Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus
Abstract
:1. Introduction
2. Results
2.1. Antagonism of Trichoderma Isolates against A. flavus
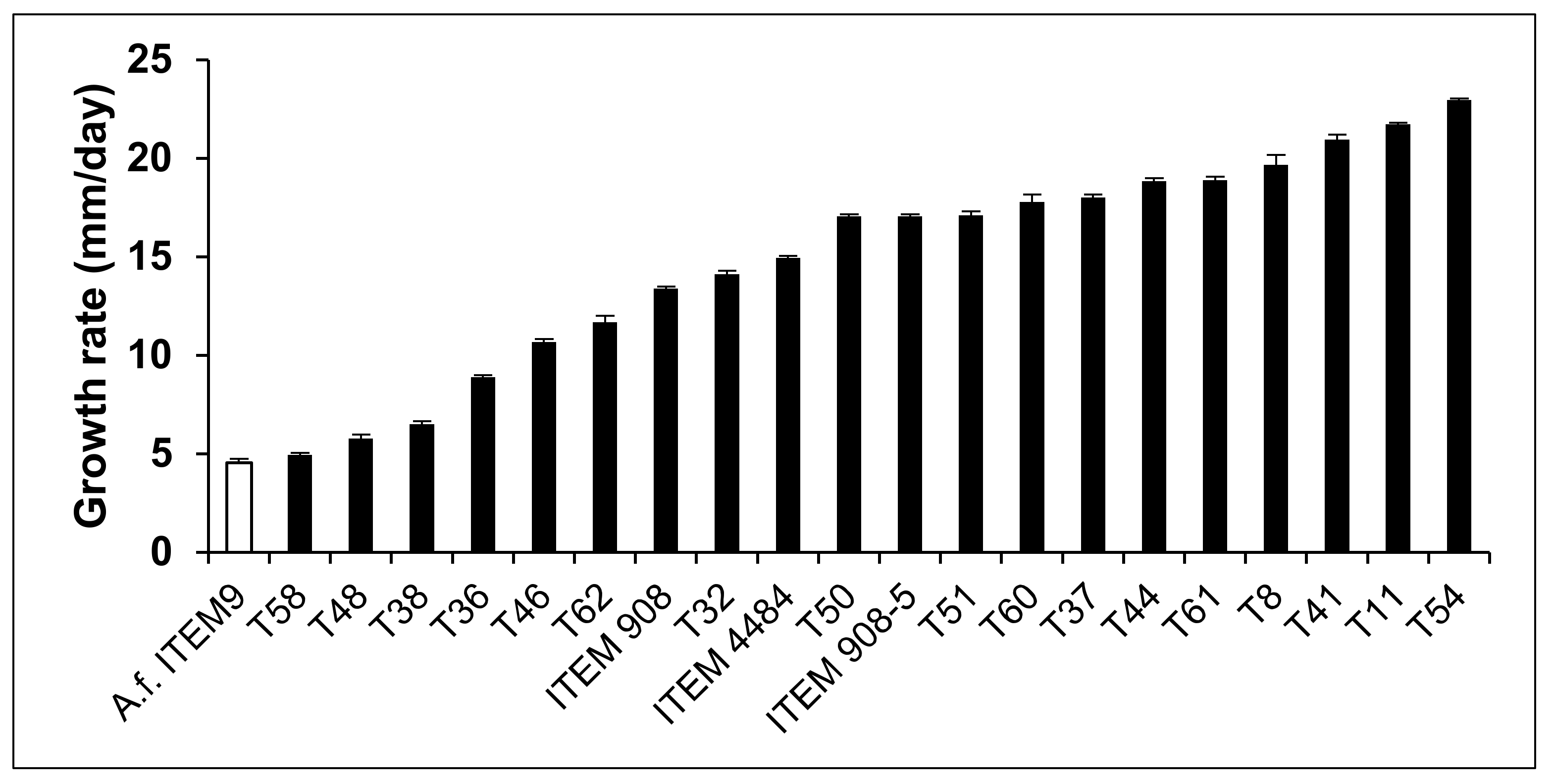
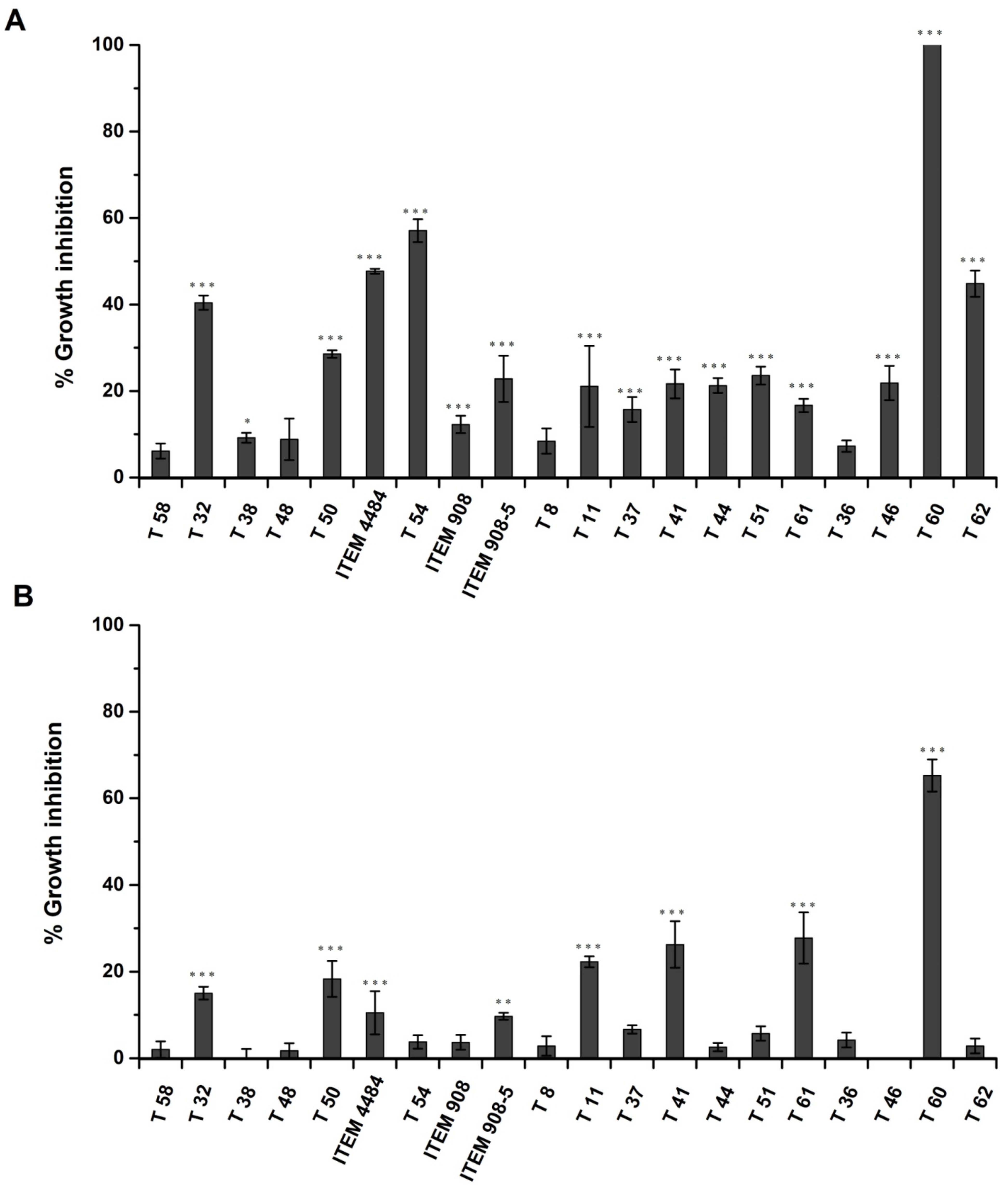
2.2. Inhibitory Effect of Trichoderma Metabolites on Growth of A. flavus
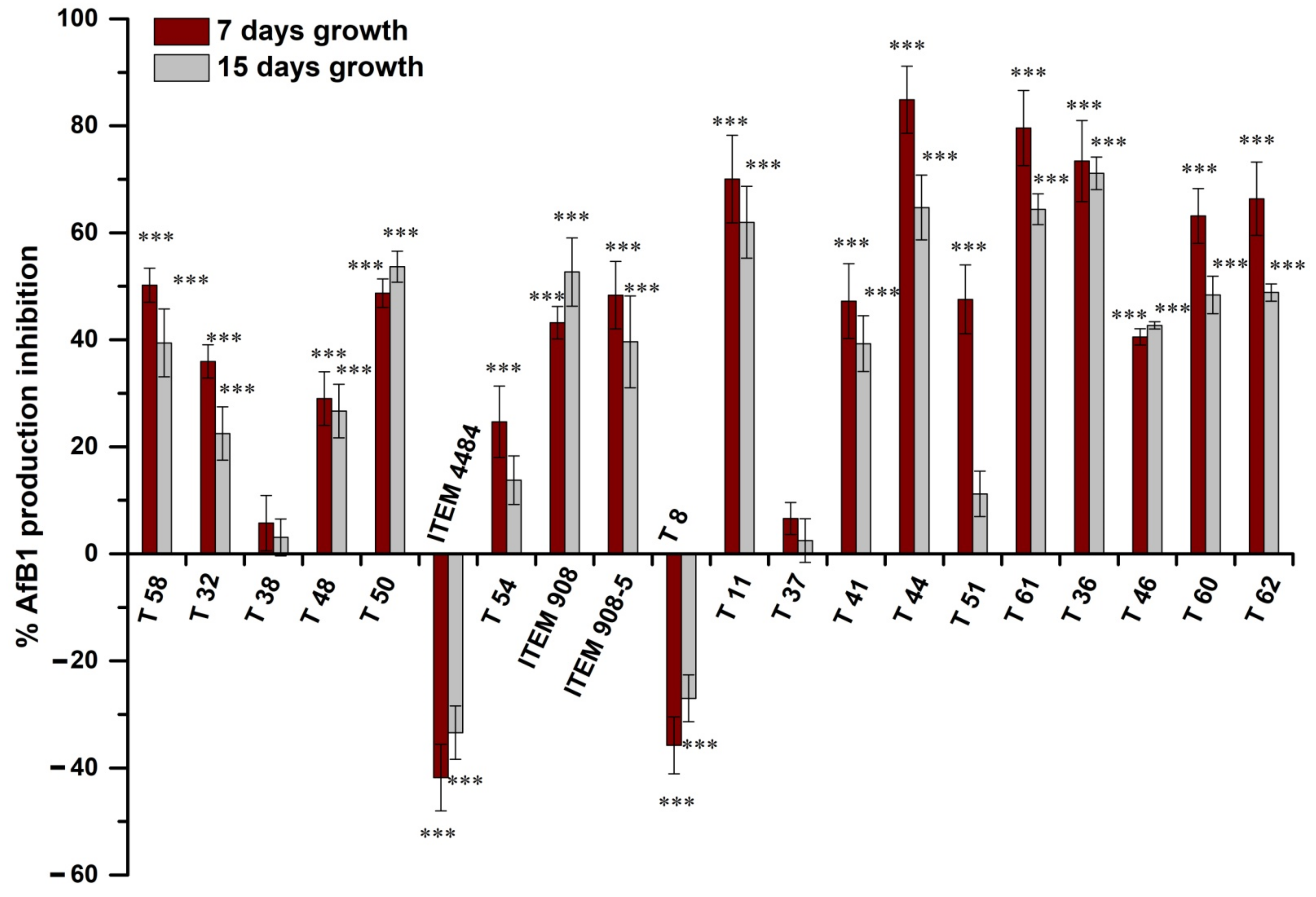
2.3. Inhibitory Effect of Trichoderma Metabolites on AfB1 Production by A. flavus
2.4. Analysis of Aflatoxin Biosynthesis Gene Expression in Relation to the Control of Trichoderma
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains
5.2. Antagonism of Trichoderma spp. against A. flavus
5.3. Effect of Non-Volatile Metabolites of Trichoderma spp. on A. flavus Growth
5.4. Determination of AfB1 by UPLC
5.5. Effect of Trichoderma Metabolites on Aflatoxin Production by A. flavus
5.6. Effect of Metabolites of Trichoderma on the Expression of Aflatoxin Biosynthesis Genes
5.6.1. Strains Culture and Sampling of Mycelia
5.6.2. RNA Extraction and cDNA Synthesis
5.6.3. Expression Analysis of Aflatoxin Biosynthesis Gene by Quantitative Reverse Transcriptase PCR (RT-qPCR)
5.7. Chemicals and Reagents
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altomare, C.; Logrieco, A.F.; Gallo, A. Mycotoxins and Mycotoxigenic Fungi: Risk and Management. A Challenge for Future Global Food Safety and Security. Encycl. Mycol. 2021, 1, 64–93. [Google Scholar]
- Bennett, J.W.; Klich, M. Clinical Microbiology Reviews. Mycotoxins 2003, 16, 497. [Google Scholar]
- Craig, A.W. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: International Agency for Research on Cancer. Food Chem. Toxicol. 1987, 25, 717. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556.
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Cotty, P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.K. Aflatoxin and fumonisin contamination of commercial corn (Zea mays) hybrids in Mississippi. J. Agric. Food Chem. 2002, 50, 5246–5254. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Locke, M.A. Spatial variability of Aspergillus flavus soil populations under different crops and corn grain colonization and aflatoxins. Can. J. Bot. 2004, 82, 1768–1775. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.M.; Barros, G.G.; Palacios, S.A.; Chulze, S.N.; Battilani, P. Review on pre- and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 2014, 62, 11–19. [Google Scholar] [CrossRef]
- Horn, B.W.; Ramirez-Prado, J.H.; Carbone, I. The sexual state of Aspergillus parasiticus. Mycologia 2017, 101, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egmond, H.P.V.; Jonker, M.A. Worldwide Regulations on Aflatoxins—The Situation in 2002. Toxin Rev. 2008, 119, 92–102. [Google Scholar] [CrossRef]
- Ministry of Health of the People’s Republic of China. GB2761-2011 National Food Safety Standard in Food. Limit of Mycotoxins; China Standard Press: Beijing, China, 2011.
- EU Commision. Commission Regulation (EC). No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- EU Commission. The Rapid Alert System for Food and Feed Annual Report. Food Saf. Brief. 2014, 16, 9–10. [Google Scholar]
- Hka, C.; Xl, B.; Jing, J.A.; Fx, A. Current status of major mycotoxins contamination in food and feed in Africa. Food Control 2019, 110, 69–75. [Google Scholar]
- Cotty, P.J.; Probst, C.; Jaime-Garcia, R. Etiology and Management of Aflatoxin Contamination. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Leslie, F., Ranajit, B., Eds.; CABI: Wallingford, UK, 2008; pp. 287–299. [Google Scholar]
- Payne, G.A. Process of contamination by aflatoxin producing fungi and their impacts on crops. In Mycotoxins in Agriculture and Food Safety; Sinha, K.K., Bhatnagar, D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Cary, J.W.; Cleveland, T.E. Prevention of preharvest aflatoxin contamination through genetic engineering of crops. Mycotoxin Res. 2006, 22, 118–124. [Google Scholar] [CrossRef]
- Cotty, P.J. Biocompetitive Exclusion of Toxigenic Fungi. In The Mycotoxin Factbook; Barug, D., Bhatnagar, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 179–197. [Google Scholar]
- Ehrlich, K.C.; Cotty, P.J. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase. Appl. Microbiol. Biotechnol. 2004, 65, 473–478. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol. Sci. Technol. 2007, 16, 437–449. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Moore, G.G.; Mellon, J.E.; Bhatnagar, D. Challenges facing the biological control strategy for eliminating aflatoxin contamination. World Mycotoxin J. 2015, 8, 225–233. [Google Scholar] [CrossRef]
- Pitt, J.I. The pros and cons of using biocontrol by competitive exclusion as a means for reducing aflatoxin in maize in Africa. World Mycotoxin J. 2019, 12, 103–112. [Google Scholar] [CrossRef]
- Wilson, B.J. Toxins Other than Aflatoxins Produced by Aspergillus flavus. Bacteriol. Rev. 1966, 30, 478–484. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Resnik, S.L.; González, H.H.L.; Pacin, A.M.; Viora, M.; Caballero, G.M.; Gros, E.G. Cyclopiazonic acid and aflatoxins production by Aspergillus flavus isolated from Argentinian corn. Mycotoxin Res. 1996, 12, 61–66. [Google Scholar] [CrossRef]
- Pinto, V.E.; Patriarca, A.; Locani, O.; Vaamonde, G. Natural co-occurrence of aflatoxin and cyclopiazonic acid in peanuts grown in Argentina. Food Addit. Contam. 2001, 18, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Siahmoshteh, F.; Hamidi-Esfahani, Z.; Spadaro, D.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 2018, 89, 300–307. [Google Scholar] [CrossRef]
- Kong, Q.; Chi, C.; Yu, J.; Shan, S.; Li, Q.; Li, Q.; Guan, B.; Nierman, W.C.; Bennett, J.W. The inhibitory effect of Bacillus megaterium on aflatoxin and cyclopiazonic acid biosynthetic pathway gene expression in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2014, 98, 5161–5172. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L.; Abbas, H.K. Isolation of maize soil and rhizosphere bacteria with antagonistic activity against Aspergillus flavus and Fusarium verticillioides. J. Food Prot. 2007, 70, 1615–1621. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Chen, Z.Y.; Liu, H.; Li, P. Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE 2017, 12, e0178810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlberg, S.; Joutsjoki, V.; Laurikkala, S.; Varmanen, P.; Korhonen, H. Aspergillus flavus growth inhibition by Lactobacillus strains isolated from traditional fermented Kenyan milk and maize products. Arch. Microbiol. 2017, 199, 457–464. [Google Scholar] [CrossRef]
- Sangmanee, P.; Hongpattarakere, T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control. 2014, 40, 224–233. [Google Scholar] [CrossRef]
- Qaiser, S.; Ang, L.; Jing, Z.; Wu, M.; Li, G.; Tom, H.; Long, Y. Biocontrol of Aspergillus flavus on Peanut Kernels Using Streptomyces yanglinensis 3–10. Front. Microbiol. 2018, 9, 1049. [Google Scholar]
- Verheecke, C.; Liboz, T.; Darriet, M.; Sabaou, N.; Mathieu, F. In vitro interaction of actinomycetes isolates with Aspergillus flavus: Impact on aflatoxins B1 and B2 production. Lett. Appl. Microbiol. 2014, 58, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, Y.; Magan, N. Impact of a Streptomyces (AS1) strain and its metabolites on control of Aspergillus flavus and aflatoxin B1 contamination in vitro and in stored peanuts. Biocontrol. Sci. Technol. 2011, 21, 1437–1455. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Armando, M.R.; Dogi, C.A.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Saccharomyces cerevisiae strains and the reduction of Aspergillus parasiticus growth and aflatoxin B1 production at different interacting environmental conditions, in vitro. Food. Addit. Contam. Part A 2012, 29, 1443–1449. [Google Scholar] [CrossRef]
- Penna, M.L.; Etcheverry, M. Impact on growth and aflatoxin B1 accumulation by Kluyveromyces isolates at different water activity conditions. Mycopathologia 2006, 162, 347–353. [Google Scholar] [CrossRef]
- Hua, S.S.T.; Baker, J.L.; Flores-Espiritu, M. Interactions of saprophytic yeasts with a nor mutant of Aspergillus flavus. Appl. Environ. Microbiol. 1999, 65, 2738–2740. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species--opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Cardoza, R.E.; Rubio, M.B.; Gutiérrez, S.; Monte, E. Secondary metabolism and antimicrobial metabolites of Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–137. [Google Scholar]
- Wicklow, D.T. Interference competition. In The Fungal Community-Its Organization and Role in the Ecosystem; Carroll, G.C., Wicklow, D.T., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 265–274. [Google Scholar]
- Whipps, J.M. Effect of media on growth and interactions between a range of soil-borne glasshouse pathogens and antagonistic fungi. New Phytol. 1987, 107, 127–142. [Google Scholar] [CrossRef]
- Mostafa, A.A. Antagonistic Activities of Some Fungal Strains against the Toxigenic Aspergillus flavus Isolate and its Aflatoxins Productivity. J. Pure Appl. Microbiol. 2013, 7, 169–178. [Google Scholar]
- Calistru, C.; McLean, M.; Berjak, P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species—A study of the production of extracellular metabolites by Trichoderma species. Mycopathologia 1997, 137, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Wang, L.; Liu, X.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Liu, Y. Aflatoxin B1 inhibition in Aspergillus flavus by Aspergillus niger through down-regulating expression of major biosynthetic genes and AFB1 degradation by atoxigenic A. flavus. Int. J. Food Microbiol. 2017, 256, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J.; Bayman, P. Competitive exclusion of a toxigenic strain of Aspergillus flavus by an atoxigenic strain. Phytopathology 1993, 83, 1283–1287. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ‘omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [Green Version]
- Gajera, H.P.; Savaliya, D.D.; Patel, S.V.; Golakiya, B.A. Trichoderma viride induces pathogenesis related defense response against rot pathogen infection in groundnut (Arachis hypogaea L.). Infect. Genet. Evol. 2015, 34, 314–325. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Trichoderma harzianum Enhances Antioxidant Defense of Tomato Seedlings and Resistance to Water Deficit. Mol. Plant-Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Viveros-Bremauntz, F.; del-Val, E.; Macías-Rodríguez, L.; López-Carmona, D.A.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Alterations of foliar arthropod communities in a maize agroecosystem induced by the root-associated fungus Trichoderma harzianum. J. Pest. Sci. 2021, 94, 363–374. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef] [Green Version]
- Anjaiah, V.; Thakur, R.P.; Koedam, N. Evaluation of bacteria and Trichoderma for biocontrol of pre-harvest seed infection by Aspergillus flavus in groundnut. Biocontrol. Sci. Technol. 2006, 16, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Sivparsad, B.J.; Laing, M.D. Pre-harvest silk treatment with Trichoderma harzianum reduces aflatoxin contamination in sweetcorn. J. Plant Dis. Prot. 2016, 123, 285–293. [Google Scholar] [CrossRef]
- Kifle, M.H.; Yobo, K.S.; Laing, M.D. Biocontol of Aspergillus flavus in groundnut using Trichoderma harzanium stain kd. J. Plant Dis. Prot. 2017, 124, 51–56. [Google Scholar] [CrossRef]
- Dania, V.O.; Eze, S.E. Using Trichoderma Species in Combination with Cattle Dung as Soil Amendment Improves Yield and Reduces Pre-Harvest Aflatoxin Contamination in Groundnut. AGRIVITA J. Agric. Sci. 2020, 42, 449–461. [Google Scholar] [CrossRef]
- Debnath, S.; Kabir, S.E. Antagonistic Behavior of Trichoderma harzianum against Aspergillus flavus and A. niger. Indian J. Fundam. Appl. Life Sci. 2017, 7, 1–4. [Google Scholar]
- Gachomo, E.W.; Kotchoni, S.O. The Use of Trichoderma harzianum and T. viride as Potential Biocontrol Agents against Peanut Microflora and Their Effectiveness in Reducing Aflatoxin Contamination of Infected Kernels. Biotechnology 2008, 7, 439–447. [Google Scholar] [CrossRef]
- Schroeder, H.W. Effect of corn steep liquor on mycelial growth and aflatoxin production in Aspergillus parasiticius. Appl. Microbiol. 1966, 14, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mohawed, S.M.; Bhatnagar, D.; Cleveland, T.E. Substrate-induced lipase gene expression and aflatoxin production in Aspergillus parasiticus and Aspergillus flavus. J. Appl. Microbiol. 2003, 95, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; White, D.G.; Gary, A.P. Corn Seed Proteins Inhibitory to Aspergillus flavus and Aflatoxin Biosynthesis. Phytopathology 1997, 87, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caceres, I.; Khoury, A.A.; Khoury, R.E.; Lorber, S.; Oswald, I.P.; Khoury, A.E.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, A.; Solfrizzo, M.; Epifani, F.; Panzarini, G.; Perrone, G. Effect of temperature and water activity on gene expression and aflatoxin biosynthesis in Aspergillus flavus on almond medium. Int. J. Food Microbiol. 2016, 217, 162–169. [Google Scholar] [CrossRef]
- Chang, P.K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genom. 2003, 268, 711–719. [Google Scholar] [CrossRef]
- Price, M.S.; Yu, J.; Nierman, W.C.; Kim, H.; Pritchard, B.; Jacobus, C.A.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol. Lett. 2006, 255, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Caceres, I.; Snini, S.P.; Puel, O.; Mathieu, F. Streptomyces Roseolus, a promising biocontrol agent against Aspergillus flavus, the main aflatoxin B1 producer. Toxins 2018, 10, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef] [Green Version]
- Khonga, E.B.; Kaundat, C.C.; Hillocks, R.J. Biocontrol of Sclerotium rolfsii Sacc. in peanuts (Arachis hypogaea L.) by Trichoderma harzianum Rifai in Malawi. Malawi J. Sci. Technol. 1998, 4, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Ganuza, M.; Pastor, N.; Erazo, J.; Andrés, J.; Reynoso, M.M.; Rovera, M.; Torres, A.M. Efficacy of the biocontrol agent Trichoderma harzianum ITEM 3636 against peanut smut, an emergent disease caused by Thecaphora frezii. Eur. J. Plant. Pathol. 2018, 151, 257–262. [Google Scholar] [CrossRef]
- Diener, U.L.; Cole, R.J.; Sanders, T.H.; Payne, G.A.; Lee, L.S.; Klich, M.A. Epidemiology of Aflatoxin Formation by Aspergillus flavus. Ann. Rev. Phytopathol. 1987, 25, 249–270. [Google Scholar] [CrossRef]
- Gams, W.; Bissett, J. Morphology and identification of Trichoderma. In Trichoderma and Gliocladium; Kubicek, C.P., Harman, G.E., Eds.; CRC Press: London, UK, 1998; Volume 1, pp. 3–34. [Google Scholar]
- Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Altomare, C. Genomic characterization of Trichoderma atrobrunneum (T. harzianum species complex) ITEM 908: Insight into the genetic endowment of a multi-target biocontrol strain. BMC Genom. 2018, 19, 662. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [Green Version]
- Marzano, M.; Gallo, A.; Altomare, C. Improvement of biocontrol efficacy of Trichoderma harzianum vs. Fusarium oxysporum f. sp. lycopersici through UV-induced tolerance to fusaric acid. Biol. Control 2013, 67, 397–408. [Google Scholar] [CrossRef]
- Altomare, C.; Perrone, G.; Zonno, M.C.; Evidente, A.; Pengue, R.; Franti, F.; Polonelli, L. Biological characterization of fusapyrone and deoxyfusapyrone, two bioactive secondary metabolites of Fusarium semitectum. J. Nat. Prod. 2000, 63, 1131–1135. [Google Scholar] [CrossRef]
- Fani, S.R.; Moradi, M.; Probst, C.; Zamanizadeh, H.R.; Mirabolfathy, M.; Haidukowski, M.; Logrieco, A.F. A critical evaluation of cultural methods for the identification of atoxigenic Aspergillus flavus isolates for aflatoxin mitigation in pistachio orchards of Iran. Eur. J. Plant Pathol. 2014, 140, 631–642. [Google Scholar] [CrossRef]
- Branà, M.T.; Cimmarusti, M.T.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Bioremediation of aflatoxin B1-contaminated maize by king oyster mushroom (Pleurotus eryngii). PLoS ONE 2017, 12, e0182574. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene 428 expression data using Real-Time Quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Antagonistic Species/Strain | %IDC (a) | p Value (b) | Interaction Type (c) |
|---|---|---|---|
| Trichodermaasperellum | |||
| T58 | 35.6 ± 3.45 | ** | 4 |
| Trichodermaatroviride | |||
| T32 | 2.88 ± 0.98 | n.s. | 1 |
| T38 | 20.0 ± 3.18 | * | 4 |
| T48 | 42.3 ± 3.21 | ** | 4 |
| T50 | 0.00 ± 0.00 | n.s. | 1 |
| Trichoderma citrinoviride | |||
| ITEM 4484 | 4.45 ± 1.93 | n.s. | 3 |
| T54 | 1.90 ± 1.65 | n.s. | 3 |
| Trichodermaharzianum species complex (incl. T. atrobrunneum) | |||
| ITEM 908 | 12.5 ± 2.43 | ** | 1 |
| ITEM 908-5 | 5.75 ± 2.00 | n.s. | 3 |
| T8 | 5.24 ± 1.75 | n.s. | 4 |
| T11 | 0.00 ± 0.00 | n.s. | 3 |
| T37 | 0.00 ± 0.00 | n.s. | 3 |
| T41 | 0.00 ± 0.00 | n.s. | 3 |
| T44 | 0.00 ± 0.00 | n.s. | 3 |
| T51 | 5.24 ± 1.75 | n.s. | 3 |
| T61 | 1.15 ± 1.20 | n.s. | 1 |
| Trichoderma inhamatum | |||
| T36 | 37.9 ± 0.01 | ** | 4 |
| Trichodermaparceramosus | |||
| T46 | 21.2 ± 0.85 | ** | 4 |
| Trichodermapolysporum | |||
| T60 | 2.30 ± 1.99 | n.s. | 1 |
| Trichodermaviride | |||
| T62 | 3.70 ± 1.60 | n.s. | 1 |
| Species/Strain (a) | Geographical Origin | Source |
|---|---|---|
| Trichoderma asperrellum | ||
| T58 | Not known | Not known |
| Trichoderma atroviride | ||
| T32 | Not known | Not known |
| T38 | Not known | Not known |
| T48 | Italy | Soil |
| T50 | USA | Corn kernel |
| Trichoderma citrinoviride | ||
| ITEM 4484 | Austria | Forest soil |
| T54 | Not known | Maize |
| Trichoderma harzianum species complex (incl. T. atrobrunneum) | ||
| ITEM 908 (b) | Italy | Olive |
| ITEM 908-5 | - | UV-mutant of ITEM 908 |
| T8 | Italy | Mushroom substrate |
| T11 | Italy | Corn kernel |
| T37 | Italy | Seedling soil mix |
| T41 | Borneo, Asia | Soil |
| T44 | Italy | Mushroom substrate |
| T51 | USA | Corn kernel |
| T61 | Not known | Not known |
| Trichoderma inhamatum | ||
| T36 | Not known | Not known |
| Trichoderma parceramosus | ||
| T46 | Italy | Mushroom substrate |
| Trichoderma polysporum | ||
| T60 | Italy | Chestnut soil |
| Trichoderma viride | ||
| T62 | Italy | Eggplant leaf |
| Gene | Primer Code | Concentration | Sequence (5′–3′) | Fragment Length |
|---|---|---|---|---|
| β-tubulin ID 64852080 | AFtub_for | 100 nM | GGTCGTTACCTCACCTGCTCT | 79 bp |
| AFtub_rev | GGATGTTGCGCATCTGGT | |||
| aflR ID 64848036 | aflR_for | 100 nM | CGGCACAGCTTGTTCTGAGT | 88 bp |
| aflR_rev | GCATCGTCTCCACCTTCTTG | |||
| aflS ID 64848035 | aflS_for | 150 nM | CTGGCAAAACTTGGGAATGG | 103 bp |
| aflS_rev | CACGAGGAAACGGAGTGATG | |||
| aflA ID 64848038 | aflA_for | 250 nM | CATGCTGTTAACCCCCGACT | 111 bp |
| aflA_rev | AATTGGGCTAGGAAACCGGG | |||
| aflD ID 64848039 | aflD_for | 100 nM | GCGCAAGTTCCACTTTGAGA | 84 bp |
| aflD_rev | CCTTGGTCGCCCATATCAGT | |||
| aflO ID 64848026 | aflO_for | 100 nM | GTGCGGTGGTGCAACTATTC | 71 bp |
| aflO_rev | TCTCTCGGCCAGGAAGTCA | |||
| aflQ ID 64848029 | aflQ_for | 250 nM | GCACCAACAATTCGGCTCTG | 134 bp |
| aflQ_rev | TGTGGAAGGGTGGAAGATGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus. Toxins 2022, 14, 86. https://doi.org/10.3390/toxins14020086
Ren X, Branà MT, Haidukowski M, Gallo A, Zhang Q, Logrieco AF, Li P, Zhao S, Altomare C. Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus. Toxins. 2022; 14(2):86. https://doi.org/10.3390/toxins14020086
Chicago/Turabian StyleRen, Xianfeng, Maria Teresa Branà, Miriam Haidukowski, Antonia Gallo, Qi Zhang, Antonio F. Logrieco, Peiwu Li, Shancang Zhao, and Claudio Altomare. 2022. "Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus" Toxins 14, no. 2: 86. https://doi.org/10.3390/toxins14020086
APA StyleRen, X., Branà, M. T., Haidukowski, M., Gallo, A., Zhang, Q., Logrieco, A. F., Li, P., Zhao, S., & Altomare, C. (2022). Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus. Toxins, 14(2), 86. https://doi.org/10.3390/toxins14020086









