source of psychrotrophic B. cereus able to grow at refrigerator temperature. Also, other products showed as a potential source of toxigenic presumptive B. cereus that can grow at refrigerator temperatures, such as mashed potato flakes (9/10), instant soups (12/12), and spices (6/7).
Although our study demonstrated that psychrotrophic B. cereus are highly prevalent among different food products, data from the literature is diverse. For example, Samapundo et al. [47] showed that among 380 isolates, none were able to grow at 5 °C in 45 days. Similar results were reported by Park et al. who isolated B. cereus from the green lettuce [59]. Isolates obtained from cooked chilled products did not grow at 4 °C; however, 10% of these strains were able to grow at 5 °C [60]. Carlin et al. reported that 9/83 of isolates obtained from food and environment were able to grow at 4 °C [61]. B. cereus obtained from condiments were all psychrotrophic [62]. Currently, there is no standardized assay for defining B. cereus temperature growth affinities. Factors such as selection of media, growth conditions (i.e., temperature, agar plate vs. broth, shaking vs. non-shaking) represent potential sources of variation among data. While some researchers have suggested usage of the “psychrotolerant signatures” obtained via cold shock protein (encoded by cspA) or panC, this approach is still questioned as some non-psychrotolerant strains also encode these “psychrotolerant signatures” or are able to grow at low temperatures but do not possess any of the genes that would indicate there temperature growth affinities [63,64]. Based on the differences in 16 s rRNA, Wijnands et al. [30] reported that 4.4% of food isolates were psychrotrophic. Furthermore, researchers estimated psychrotrophic profiles by exposure of isolates at 6 °C. Isolates obtained from food products in Morocco had ability to grow at 6 °C (37/52) [65]. Also, Stenfors and Granum found that 17/26 were able to grow at 6 °C among dairy, food, and clinical isolates [66]. Jan et al. [64] reported that all isolates from egg products multiplied at 6 °C. Similarly, Torkar and Seme [67] detected 56.7% of B. cereus food and clinical isolates at 6.5 °C.
At 7 °C, 104/121 of presumptive B. cereus were detected. The GDT range and average were similar to results obtained at 4 °C (1–22 and 10 days, respectively); however, the median value was lower (11 days). In the first five days, 28/104 of tested B. cereus were detected. After 6–10 and 11–15 days, 15 and 21/104 of B. cereus were observed. The highest number of B. cereus was able to grow in the range between 16–20 days (34/104), while 6/104 were detected after 20 days of exposure at 7 °C. In the category of cereal products, including rice and seeds/pulses, B. cereus was detected in 58/70. Specifically, colonies from rice and pasta were detected in 7/8 and 8/10 of tested products. Further, presumptive B. cereus obtained from milk powder and powders containing milk or whey protein had the same ability to grow at 7 °C as at 4 °C. For colonies obtained from mashed potato flakes, instant soups, seeds, and herbs, and spices growth potential at 7 °C was slightly higher (10/10, 12/12, and 7/7, respectively). Berthold-Pluta et al. [38] tested the psychrotrophic properties of 1022 isolates obtained from different food products at 7 °C for 10 days. Results showed that 25% (256) isolates were able to grow at 7 °C, which is in line with our study where 21/104 (20.2%) grew at 7 °C in 10 days. However, the majority of tested B. cereus communities started to grow after 10 days. Exposure time in our study was longer; therefore we could observe more psychrotrophs. Similar results were reported for isolates obtained from refrigerated dairy products. Four out of 15 isolates (26.7%) were detected at 7 °C after 10 days of exposure. Carlin et al. [61] reported that 41/83 (49%) diarrheal and food–environment strains grew at 7 °C after 12 days of exposure. Among the 101 isolated B. cereus from green lettuce, only 18 were capable of growing at 7 °C [59].
Results obtained at 10 °C indicate that all presumptive B. cereus exhibit mesophilic character considering that 121/121 were able to grow at this temperature. On the 16th day, presumptive B. cereus were detected in all tested samples, with an average of seven days and a median value of four days. For the near half of the inoculated agar plates (45%), the GDT was in the first five days. After, 21 and 19/121 were detected to grow between 6–10 and 11–15 days. Other studies also reported that many B. cereus can grow at 10 °C. Samapundo et al. [47] detected the majority of the isolates (87.9%) during 45 days of exposure. Isolates obtained from dairy products also displayed psychrotrophic character (12/15) [68].
Temperature profiling of food isolates represents an important factor for food safety and quality. Determination of potential growth or toxin production at a given temperature may provide information about behavior in a specific food product that is directly related to food storage recommended temperature. Especially, spore formers are a significant risk in the case of temperature/time storage abuse. Although they grow very slowly at 4 and 7 °C; at 10 °C, psychrotrophs can grow fast and may produce toxins.
2.3. Toxigenic Profiles of Psychrotrophic Presumptive B. cereus Grown at 4 °C
Several studies have shown that psychrotrophic B. cereus carry toxin encoding genes associated with both diarrheal and emetic disease. To assess the potential hazard of psychrotrophic presumptive B. cereus that are able to grow at refrigerated temperatures (n = 94), their toxigenic profiles were determined. Enterotoxin and cereulide encoding genes were detected in 81% (76/94) of psychrotrophic presumptive B. cereus biomasses isolated from food. The nheA gene was the most prevalent at 70.2% (66/94), followed the by hblA gene at 55% (52/94), and the ces encoding gene at 11.7% (11/94). None of the psychrotrophic presumptive B. cereus were cytotoxin K-1 encoding gene (cytK-1) positive. The main carrier of this gene is B. cytotoxicus species, which has been characterized by thermotolerant growth affinities. Presumably, ribosomes in thermophilic bacteria are non-functional at lower temperatures [69]; therefore, the absence of this microorganism among psychrotrophs was expected. Park et al. [59] reported that 94% of the psychrotolerant B. cereus isolates obtained from green lettuce harbored the nheABC genes, 44% hbl, while ces was not detected. Similarly, Bartoszewicz et al. [70] reported that 85 and 30% of psychrotrophic isolates were nheA, hblA positive, respectively. Together, hblA and nheA were present in 40% (38/94) of B. cereus communities that were able to grow at 4 °C, while the combination of hblA and ces was not noted. hblA, nheA, and ces psychrotrophic presumptive B. cereus were found together in 5.3% (5/94) of tested food products. Our data are consistent with previous reports demonstrating that enterotoxin encoding genes are prevalent among psychrotrophic B. cereus. Simultaneously, ces and nheA were detected in 7.44% (7/94), while ces was found alone only in one sample. Frequency distributions of the growth detection time (GDT, day) of enterotoxigenic and emetic psychrotrophic presumptive B. cereus are shown in Figure 1.
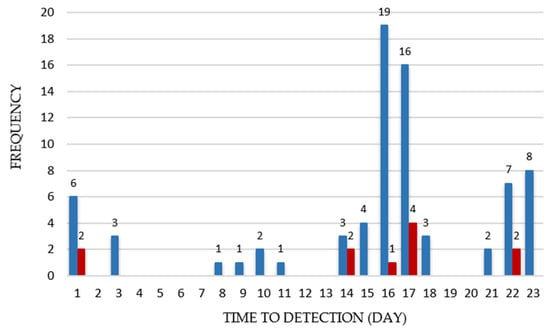
Figure 1.
Frequency Distributions of the Time to Detection (GDT, day) of Enterotoxigenic (n = 76) and Emetic (n = 11) Psychrotrophic Presumptive B. cereus. Blue columns represent the number of enterotoxigenic B. cereus, and the orange columns represent the number of emetic B. cereus.
Among 52 presumptive B. cereus isolated from the category cereal products, including rice and seeds/pulses, hblA, nheA, and ces genes were detected in 34.5% (20/58), 37.9% (22/58), and 8.6% (5/58). In 27.6.% (16/58) of tested psychrotrophs, hblA and nheA were detected together, and nheA and ces in 8.6% (5/58). A combination of hblA, nheA, and ces was found in 7.7% (4/52). Specifically, bulgur (2/3) was contaminated with all potential toxin-producers that were able to grow at 4 °C, as well as couscous (1/5) and muesli (1/5), indicating that this type of food can be a significant source of both enterotoxigenic and emetic B. cereus at refrigerator temperatures. ces encoding gene in combination with nheA was found in communities obtained from buckwheat (1/2), muesli (1/5), and pasta (1/11). Further, a combination of hblA and nheA was found in millet (1/2), polenta (3/3), semolina (2/4), muesli (1/5), flour (3/12), and rice (4/8). hblA gene was rarely found alone-only in millet (1/2), flour (1/12), and semolina (1/4). nheA alone was found in oat flakes (1/5), rice waffles (1/3), flour (1/12), pasta (1/10), and rice (1/8). Seeds were contaminated with nheA, and hblA, positive B. cereus (3 and 2/6 samples, respectively). Dairy-based powdered products such as milk powder and derivatives of milk powder were also contaminated with psychrotrophic toxigenic B. cereus (9/16). From 2/16 tested products, a combination of hblA, nheA, and ces was found in milk powder (1/7) and coffee creamer (1/6). hblA and nheA were found in communities obtained from milk powder (1/6) and coffee creamer (2/6). nheA alone was detected in the sample obtained from chantilly cream (1/3), as well as the ces gene (1/3).
According to results obtained in this study, mashed potato flakes seem like the significant carriers of psychrotrophic enterotoxigenic B. cereus. Five out of nine psychrotrophic presumptive B. cereus were both hblA and nheA positive, while one out of nine was only nheA positive. Similarly, instant soups showed as a great source of toxigenic psychrotrophic B. cereus considering that 10/12 psychrotrophic communities were positive on some of the tested genes. Eight out of twelve were hblA and nheA positive, one out of twelve was ces and nheA positive. In one sample nheA positive psychrotrophic B. cereus were detected. Further, herbs and spices were contaminated with enterotoxigenic B. cereus (five out of six). While not many studies were conducted to examine toxigenic properties of psychrotrophic B. cereus isolated from specific food groups, concerns have been raised for many years due to discrepancies among data. Investigations related to foodborne outbreaks did not report properly chilled food as the main cause of B. cereus poisoning [29]. At low temperatures, B. cereus proliferates slowly; however, the effects of the food matrix seem to play an important role in toxin production.
2.4. Cereulide Production by Psychrotropic Presumptive B. cereus at 37 °C and 4 °C
Cereulide producers are characterized by mesophilic and psychrotrophic growth capacities [68]. Psychrotrophs mainly belong to the B. weihenstephanensis species. However, the potential for psychrotrophic B. cereus group strains to acquire the ces genes by horizontal gene transfer exists. Therefore it has been assumed that potential cereulide psychrotrophic might not all belong strictly to this species [29]. In our study, we identified potential cereulide producers that can grow at refrigerator temperatures with the aim to examine the ability to produce cereulide. Presumptive psychrotrophic B. cereus identified as ces gene positive were tested by the computer-aided semen analysis study of the boar semen motility described by Rajkovic et al. [69]. After exposure to 4 °C, 11 out of 38 examined ces positive B. cereus were detected to grow. These B. cereus were isolated from the following food products: buckwheat, chantilly cream, coffee creamer, couscous, milk powder, muesli, polenta, instant vermicelli soup, and instant meat soup (Table 3).

Table 3.
GDT and cereulide production at 37 and 4 °C of psychrotrophic ces-positive B. cereus.
Based on the motility assay, it was seen that 5/11 of ces-positive presumptive B. cereus were able to produce cereulide at 37 °C. Our results are consistent with the study of Biest-Pieters et al. [39], who reported that emetic strains do not always display their pathogenic potential. Food products from which psychrotophic cereulide producers were obtained are buckwheat, chantilly cream, coffee creamer, muesli, and instant vermicelli soup. Further, to estimate the ability to produce cereulide in small quantities at 4 °C, we used LC-MS2. None of the ces-positive samples were able to produce any significant amounts of cereulide at 4 °C after one month of exposure.
Only in one sample 0.79 ng/mL of cereulide was quantified. Limited data are available about cereulide production at refrigerator temperatures. It has been noted that at 8 °C emetic strains produce minimum amounts of toxin [29,71]. This can explain why correctly stored products were never reported as a cause of intoxication with cereulide. However, it should not be ignored that, in many cases, food products are subjected to a temperature that often surpasses recommended refrigerator temperatures and, in numerous cases, can be higher than 10 °C. Cereulide synthesis is a multifactorial process that is not strictly dependent on the cell number. Present nutrients can trigger production even before the exponential growth phase [72]. Moreover, cereulide at low temperatures can be produced in high amounts [73] or in more potent forms (isocereulide A) [74,75].
3. Conclusions
This study demonstrated that selected food ingredients and products were significantly contaminated with toxigenic B. cereus. Many of them were concomitantly contaminated with different potentially pathogenic B. cereus gene carriers, indicating that one product may pose a risk for both diarrheal and emetic disease. The relatively high number of isolated psychrotrophic presumptive B. cereus were enterotoxin and cereulide gene carriers, indicating that refrigeration cannot ensure complete growth prevention. Due to the increasing number of foodborne outbreaks caused by B. cereus toxins, it is necessary to monitor the presence of this pathogen among food products more regularly. Moreover, in order to comply with an appropriate level of consumers protection, relevant parties such as food business operators and health authorities must take into account risks present during food handling. Since toxigenic psychrotrophic B. cereus is abundant in food products and ingredients, it is important to estimate possible risks related to the refrigerator and temperature abuse conditions in regards to both enterotoxin and cereulide producing B. cereus. Therefore, additional studies on factors that affect B. cereus growth and toxin production are needed.
4. Materials and Methods
4.1. Collection of Food Products
A total of 250 food products were collected from Belgian, Dutch, and Serbian retail markets. Selected products are mainly used as ingredients in mixed food products (n = 250). Products were classified into five categories: ‘cereal products, including rice and seeds/pulses’, ‘dairy-based products’, ‘dehydrated vegetables, ‘mixed food’, ‘herbs and spices’ according to the Manual for reporting on food-borne outbreaks in accordance with Directive 2003/99/EC published by EFSA (Table 4) [76]. Individual products within group were selected from different brands available in retail shops during 2019 and stored at recommended conditions prior to analysis. Analysis was performed a maximum of one day after purchase of ready-to-eat products and sprouted seeds or five days upon arrival of dry food products in the Laboratory of Food Microbiology and Food Preservation (Ghent University).

Table 4.
Categories of Samples Analyzed in This Study.
4.2. Detection of Presumptive B. cereus
Detection of presumptive B. cereus was performed as previously described [47]. In brief, 25 g of selected food product was placed in a sterile filter stomacher and enrichment broth composed of Tryptone Soy Broth (TSB) (Oxoid, UK) and 50,000 IU of polymyxin B supplement (Oxoid, Hampshire, UK) was added to the total of 250 g. Enrichment of homogenized samples was performed at 30 °C for 24 h, after which 0.1 mL was inoculated onto a mannitol-egg yolk-phenol-red polymyxin-agar medium (Oxoid, Hampshire, UK), supplemented with polymyxin B according to manufacturer instructions and incubated at 30 °C for 48 h.
4.3. Toxin Gene Profiling
DNA isolation was performed using Prepman lysis solution (Applied Biosystems, Waltham, MA, USA). Fresh culture of presumptive B. cereus was collected and placed in a lysis buffer in 2 mL tube, mixed thoroughly, and incubated at 95 °C for 15 min. Afterwards, supernatants with isolated DNA were stored at −20 °C and screened for the presence of enterotoxin and cereulide encoding genes using primers as previously described (Table 5), namely the nheA, hblA, cytK-1, and cereulide toxin-related genes ces. The specificity of used primers was primarily tested in Basic Local Alignment Search Tool Genomic (https://blast.ncbi.nlm.nih.gov/Blast.cgi (5 April 2019). Further, they were checked by testing 30 reference strains with known toxigenic properties obtained from the culture collection of Laboratory of Food Microbiology and Food Preservation (Ghent University). Genomic DNA extracted from strains ATCC 14579, DSM 4384, NVH 1230-88, NCTC 11145, NS115, NS117, LMG 12334, F4346/75, NVH0075/95, NVH0500/00, F0285/78 and NVH0391-98 were used as positive or negative controls in this study (also for cereulide production in Section 4.5), depending on their toxigenic properties, while the blank control was done using nuclease-free water.

Table 5.
Primer Pairs and Annealing Temperature Conditions.
A typical 50 μL PCR mixture for the SYBR green I real-time PCR assay consisted of 2 μL of template DNA, 25 μL of 2x concentrated PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Vilnius, Lithuania), 500 nM of reverse and forward primers (IDT Technologies, Leuven, Belgium) and 18 μL of nuclease-free water (Ambion, Austin, TX, USA). Primarily, isolated DNA was exposed to 95 °C for 10 min, followed by 40 cycles of 95 °C/15 s for denaturation, and annealing and extension at temperatures shown in Table 5. In the subsequent dissociation stage for the analysis of melting curve, the temperature was raised from 60 °C to 95 °C. Further, 20 µL PCR reaction mixture for the probe-based assay contained 10 µL of GoTaq Probe qPCR (Promega, Madison, WI, USA), 500 nM of each primer, 200 nM probe, and 2 µL of the template DNA. All PCR reactions were performed and analyzed in ABI 7300 Real Time PCR system (Applied Biosystems, Waltham, MA, USA) using MicroAmp 96-well plates (Applied Biosystems, Waltham, MA, USA). For the ces gene detection, two pair of primers were used (Table 5).
4.4. Refrigeration and Mild Abuse Temperature Exposure
Collected presumptive B. cereus communities, which possessed at least one virulence gene, were tested for the capability to grow at psychotropic conditions at 4, 7, and mild abuse temperature (10 °C). Prior to exposure to respective temperatures, cultures were reactivated in TSB. After, culture was transferred onto double Tryptone Soya Agar (TSA) and incubated for 30 days at 4, 7, and 10 °C.
4.5. Emetic Toxin Detection via Boar Semen Motility Assay
The procedure was performed according to Rajkovic, Uyttendaele, and Debevere [80]. Primarily, ces-positive B. cereus samples were incubated for 24 h at 37 °C, in TSB after which 1 mL of each sample was inoculated onto TSA (in triplicates), air-dried and incubated again at 30 °C for 24 h. Grown colonies were flooded with 3 mL of methanol (≥99.9%, Sigma-Aldrich, St. Louis, MI, USA) and transferred to the 500 mL Erlenmeyer flask. Methanol was added to the final dilution of 1:3 of biomass and placed in a water bath at 100 °C for 1 h. Extracted cereulide was stored in glass vials at −20 °C until analysis. Further, five microliters of cereulide extract were mixed with 195 µL of the fresh boar semen (ca. 30 million cells per mL) in a microtiter well, and then 5 µL of suspension was immediately transferred into Leja Slides (2-chambered 20 mm slide, Leja, Nieuw-Vennep, The Netherlands). The sample was considered cereulide-positive when interruption of semen motility occurred within less than 10 min. Motility was microscopically analyzed (Zeiss, AxioCam Mrm Imager A.1, Jena, Germany) with a MiniTherm stage warmer providing an optimal temperature of 37 °C. The images were examined via the AxioVision program. Experiments were performed in three repetitions.
4.6. Quantification of Cereulide Production at 4 °C by LC–MS² Analysis
ces positive samples that were able to grow at 4 °C were tested for the cereulide production at 4 °C by LC-MS2. After one month of exposure, we extracted cereulide as previously described (Section 4.5.) and subjected to further analysis according to Delbrassinne et al. [81]. Briefly, the LC-MS LCQ Deca-XP Plus ion trap mass analyzer (ThermoFinnigan, San Jose, CA, USA) was used for analysis. A full mass spectrum from 500 to 1300 m/z values and an MS² fragmentation mass spectrum were recorded in positive electrospray mode (ESI+). The peaks corresponding to the loss of a CO (m/z values 1125.3 and 1083.3, respectively) were always the highest peaks generated through the fragmentation. The chromatograms were smoothed thanks to a Gaussian function.
Author Contributions
Conceptualization, A.R.; Data curation, J.J.; Formal analysis, J.J. and S.T.; Investigation, J.J., S.T., K.B. and A.R.; Methodology, J.J., K.B. and A.R.; Supervision, A.R.; Visualization, J.J. and S.T.; Writing—original draft, J.J.; Writing—review & editing, J.J. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work received funding from the Ghent University Special Research Fund (BOF), grant number BOFSTA2017004201.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support from the Special Research Fund (Ghent University) for research grant BOFSTA2017004201 given to Andreja Rajkovic, which provided a PhD fellowship to Jelena Jovanovic. The authors would like to thank VLIR-UOS and DGD Belgium for the GlobalMinds operational grant awarded to Jelena Jovanovic. Authors also acknowledge contribution of Julien Masquelier for the LC-MS2 analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). Risks for Public Health Related to the Presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in Foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar] [CrossRef]
- Jung, M.Y.; Paek, W.K.; Park, I.S.; Han, J.R.; Sin, Y.; Paek, J.; Rhee, M.S.; Kim, H.; Song, H.S.; Chang, Y.H. Bacillus gaemokensis sp. nov., Isolated from Foreshore Tidal Flat Sediment from the Yellow Sea. J. Microbiol. 2010, 48, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Kim, J.S.; Paek, W.K.; Lim, J.; Lee, H.; Kim, P., II; Ma, J.Y.; Kim, W.; Chang, Y.H. Bacillus manliponensis sp. nov., a New Member of the Bacillus cereus Group Isolated from Foreshore Tidal Flat Sediment. J. Microbiol. 2011, 49, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, G.H.; Hu, G.P.; Cetin, S.; Lin, N.Q.; Tang, J.Y.; Tang, W.Q.; Lin, Y.Z. Bacillus bingmayongensis sp. nov., Isolated from the Pit soil of Emperor Qin’s Terra-cotta Warriors in China. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2014, 105, 501–510. [Google Scholar] [CrossRef]
- Miller, R.A.; Beno, S.M.; Kent, D.J.; Carroll, L.M.; Martin, N.H.; Boor, K.J.; Kovac, J. Bacillus wiedmannii sp. nov., a Psychrotolerant and Cytotoxic Bacillus cereus group species Isolated from Dairy Foods and Dairy Environments. Int. J. Syst. Evol. Microbiol. 2016, 66, 4744–4753. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of Nine Novel Species of the Bacillus cereus Group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Yang, G.; Chen, Y.; Zhou, S.; Zhao, Y.; Zhuang, L. Bacillus Nitroreducens sp. nov., a Humus-Reducing Bacterium Isolated from a Compost. Arch. Microbiol. 2016, 198, 347–352. [Google Scholar] [CrossRef]
- Helgason, E.; Økstad, O.L.E.A.; Caugant, D.A.; Mock, L.E.; Hegna, I.D.; Johansen, H.A.; Fouet, A.; Mock, M.; Hegna, I.; Kolstø, A.B. One Species on the Basis of Genetic Evidence. Appl. Environ. Microbiol. 2000, 66, 2627–2630. [Google Scholar] [CrossRef] [Green Version]
- Guinebretière, M.H.; Velge, P.; Couvert, O.; Carlin, F.; Debuyser, M.L.; Nguyen-The, C. Ability of Bacillus cereus group strains to cause Food Poisoning Varies According to Phylogenetic Affiliation (groups I to VII) Rather Than Species Affiliation. J. Clin. Microbiol. 2010, 48, 3388–3391. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, J.; Ornelis, V.F.M.; Madder, A.; Rajkovic, A. Bacillus cereus Food Intoxication and Toxicoinfection. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3719–3761. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M. The Effect of Selected Factors on the Survival of Bacillus cereus in the Human Gastrointestinal Tract. Microb. Pathog. 2015, 82, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; de Buyser, M.; Einar, P.; Aliments, Â.S. A New Cytotoxin from Bacillus cereus That May Cause Necrotic Enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Senesi, S.; Ghelardi, E. Production, Secretion and Biological activity of Bacillus cereus enterotoxins. Toxins 2010, 2, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Frenzel, E.; Gohar, M. Food-bacteria interplay: Pathometabolism of Emetic Bacillus cereus. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkovic, A.; Uyttendaele, M.; Dierick, K.; Samapundo, S.; Botteldoorn, N.; Mahillon, J.; Heyndrickx, M. Risk Profile of the Bacillus cereus Group Implicated in Food Poisoning. Rep. Super. Health Counc. Belg. 2008, 1, 1–80. [Google Scholar]
- Mahler, H.; Pasi, A.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Bär, W.; Krähenbühl, S. Fulminant Liver Failure in Association with the Emetic Toxin of Bacillus cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef]
- Vangoitsenhoven, R.; Rondas, D.; Crèvecoeur, I.; D’Hertog, W.; Baatsen, P.; Masini, M.; Van der Schueren, B. Foodborne Cereulide Causes beta-cell Dysfunction and Apoptosis. PLoS ONE 2014, 9, e104866. [Google Scholar] [CrossRef]
- Dierick, K.; van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal Family Outbreak of Bacillus cereus. Assoc. Food Poisoning 2005, 43, 4277–4279. [Google Scholar]
- Bauer, T.; Sipos, W.; Stark, T.D.; Käser, T.; Knecht, C.; Brunthaler, R.; Saalmüller, A.; Hofmann, T.; Ehling-Schulz, M. First Insights Into within Host Translocation of the Bacillus cereus Toxin Cereulide Using a Porcine Model. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, M.; Denayer, S.; Botteldoorn, N.; Delbrassinne, L.; Veys, J.; Waegenaere, J.; Sirtaine, N.; Driesen, R.B.; Sipido, K.R.; Mahillon, J.; et al. Sudden Death of a Young Adult Associated with Bacillus cereus Food Poisoning. J. Clin. Microbiol. 2011, 49, 4379–4381. [Google Scholar] [CrossRef] [Green Version]
- Shiota, M.; Saitou, K.; Mizumoto, H.; Matsusaka, M.; Agata, N.; Nakayama, M.; Kage, M.; Tatsumi, S.; Okamoto, A.; Yamaguchi, S.; et al. Rapid Detoxification of Cereulide in Bacillus cereus Food Poisoning. Pediatrics 2010, 125, e951–e955. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency (EFSA). Opinion of the Scientific Panel on Biological Hazards on Bacillus cereus and Other Bacillus spp. in Foodstuffs. EFSA J. 2005, 175, 1–48. [Google Scholar]
- Schreiber, N.; Hackl, G.; Reisinger, A.C.; Zollner-schwetz, I.; Eller, K.; Schlagenhaufen, C.; Pietzka, A.; Czerwenka, C.; Stark, T.D.; Kranzler, M.; et al. Acute Liver Failure after Ingestion of Fried Rice Balls: A Case Series of Bacillus cereus Food Poisonings. Toxins 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne outbreaks in 2015. EFSA J. 2016, 14, e20449. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in in 2016. EFSA J. 2017, 15, 5077. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses report. EFSA J. 2019, 17, e05926. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2019 Zoonoses Report 2020. EFSA J. 2021, 19, 6406. [Google Scholar]
- Webb, M.D.; Barker, G.C.; Goodburn, K.E.; Peck, M.W. Risk Presented to Minimally Processed Chilled Foods by Psychrotrophic Bacillus cereus. Trends Food Sci. Technol. 2019, 93, 94–105. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2010. EFSA J. 2012, 17, 2597. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- James, C.; Onarinde, B.A.; James, S.J. The Use and Performance of Household Refrigerators: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.; Djekic, I.; Smigic, N.; Tomic, N.; Rajkovic, A. Temperature Profile and Hygiene in Household Refrigerators in Belgrade, Serbia and Their Relation to Consumers Food Safety Knowledge and Characteristics of the Refrigerators. Food Control 2022, 136, 108813. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2012. EFSA J. 2014, 12, 3547. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). Analysis of the Baseline Survey on the Prevalence of Listeria monocytogenes in Certain Ready-to-Eat Foods in the EU, 2010–2011 Part A: Listeria Monocytogenes Prevalence Estimates. EFSA J. 2013, 11, 3241. [Google Scholar] [CrossRef]
- Forghani, F.; Kim, J.B.; Oh, D.H. Enterotoxigenic Profiling of Emetic Toxin- and Enterotoxin-Producing Bacillus cereus, isolated from Food, Environmental, and Clinical Samples by Multiplex PCR. J. Food Sci. 2014, 79, M2288–M2293. [Google Scholar] [CrossRef]
- Wijnands, L.M.; Dufrenne, J.B.; Rombouts, F.M.; in’t Veld, P.H.; van Leusden, F.M. Prevalence of Potentially Pathogenic Bacillus cereus in Food Commodities in The Netherlands. J. Food Prot. 2006, 69, 2587–2594. [Google Scholar] [CrossRef]
- Berthold-pluta, A.; Pluta, A.; Garbowska, M. Prevalence and Toxicity Characterization of Bacillus cereus in Food Products from Poland. Foods 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Biesta-Pieters, E.G.; Dissel, S.; Reij, M.W.; Zwietering, M.H.; In’t Veld, P.H. Characterization and Exposure Assessment of Emetic Bacillus cereus and Cereulide Production in Food Products on the Dutch Market. J. Food Prot. 2016, 79, 230–238. [Google Scholar] [CrossRef]
- Böhm, M.; Huptas, C.; Krey, V.M.; Scherer, S. Massive Horizontal Gene Transfer, Strictly Vertical Inheritance and Ancient Duplications Differentially Shape the Evolution of Bacillus cereus Enterotoxin Operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15, 246. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.S.; Huch, M.; Franz, C.M.A.P. Antibiotics Resistance and Toxin Profiles of Bacillus cereus-Group Isolates from Fresh Vegetables from German Retail Markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bang, J.; Kim, H.; Kim, Y.; Kim, B.; Beuchat, L.R.; Ryu, J. Bacillus cereus and Bacillus thuringiensis Spores in Korean Rice: Prevalence and Toxin Production as Affected by Production Area and Degree of Milling. Food Microbiol. 2014, 42, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Glasset, B.; Herbin, S.; Guillier, L.; Cadel-Six, S.; Vignaud, M.; Grout, J.; Pairaud, S.; Michel, V.; Hennekinne, J.; Ramarao, N.; et al. Bacillus cereus-Induced Food-Borne Outbreaks in France, 2007 to 2014: Epidemiology and Genetic Characterisation. Eurosurveillance 2016, 21, 30413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtscher, J.; Etter, D.; Biggel, M.; Schlaepfer, J.; Johler, S. Further Insights Into the Toxicity of Bacillus cytotoxicus Based on Toxin Gene Profiling and Vero Cell Cytotoxicity Assays. Toxins 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Cairo, J.; Gherman, I.; Day, A.; Cook, P.E. Bacillus cytotoxicus—A Potentially Virulent Food-Associated Microbe. J. Appl. Microbiol. 2022, 132, 31–40. [Google Scholar] [CrossRef]
- Gamage, N.W.; Bamforth, J.; Ashfaq, T.; Bernard, K.; Gräfenhan, T.; Walkowiak, S. Profiling of Bacillus cereus on Canadian Grain. PLoS ONE 2021, 16, e0259209. [Google Scholar] [CrossRef]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; Devlieghere, F. Incidence, Diversity and Toxin Gene Characteristics of Bacillus cereus Group Strains Isolated from Food Products Marketed in Belgium. Int. J. Food Microbiol. 2011, 150, 34–41. [Google Scholar] [CrossRef]
- Ankolekar, C.; Rahmati, T.; Labbé, R.G. Detection of Toxigenic Bacillus cereus and Bacillus thuringiensis Spores in U.S. Rice. Int. J. Food Microbiol. 2009, 128, 460–466. [Google Scholar] [CrossRef]
- Koné, K.M.; Douamba, Z.; De Halleux, M.; Bougoudogo, F.; Mahillon, J. Prevalence and Diversity of the Thermotolerant Bacterium Bacillus cytotoxicus Among Dried Food Products. J. Food Prot. 2019, 82, 1210–1216. [Google Scholar] [CrossRef]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A Study on Prevalence and Characterization of Bacillus cereus in Ready-to-Eat Foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef] [Green Version]
- Rana, N.; Panda, A.K.; Pathak, N.; Gupta, T.; Thakur, S.D. Bacillus cereus: Public Health Burden Associated with Ready-to-Eat Foods in Himachal Pradesh, India. J. Food Sci. Technol. 2020, 57, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Dufrenne, J.; Tatini, S.; Notermans, S. Stability of Spores of Bacillus cereus Stored on Silicagel. Int. J. Food Microbiol. 1994, 23, 111–116. [Google Scholar] [CrossRef]
- Messelhäusser, U.; Frenzel, E.; Blöchinger, C.; Zucker, R.; Kämpf, P.; Ehling-Schulz, M. Emetic Bacillus cereus are More Volatile than Thought: Recent Foodborne Outbreaks and Prevalence Studies in Bavaria (2007–2013). BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, H.; Schaller, G.; von Wiese, W.; Terplan, G. Bacillus cereus in Infant Foods and Dried Milk Products. Int. J. Food Microbiol. 1994, 23, 1–15. [Google Scholar] [CrossRef]
- Tatsinkou Fossi, B.; Tatah Kihla Akoachere, J.F.; Nchanji, G.T.; Wanji, S. Occurrence, Heat and Antibiotic Resistance Profile of Bacillus cereus Isolated from Raw Cow and Processed Milk in Mezam Division, Cameroon. Int. J. Dairy Technol. 2017, 70, 43–51. [Google Scholar] [CrossRef]
- Pei, X.; Yang, S.; Zhan, L.; Zhu, J.; Song, X.; Hu, X.; Liu, G.; Ma, G.; Li, N.; Yang, D. Prevalence of Bacillus cereus in Powdered Infant and Powdered Follow-Up Formula in China. Food Control 2018, 93, 101–105. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, Q.; Xu, F.; Ding, S.Y.; Zhu, K. Characterization of Bacillus cereus in Dairy Products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef]
- Heini, N.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Characterization of Bacillus cereus Group Isolates from Powdered Food Products. Int. J. Food Microbiol. 2018, 283, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Kim, H.J. Biofilm Formation of Low-Temperature-Tolerant Bacillus cereus Isolated from Green Leaf Lettuce in the Cold Chain. Foods 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Choma, C.; Guinebretière, M.H.; Carlin, F.; Schmitt, P.; Velge, P.; Granum, P.E.; Nguyen-The, C. Prevalence, Characterization and Growth of Bacillus cereus in Commercial Cooked Chilled Foods Containing Vegetables. J. Appl. Microbiol. 2000, 88, 617–625. [Google Scholar] [CrossRef]
- Carlin, F.; Fricker, M.; Pielaat, A.; Heisterkamp, S.; Shaheen, R.; Salkinoja Salonen, M.; Svensson, B.; Nguyen-the, C.; Ehling-Schulz, M. Emetic Toxin-Producing Strains of Bacillus cereus Show Distinct Characteristics within the Bacillus cereus Group. Int. J. Food Microbiol. 2006, 109, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okanlawon, B.M.; Ogunbanwo, S.T.; Okunlola, A.O. Growth of Bacillus cereus Isolated from Some Traditional Condiments Under Different Regimens. Afr. J. Biotechnol. 2010, 9, 2129–2135. [Google Scholar]
- Jan, S.; Brunet, N.; Techer, C.; Le Maréchal, C.; Koné, A.Z.; Grosset, N.; Cochet, M.F.; Gillard, A.; Gautier, M.; Puterflam, J.; et al. Biodiversity of Psychrotrophic Bacteria of the Bacillus cereus Group Collected in Farm and Egg Product Industry. Food Microbiol. 2011, 28, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Cheng, R.A.; Wiedmann, M.; Kovac, J. Keeping Up with the Bacillus cereus Group: Taxonomy Through the Genomics Era and Beyond. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Merzougui, S.; Cohen, N.; Grosset, N.; Gautier, M.; Lkhider, M. Enterotoxigenic Profiles of Psychrotolerant and Mesophilic Strains of the Bacillus cereus Group Isolated from Food in Morocco. Int. J. Eng. Res. Appl. 2013, 3, 964–970. [Google Scholar]
- Stenfors, L.P.; Granum, P.E. Psychrotolerant Species from the Bacillus cereus Group are not Necessarily Bacillus weihenstephanensis. FEMS Microbiol. Lett. 2001, 197, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Godič Torkar, K.; Seme, K. Antimicrobial Susceptibility, β-Lactamase and Enterotoxin Production in Bacillus cereus Isolates from Clinical and Food Samples. Folia Microbiol. 2009, 54, 233–238. [Google Scholar] [CrossRef]
- Montanhini, M.T.M.; dos Santos Bersot, L. Avaliação do Comportamento Psicrotrófico e Atividade Lipolítica e Proteolítica de Bacillus cereus Isolado de Produtos Lácteos Refrigerados. Acta Sci. Technol. 2013, 35, 163–167. [Google Scholar]
- Fiedoruk, K.; Drewnowska, J.M.; Daniluk, T.; Leszczynska, K.; Iwaniuk, P.; Swiecicka, I. Ribosomal Background of the Bacillus cereus Group Thermotypes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Bideshi, D.K.; Kraszewska, A.; Modzelewska, E.; Swiecicka, I. Natural Isolates of Bacillus thuringiensis Display Genetic and Psychrotrophic Properties Characteristic of Bacillus weihenstephanensis. J. Appl. Microbiol. 2009, 106, 1967–1975. [Google Scholar] [CrossRef]
- Thorsen, L.; Budde, B.B.; Henrichsen, L.; Martinussen, T.; Jakobsen, M. Cereulide Formation by Bacillus Weihenstephanensis and Mesophilic Emetic Bacillus cereus at Temperature Abuse Depends on Pre-Incubation conditions. Int. J. Food Microbiol. 2009, 134, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, M.; Buss Da Silva, N.; Rouzeau-Szynalski, K.; Coisne, L.; Cantergiani, F.; Baranyi, J. Modeling Bacillus cereus Growth and Cereulide Formation in Cereal-, Dairy-, Meat-, Vegetable-Based Food and Culture Medium. Front. Microbiol. 2021, 12, 155. [Google Scholar]
- Finlay, W.J.J.; Logan, N.A.; Sutherland, A.D. Bacillus cereus Produces Most Emetic toxin at Lower Temperatures. Lett. Appl. Microbiol. 2000, 31, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzler, M.; Stollewerk, K.; Rouzeau-szynalski, K.; Blayo, L.; Sulyok, M.; Ehling-Schulz, M. Temperature Exerts Control of Bacillus cereus Emetic Toxin Production on Post-Transcriptional Levels. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marxen, S.; Stark, T.D.; Frenzel, E.; Rütschle, A.; Lücking, G.; Pürstinger, G.; Pohl, E.E.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. Chemodiversity of Cereulide, the Emetic Toxin of Bacillus cereus. Anal. Bioanal. Chem. 2015, 407, 2439–2453. [Google Scholar] [CrossRef]
- European Food Safety Agency (EFSA). Manual for Reporting on Food-Borne Outbreaks in Accordance with Directive 2003/99/EC for Information Deriving from the Year 2016. EFSA Support. Publ. 2017, 14, 1174E. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.C.; Shih, D.Y.C.; Huang, T.P.; Huang, Y.P.; Wang, J.Y.; Pan, T.M. Establishment of a Novel Multiplex PCR Assay and Detection of Toxigenic Strains of the Species in the Bacillus cereus Group. J. Food Prot. 2005, 68, 2123–2130. [Google Scholar] [CrossRef]
- Wehrle, E.; Didier, A.; Moravek, M.; Dietrich, R.; Märtlbauer, E. Detection of Bacillus cereus with Enteropathogenic Potential by Multiplex Real-Time PCR-Based on SYBR Green I. Mol. Cell. Probes 2010, 24, 124–130. [Google Scholar] [CrossRef]
- Fricker, M.; Messelhäußer, U.; Busch, U.; Scherer, S.; Ehling-Schulz, M. Diagnostic Real-Time PCR Assays for the Detection of Emetic Bacillus cereus Strains in Foods and Recent Food-Borne Outbreaks. Appl. Environ. Microbiol. 2007, 73, 1892–1898. [Google Scholar] [CrossRef] [Green Version]
- Rajkovic, A.; Uyttendaele, M.; Debevere, J. Computer Aided Boar Semen Motility Analysis for Cereulide Detection in Different Food Matrices. Int. J. Food. Microl. 2007, 114, 92–99. [Google Scholar] [CrossRef]
- Delbrassinne, L.; Andjelkovic, M.; Rajkovic, A.; Dubois, P.; Nguessan, E.; Mahillon, J.; van Loco, J. Determination of Bacillus cereus Emetic Toxin in Food Products by Means of LC-MS2. Food Anal. Methods 2012, 5, 969–979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).