Application of Wastewater-Based Epidemiology for Tracking Human Exposure to Deoxynivalenol and Enniatins
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Sample Preparation and Extraction Procedure
2.2. Chromatographic Separation and MS Detection
2.3. Validation of the Optimized Method
2.3.1. Linearity
2.3.2. Evaluation of Matrix Effects
2.3.3. Absolute and Relative Method Recoveries
2.3.4. LOD, LOQ and Precision
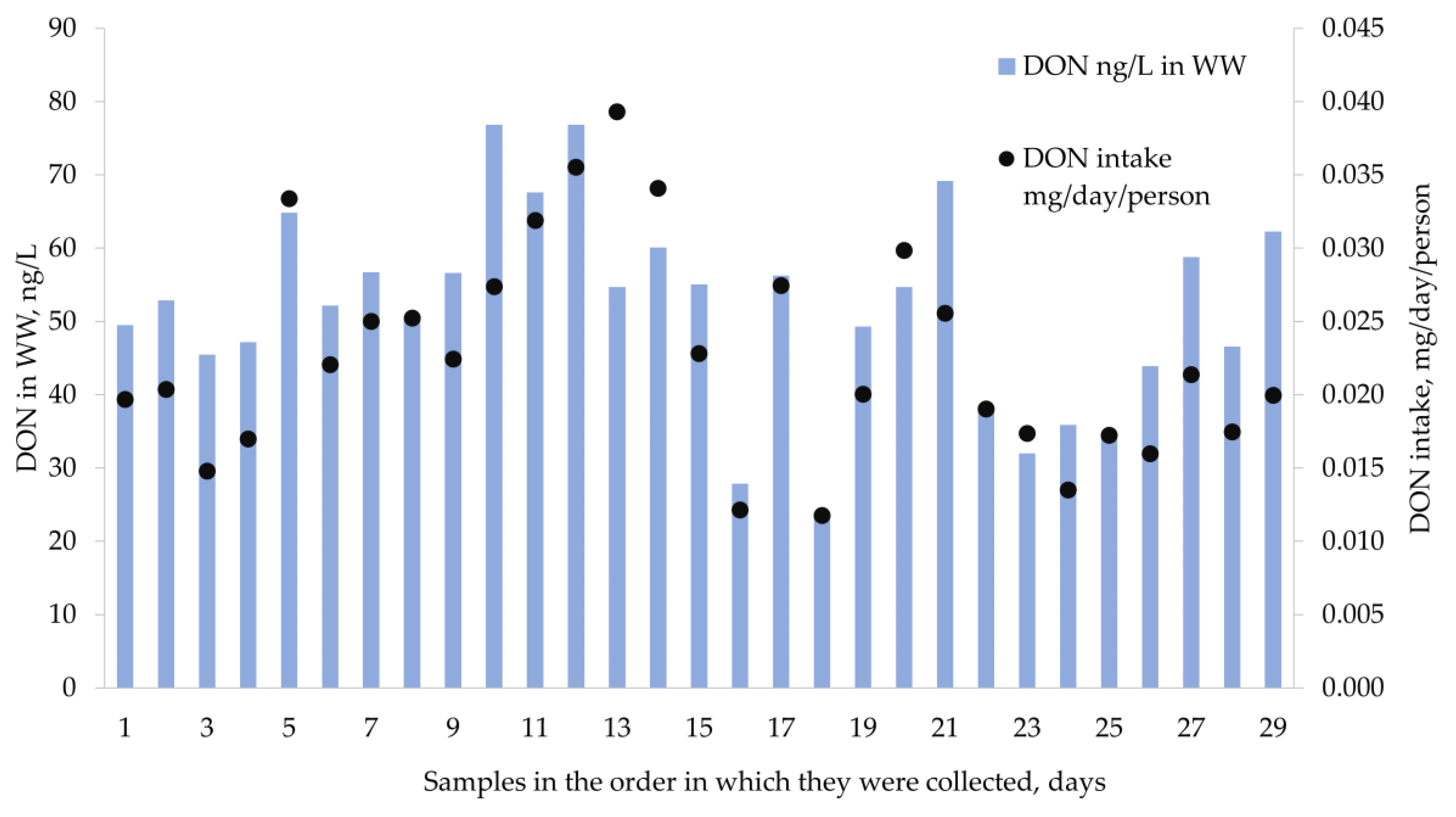
2.4. Occurrence of Mycotoxins in Wastewater
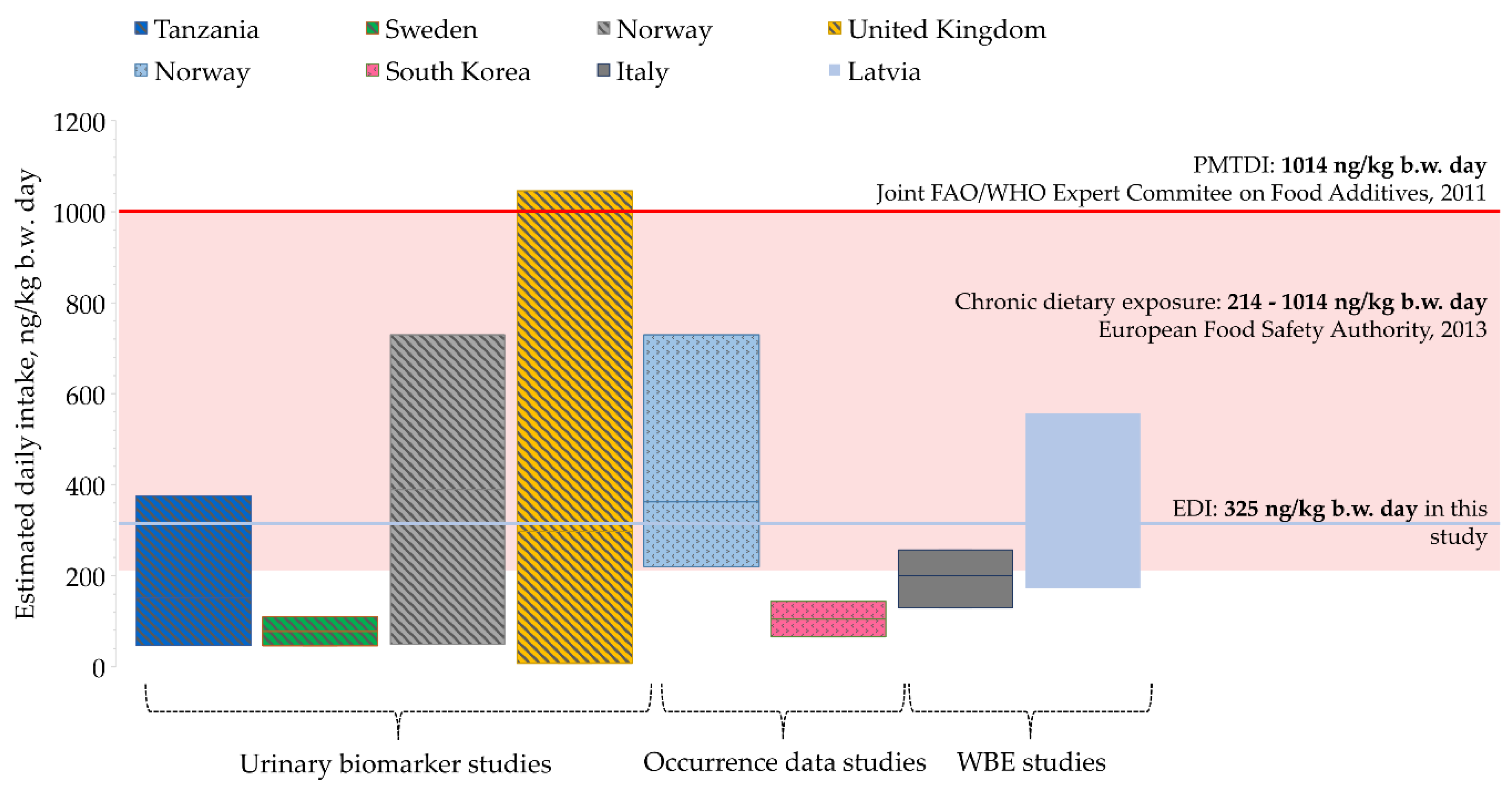
2.5. Wastewater Based Epidemiology for Tracking Human Exposure to Mycotoxins
3. Conclusions
4. Materials and Methods
4.1. Reagents and Analytical Standards
4.2. Wastewater Sampling
4.3. Sample Preparation
4.4. Solid Phase Extraction
4.5. LC-MS/MS Analysis
4.5.1. Analysis of Deoxynivalenol
4.5.2. Analysis of the Mycotoxin Group including Beauvericin and Enniatins
4.6. Method Validation Parameters
4.6.1. Linearity
4.6.2. Matrix Effect
4.6.3. Absolute SPE Recoveries
4.6.4. Absolute and Relative Recoveries of Methods
4.6.5. Method Precision and Detection Limits
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA CONTAM Panel. Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 1–97. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission Regulation (EC): Brussels, Belgium, 2006; pp. 5–24.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) Safety evaluation of certain contaminants in food: Deoxynivalenol (addendum). World Health Organ. 2007, 317–485. [CrossRef]
- EFSA. Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA J. 2014, 12, 1–61. [Google Scholar] [CrossRef]
- Hyun, E.O.; Hyun, J.K.; Tae, Y.C.; Keum, S.O.; Hyang, S.C. Determination of deoxynivalenol in cereal-based foods and estimation of dietary exposure. J. Toxicol. Environ. Health. A 2009, 72, 1424–1430. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Knutsen, H.K.; Sandvik, M.; Brantsæter, A.-L. Urinary deoxynivalenol as a biomarker of exposure in different age, life stage and dietary practice population groups. Environ. Int. 2021, 157, 106804. [Google Scholar] [CrossRef]
- Warensjö Lemming, E.; Montano Montes, A.; Schmidt, J.; Cramer, B.; Humpf, H.U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents—Possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef]
- Turner, P.; White, K.; Burley, V.; Hopton, R.; Rajendram, A.; Fisher, J.; Cade, J.; Wild, C. A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers 2010, 15, 553–562. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Zuccato, E.; Hernández, F.; Castiglioni, S. Wastewater-based epidemiology for tracking human exposure to mycotoxins. J. Hazard. Mater. 2020, 382, 121108. [Google Scholar] [CrossRef]
- Hou, C.; Chu, T.; Chen, M.; Hua, Z.; Xu, P.; Xu, H.; Wang, Y.; Liao, J.; Di, B. Application of multi-parameter population model based on endogenous population biomarkers and flow volume in wastewater epidemiology. Sci. Total Environ. 2021, 759, 143480. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Schenzel, J.; Meyer, M.T.; Phillips, P.J.; Hubbard, L.E.; Scott, T.-M.; Bucheli, T.D. Mycotoxins: Diffuse and point source contributions of natural contaminants of emerging concern to streams. Sci. Total Environ. 2014, 470, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, K.; Waśkiewicz, A.; Świetlik, J.; Bocianowski, J.; Goliński, P. The role of wastewater treatment in reducing pollution of surface waters with zearalenone/Uloga pročišćavanja otpadnih voda u smanjenju onečišćenja površinskih voda zearalenonom. Arch. Ind. Hyg. Toxicol. 2015, 66, 159–164. [Google Scholar] [CrossRef][Green Version]
- Gromadzka, K.; Waśkiewicz, A.; Goliński, P.; Świetlik, J. Occurrence of estrogenic mycotoxin—Zearalenone in aqueous environmental samples with various NOM content. Water Res. 2009, 43, 1051–1059. [Google Scholar] [CrossRef]
- Schenzel, J.; Schwarzenbach, R.P.; Bucheli, T.D. Multi-residue Screening Method To Quantify Mycotoxins in Aqueous Environmental Samples. J. Agric. Food Chem. 2010, 58, 11207–11217. [Google Scholar] [CrossRef]
- Wettstein, F.E.; Bucheli, T.D. Poor elimination rates in waste water treatment plants lead to continuous emission of deoxynivalenol into the aquatic environment. Water Res. 2010, 44, 4137–4142. [Google Scholar] [CrossRef]
- Schenzel, J.; Forrer, H.R.; Vogelgsang, S.; Hungerbühler, K.; Bucheli, T.D. Mycotoxins in the environment: I. Production and emission from an agricultural test field. Environ. Sci. Technol. 2012, 46, 13067–13075. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects. Toxins 2017, 9, 246. [Google Scholar] [CrossRef]
- Mata, A.T.; Ferreira, J.P.; Oliveira, B.R.; Batoréu, M.C.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Izzo, L.; Gaspari, A.; Graziani, G.; Mañes, J.; Ritieni, A. Urinary levels of enniatin B and its phase I metabolites: First human pilot biomonitoring study. Food Chem. Toxicol. 2018, 118, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Narváez, A.; Izzo, L.; Gaspari, A.; Graziani, G.; Ritieni, A. Biomonitoring of Enniatin B1 and Its Phase I Metabolites in Human Urine: First Large-Scale Study. Toxins 2020, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.K.; O’Brien, J.W.; Banks, A.P.W.; Jiang, G.; Gao, J.; Choi, P.M.; Yuan, Z.; Mueller, J.F. Evaluating the in-sewer stability of three potential population biomarkers for application in wastewater-based epidemiology. Sci. Total Environ. 2019, 671, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kostakis, C.; Gerber, J.P.; Tscharke, B.J.; Irvine, R.J.; White, J.M. Towards finding a population biomarker for wastewater epidemiology studies. Sci. Total Environ. 2014, 487, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.; Walsh, G.; Tokmakejian, S.; Van Uum, S.H.M. Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin A following over-the-counter 5-hydroxytryptophan intake. Can. J. Gastroenterol. 2008, 22, 49–53. [Google Scholar] [CrossRef][Green Version]
- University of Rochester Medical Center. 5-Hydroxyindoleacetic Acid (Urine)—Health Encyclopedia. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=5_hiaa (accessed on 29 September 2021).
- Mayo Clinic Laboratories. HIAA—Clinical: 5-Hydroxyindoleacetic Acid, 24 Hour, Urine. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/9248 (accessed on 29 September 2021).
- Gracia-Lor, E.; Zuccato, E.; Castiglioni, S. Refining correction factors for back-calculation of illicit drug use. Sci. Total Environ. 2016, 573, 1648–1659. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef]
- Mengelers, M.; Zeilmaker, M.; Vidal, A.; De Boevre, M.; De Saeger, S.; Hoogenveen, R. Biomonitoring of Deoxynivalenol and Deoxynivalenol-3-glucoside in Human Volunteers: Renal Excretion Profiles. Toxins 2019, 11, 466. [Google Scholar] [CrossRef]
- McCall, A.-K.; Palmitessa, R.; Blumensaat, F.; Morgenroth, E.; Ort, C. Modeling in-sewer transformations at catchment scale—Implications on drug consumption estimates in wastewater-based epidemiology. Water Res. 2017, 122, 655–668. [Google Scholar] [CrossRef]
- EFSA. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 1–32. [Google Scholar] [CrossRef]
- Srey, C.; Kimanya, M.E.; Routledge, M.N.; Shirima, C.P.; Gong, Y.Y. Deoxynivalenol exposure assessment in young children in Tanzania. Mol. Nutr. Food Res. 2014, 58, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Ikkere, L.E.; Jansons, M.; Perkons, I.; Sukajeva, V.; Bartkevics, V. Two-dimensional liquid chromatography—Mass spectrometry as an effective tool for assessing a wide range of pharmaceuticals and biomarkers in wastewater-based epidemiology studies. J. Pharm. Biomed. Anal. 2021, 205, 114295. [Google Scholar] [CrossRef] [PubMed]
| Frequency of Detection (>LOD) | Average, ng/L | Range, ng/L | Median, ng/L | LOD, ng/L | LOQ, ng/L | Precision, % | |
|---|---|---|---|---|---|---|---|
| DON | 100% | 51.7 | 23.2–76.9 | 52.9 | 1.9 | 6.4 | 6 |
| ENNA | 90% | 3.0 * | LOQ–16.7 | 2.0 * | 0.12 | 0.40 | 3 |
| ENNA1 | 86% | 4.2 * | LOQ–27.7 | 2.5 * | 0.14 | 0.47 | 4 |
| ENNB | 100% | 4.6 | 0.6–9.9 | 4.1 | 0.04 | 0.15 | 1.3 |
| ENNB1 | 100% | 3.9 | 1.4–10.1 | 3.4 | 0.13 | 0.43 | 3 |
| BEA | 14% | <LOQ | <LOQ | <LOQ | 0.04 | 0.13 | 7 |
| ENNA | ENNA1 | |||||
| Assumed Excretion Factors | 5% | 25% | 50% | 5% | 25% | 50% |
| Average | 0.25 | 0.050 | 0.025 | 0.36 | 0.072 | 0.036 |
| Max | 1.18 | 0.24 | 0.12 | 1.95 | 0.39 | 0.19 |
| Min | 0.012 | 0.0024 | 0.0013 | 0.017 | 0.0034 | 0.0017 |
| Median | 0.15 | 0.029 | 0.015 | 0.21 | 0.042 | 0.021 |
| ENNB | ENNB1 | |||||
| Average | 0.39 | 0.079 | 0.039 | 0.35 | 0.069 | 0.035 |
| Max | 0.84 | 0.17 | 0.084 | 1.05 | 0.21 | 0.105 |
| Min | 0.048 | 0.0097 | 0.0049 | 0.109 | 0.022 | 0.0109 |
| Median | 0.34 | 0.067 | 0.034 | 0.30 | 0.060 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzina, Z.; Pavlenko, R.; Jansons, M.; Bartkiene, E.; Neilands, R.; Pugajeva, I.; Bartkevics, V. Application of Wastewater-Based Epidemiology for Tracking Human Exposure to Deoxynivalenol and Enniatins. Toxins 2022, 14, 91. https://doi.org/10.3390/toxins14020091
Berzina Z, Pavlenko R, Jansons M, Bartkiene E, Neilands R, Pugajeva I, Bartkevics V. Application of Wastewater-Based Epidemiology for Tracking Human Exposure to Deoxynivalenol and Enniatins. Toxins. 2022; 14(2):91. https://doi.org/10.3390/toxins14020091
Chicago/Turabian StyleBerzina, Zane, Romans Pavlenko, Martins Jansons, Elena Bartkiene, Romans Neilands, Iveta Pugajeva, and Vadims Bartkevics. 2022. "Application of Wastewater-Based Epidemiology for Tracking Human Exposure to Deoxynivalenol and Enniatins" Toxins 14, no. 2: 91. https://doi.org/10.3390/toxins14020091
APA StyleBerzina, Z., Pavlenko, R., Jansons, M., Bartkiene, E., Neilands, R., Pugajeva, I., & Bartkevics, V. (2022). Application of Wastewater-Based Epidemiology for Tracking Human Exposure to Deoxynivalenol and Enniatins. Toxins, 14(2), 91. https://doi.org/10.3390/toxins14020091








