Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells
Abstract
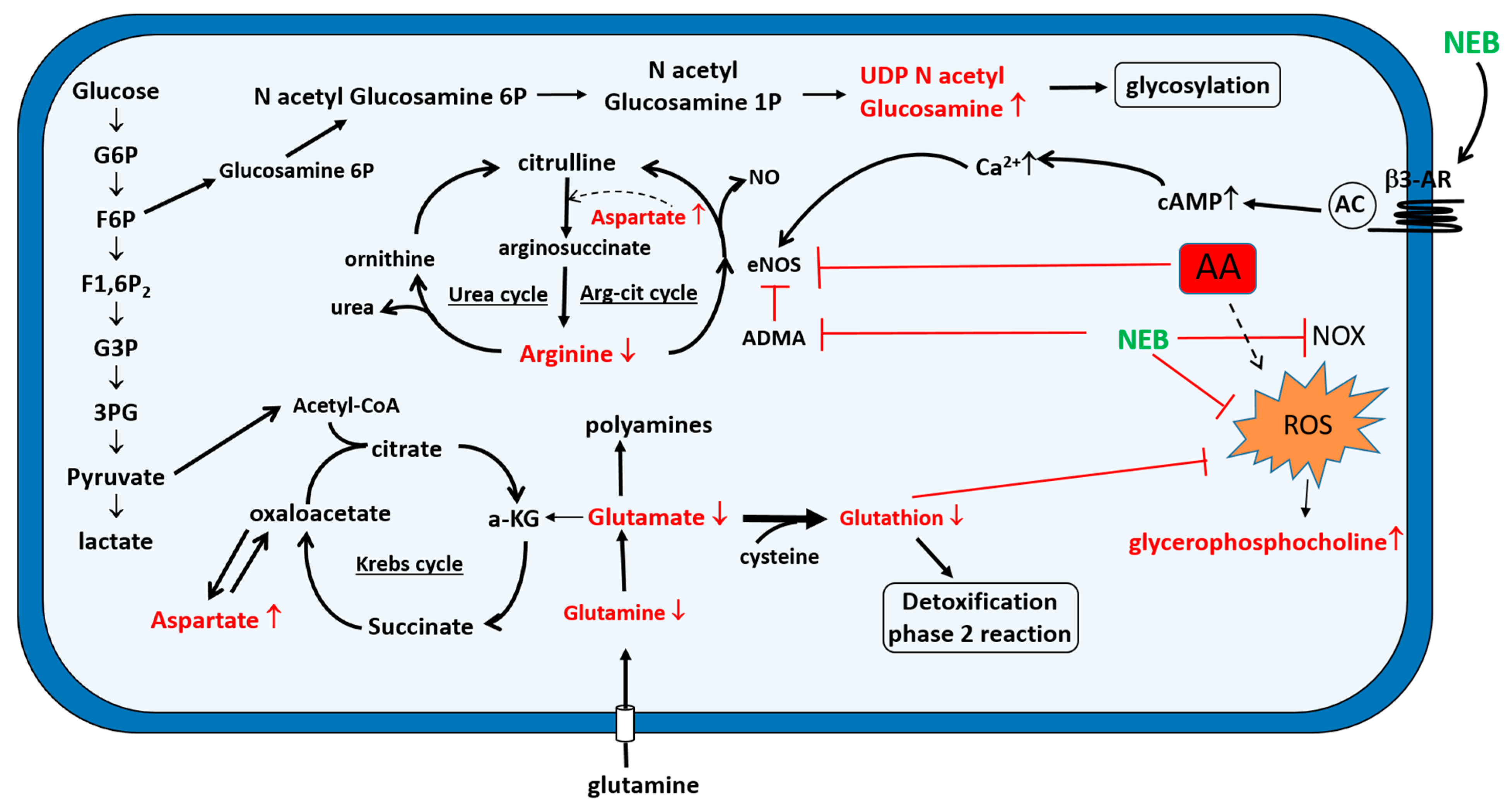
:1. Introduction
2. Results
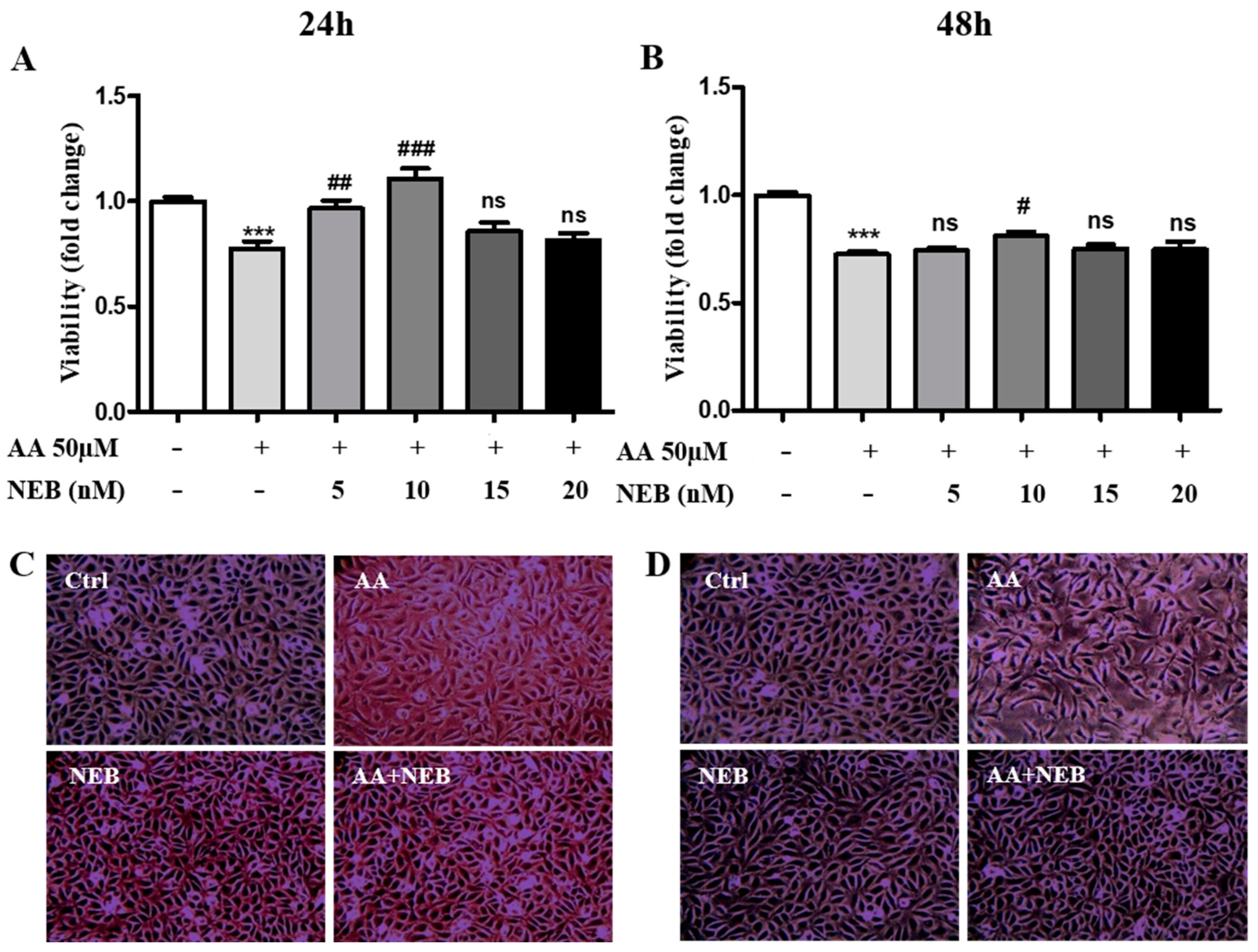
2.1. Nebivolol Protects EAhy926 Cells against AA-Induced Cytotoxicity
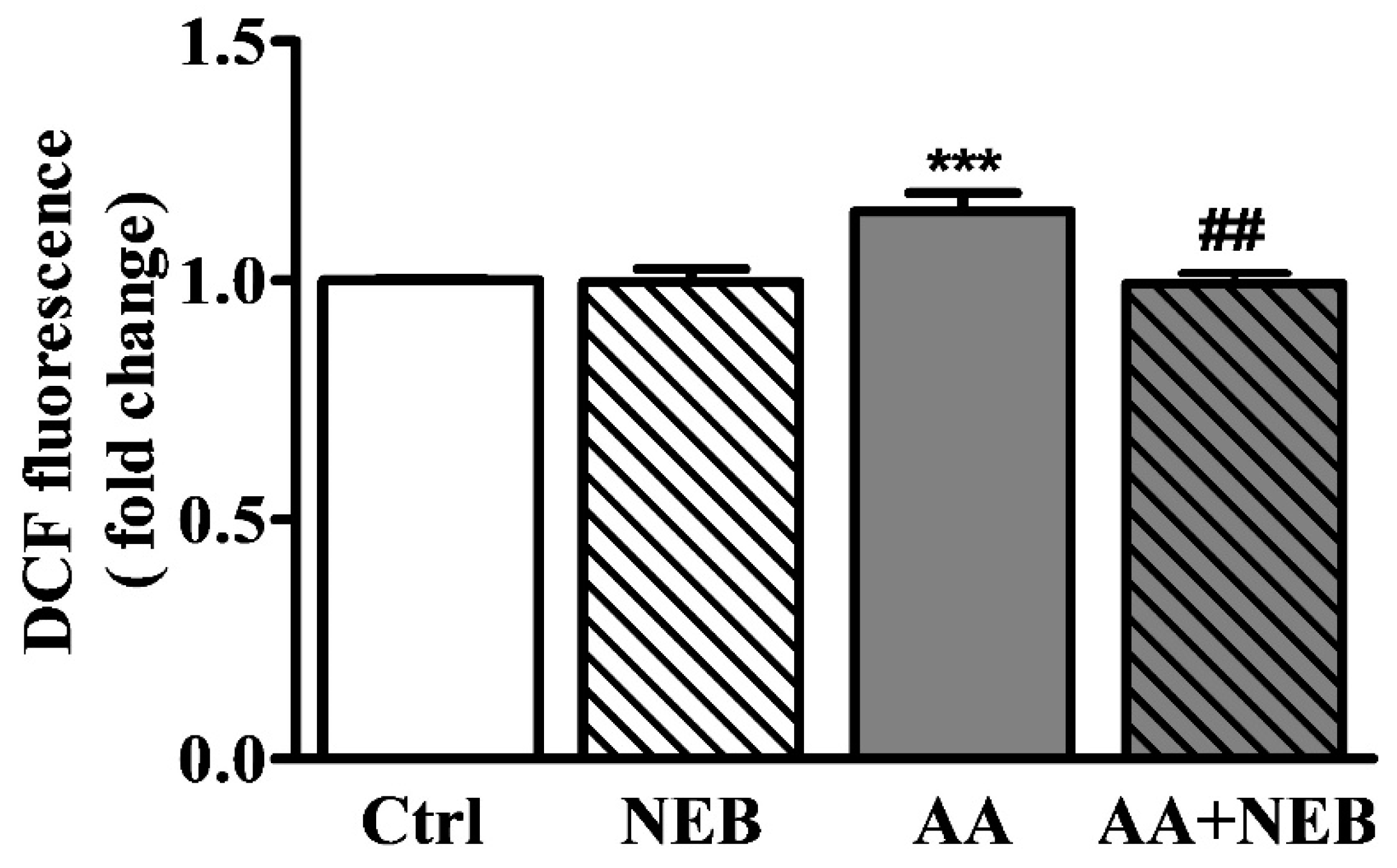
2.2. Nebivolol Attenuates ROS Levels
2.3. Metabolism of EAhy926 Endothelial Cells under Baseline Conditions
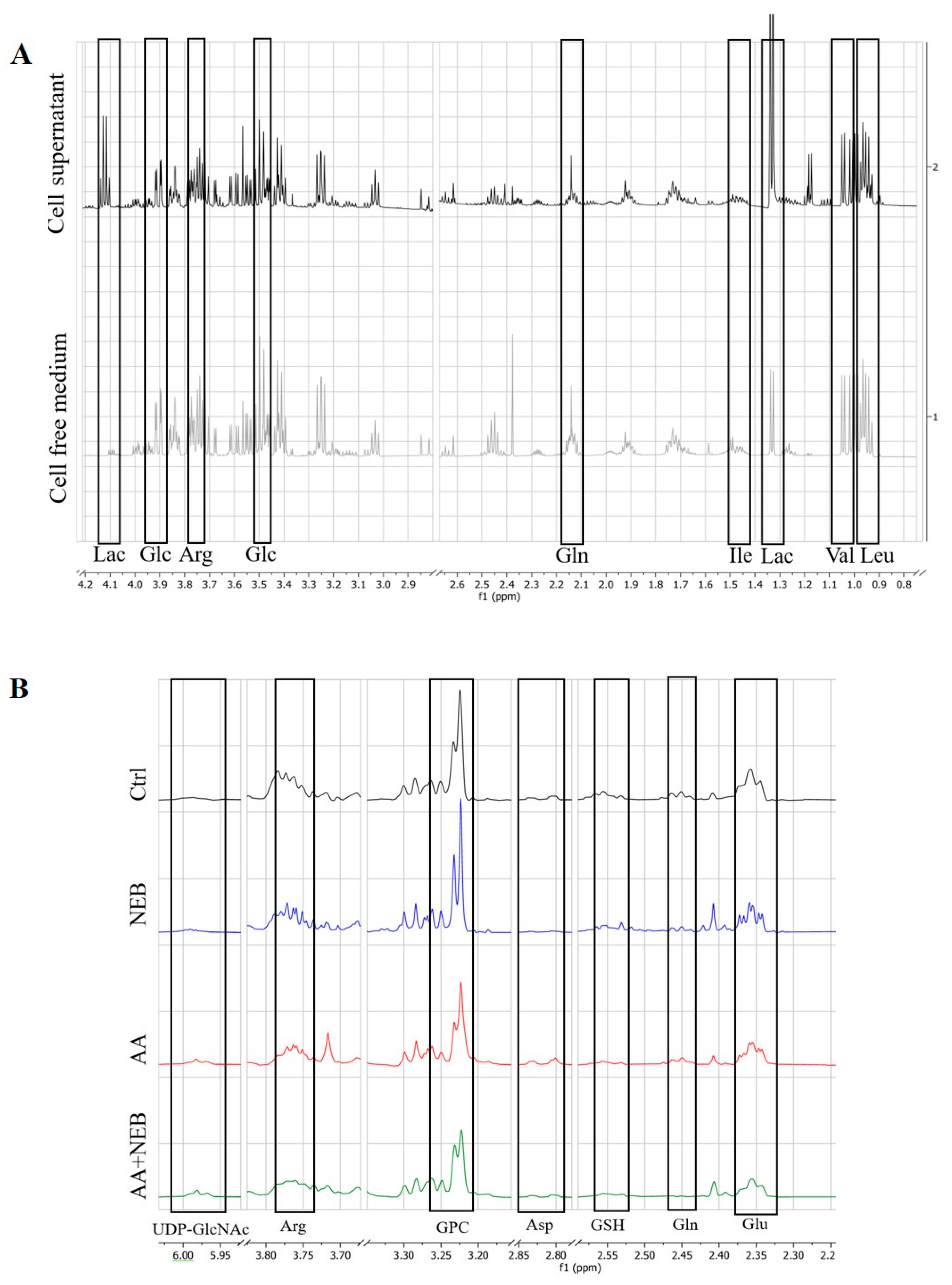
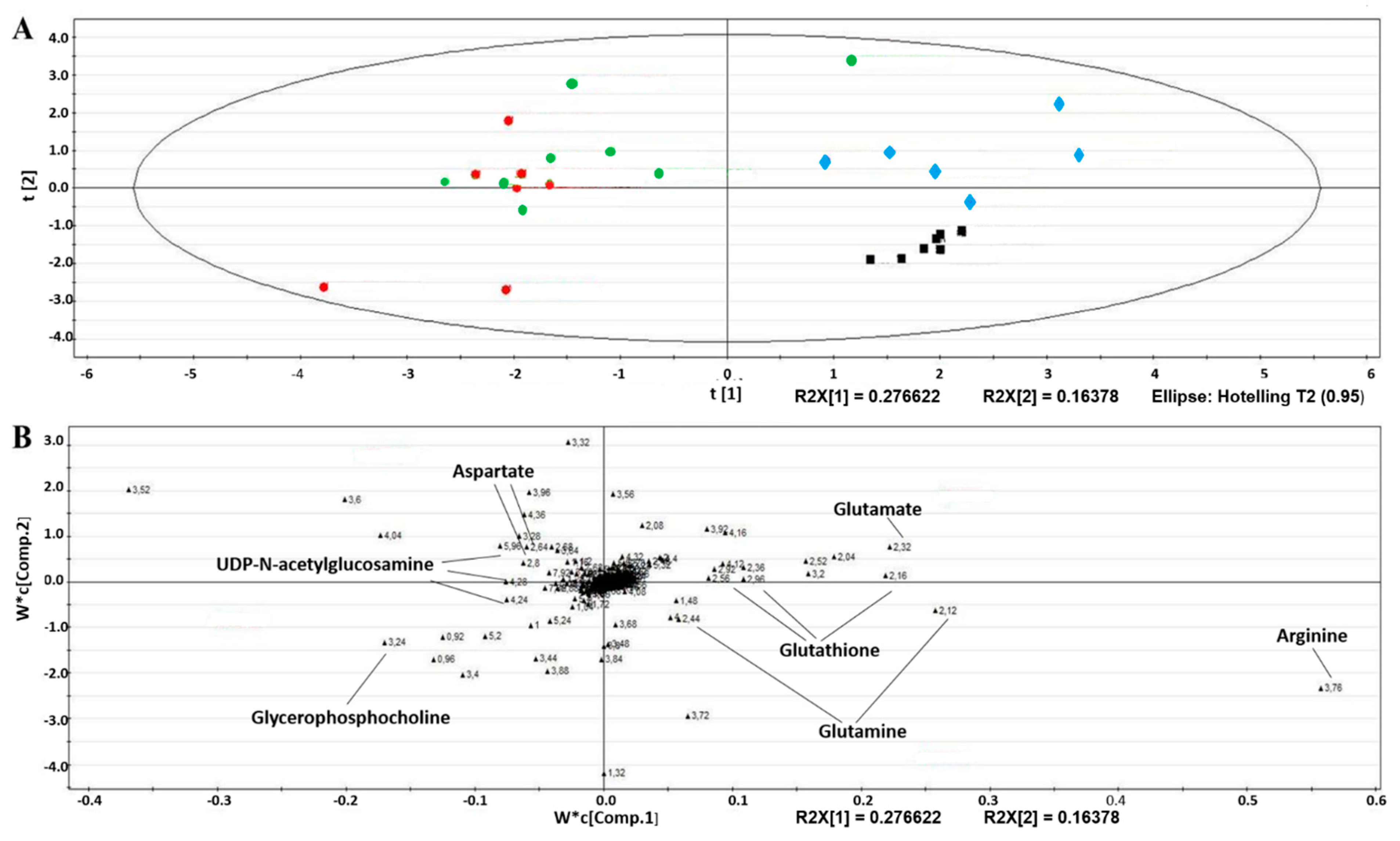
2.4. Intracellular Metabolic Alterations Induced by AA Exposure
2.5. Extracellular Metabolic Alterations Induced by AA Exposure
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Cell Cultures
5.3. Cell Viability
5.4. Microscopic Examination
5.5. Measurement of ROS
5.6. 1H-NMR Samples’ Collection and Extraction
5.7. 1H-NMR Spectroscopy
5.8. Multivariate Data Analyses and Metabolite Identification
5.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 13, 387–391. [Google Scholar] [CrossRef]
- Bunel, V.; Souard, F.; Antoine, M.H.; Stevigny, C.; Nortier, J.L. Nephrotoxicity of Natural Products: Aristolochic Acid and Fungal Toxins. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier Ltd.: Oxford, UK, 2018; Volume 14, pp. 340–379. [Google Scholar]
- Debelle, F.D.; Nortier, J.L.; De Prez, E.G.; Garbar, C.H.; Vienne, A.R.; Salmon, I.J.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L. Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J. Am. Soc. Nephrol. 2002, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Feng, J.; Dai, C.; Sun, L.; Jin, T.; Ma, J.; Wang, L. Role of peritubular capillary loss and hypoxia in progressive tubulointerstitial fibrosis in a rat model of aristolochic acid nephropathy. Am. J. Nephrol. 2006, 26, 363–371. [Google Scholar] [CrossRef]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Menshikh, A.; Scarfe, L.; Delgado, R.; Finney, C.; Zhu, Y.; Yang, H.; de Caestecker, M.P. Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am. J. Physiol. Renal Physiol. 2019, 317, F1383–F1397. [Google Scholar] [CrossRef] [PubMed]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Basile, D.P. Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr. Opin. Nephrol. Hypertens. 2004, 13, 1–7. [Google Scholar] [CrossRef]
- Shi, H.; Feng, J.M. Aristolochic acid induces apoptosis of human umbilical vein endothelial cells in vitro by suppressing PI3K/Akt signaling pathway. Acta Pharmacol. Sin. 2011, 32, 1025–1030. [Google Scholar] [CrossRef]
- Youl, E.N.H.; Husson, C.; El Khattabi, C.; El Mere, S.; Declèves, A.E.; Pochet, S.; Nortier, J.L.; Antoine, M.H. Characterization of cytotoxic effects of aristolochic acids on the vascular endothelium. Toxicol. In Vitro 2020, 65, 104811. [Google Scholar] [CrossRef]
- Bertocchi, C.; Schmid, M.; Hasslacher, J.; Dunzendorfer, S.; Patsch, J.R.; Joannidis, M. Differential effects of NO inhibition in renal epithelial and endothelial cells in mono-culture vs. co-culture conditions. Cell Physiol. Biochem. 2010, 26, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Thorngren, D.; Chen, Q.; Wang, M.; Xie, X. Protective role of relaxin in a mouse model of aristolochic acid nephropathy. Biomed. Pharmacother. 2019, 115, 108917. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.É.; Jadot, I.; Colombaro, V.; Martin, B.; Voisin, V.; Nortier, J.; Caron, N. Protective effect of nitric oxide in aristolochic acid-induced toxic acute kidney injury: An old friend with new assets. Exp. Physiol. 2016, 101, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.; Jain, S. Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J. Hum. Hypertens. 2017, 31, 376–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, S.; Garbin, U.; Pasini, A.F.; Stranieri, C.; Boccioletti, V.; Cominacini, L. Effect of DL-nebivolol, its enantiomers and metabolites on the intracellular production of superoxide and nitric oxide in human endothelial cells. Pharmacol. Res. 2007, 55, 303–309. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Gao, Y. Beta blockers, nitric oxide, and cardiovascular disease. Curr. Opin. Pharmacol. 2013, 13, 265–273. [Google Scholar] [CrossRef]
- Ladage, D.; Brixius, K.; Hoyer, H.; Steingen, C.; Wesseling, A.; Malan, D.; Bloch, W.; Schwinger, R.H. Mechanisms underlying nebivolol-induced endothelial nitric oxide synthase activation in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2006, 33, 720–724. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Colet, J.M. Metabonomics in the preclinical and environmental toxicity field. Drug Discov. Today Technol. 2015, 13, 3–10. [Google Scholar] [CrossRef]
- Duquesne, M.; Declèves, A.E.; De Prez, E.; Nortier, J.L.; Colet, J.M. Interest of metabonomic approach in environmental nephrotoxicants: Application to aristolochic acid exposure. Food Chem. Toxicol. 2017, 108, 19–29. [Google Scholar] [CrossRef]
- Mantle, P.; Modalca, M.; Nicholls, A.; Tatu, C.; Tatu, D.; Toncheva, D. Comparative (1)H NMR metabolomic urinalysis of people diagnosed with Balkan endemic nephropathy, and healthy subjects, in Romania and Bulgaria: A pilot study. Toxins 2011, 3, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Briciu, C.; Neag, M.; Muntean, D.; Bocsan, C.; Buzoianu, A.; Antonescu, O.; Gheldiu, A.M.; Achim, M.; Popa, A.; Vlase, L. Phenotypic differences in nebivolol metabolism and bioavailability in healthy volunteers. Clujul Med. 2015, 88, 208–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozdzik, A.A.; Giordano, L.; Li, G.; Antoine, M.H.; Quellard, N.; Godet, J.; De Prez, E.; Husson, C.; Declèves, A.E.; Arlt, V.M.; et al. Blocking TGF-β Signaling Pathway Preserves Mitochondrial Proteostasis and Reduces Early Activation of PDGFRβ+ Pericytes in Aristolochic Acid Induced Acute Kidney Injury in Wistar Male Rats. PLoS ONE 2016, 11, e0157288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Wang, Y.; Jin, J.; Guan, C.; Li, M.; Xi, C.; Ouyang, Z.; Chen, M.; Qiu, Y.; Huang, M.; et al. Endoplasmic reticulum stress mediates aristolochic acid I-induced apoptosis in human renal proximal tubular epithelial cells. Toxicol. In Vitro 2012, 26, 663–671. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, J.; Feng, X.; Wu, H.; Huang, R.; Fan, J.; Yu, X.; Yang, X. Mitochondrial dysfunction is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Hum. Exp. Toxicol. 2020, 39, 673–682. [Google Scholar] [CrossRef]
- Mason, R.P.; Kubant, R.; Jacob, R.F.; Walter, M.F.; Boychuk, B.; Malinski, T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: Role of antioxidant activity. J. Cardiovasc. Pharmacol. 2006, 48, 862–869. [Google Scholar] [CrossRef]
- De Groot, A.A.; Mathy, M.J.; van Zwieten, P.A.; Peters, S.L. Antioxidant activity of nebivolol in the rat aorta. J. Cardiovasc. Pharmacol. 2004, 43, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; An, W.; Zhang, F.; Niu, M.; Liu, Y.; Shi, R. Nebivolol ameliorated kidney damage in Zucker diabetic fatty rats by regulation of oxidative stress/NO pathway: Comparison with captopril. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1135–1148. [Google Scholar] [CrossRef]
- Dallons, M.; Schepkens, C.; Dupuis, A.; Tagliatti, V.; Colet, J.M. New Insights About Doxorubicin-Induced Toxicity to Cardiomyoblast-Derived H9C2 Cells and Dexrazoxane Cytoprotective Effect: Contribution of In Vitro (1)H-NMR Metabonomics. Front Pharmacol. 2020, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- Pink, M.; Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Integrated proteomic and metabolomic analysis to assess the effects of pure and benzo[a]pyrene-loaded carbon black particles on energy metabolism and motility in the human endothelial cell line EA.hy926. Arch. Toxicol. 2014, 88, 913–934. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groschner, L.N.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Endothelial mitochondria—Less respiration, more integration. Pflugers Arch. 2012, 464, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chan, C.K.; Ham, Y.H.; Chan, W. Identifying Cysteine, N-Acetylcysteine, and Glutathione Conjugates as Novel Metabolites of Aristolochic Acid I: Emergence of a New Detoxification Pathway. Chem. Res. Toxicol. 2020, 33, 1374–1381. [Google Scholar] [CrossRef]
- Wu, X.; Sun, X.; Sharma, S.; Lu, Q.; Yegambaram, M.; Hou, Y.; Wang, T.; Fineman, J.R.; Black, S.M. Arginine recycling in endothelial cells is regulated BY HSP90 and the ubiquitin proteasome system. Nitric Oxide 2021, 1, 12–19. [Google Scholar] [CrossRef]
- Hecker, M.; Sessa, W.C.; Harris, H.J.; Anggård, E.E.; Vane, J.R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. USA 1990, 87, 8612–8616. [Google Scholar] [CrossRef] [Green Version]
- Mihout, F.; Shweke, N.; Bigé, N.; Jouanneau, C.; Dussaule, J.C.; Ronco, P.; Chatziantoniou, C.; Boffa, J.J. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-β1 synthesis. J. Pathol. 2011, 223, 37–45. [Google Scholar] [CrossRef]
- Cui, Y.; Han, J.; Ren, J.; Chen, H.; Xu, B.; Song, N.; Li, H.; Liang, A.; Shen, G. Untargeted LC-MS-based metabonomics revealed that aristolochic acid I induces testicular toxicity by inhibiting amino acids metabolism, glucose metabolism, beta-oxidation of fatty acids and the TCA cycle in male mice. Toxicol. Appl. Pharmacol. 2019, 373, 26–38. [Google Scholar] [CrossRef]
- Hertz, L.; Song, D.; Peng, L.; Chen, Y. Multifactorial Effects on Different Types of Brain Cells Contribute to Ammonia Toxicity. Neurochem. Res. 2017, 42, 721–736. [Google Scholar] [CrossRef]
- Martínek, V.; Sklenár, J.; Dracínsky, M.; Sulc, M.; Hofbauerová, K.; Bezouska, K.; Frei, E.; Stiborová, M. Glycosylation protects proteins against free radicals generated from toxic xenobiotics. Toxicol. Sci. 2010, 117, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; He, B.; Liu, Y.; Huo, M.; Fu, W.; Yang, C.; Wei, J.; Abliz, Z. In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta Pharm. Sin. B. 2020, 10, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, H.L.; Cheng, X.L.; Wei, F.; Bai, X.; Lin, R.C.; Vaziri, N.D. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci. Rep. 2015, 7, 12936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J. Membrane breakdown in acute and chronic neurodegeneration: Focus on choline-containing phospholipids. J. Neural. Transm. 2000, 107, 1027–1063. [Google Scholar] [CrossRef] [PubMed]
- Bonvallot, N.; Tremblay-Franco, M.; Chevrier, C.; Canlet, C.; Warembourg, C.; Cravedi, J.P.; Cordier, S. Metabolomics tools for describing complex pesticide exposure in pregnant women in Brittany (France). PLoS ONE 2013, 8, e64433. [Google Scholar]
- Zhang, Y.X.; Yang, X.; Zou, P.; Du, P.F.; Wang, J.; Jin, F.; Jin, M.J.; She, Y.X. Nonylphenol Toxicity Evaluation and Discovery of Biomarkers in Rat Urine by a Metabolomics Strategy through HPLC-QTOF-MS. Int. J. Environ. Res. Public Health 2016, 13, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozdzik, A.A.; Salmon, I.J.; Debelle, F.D.; Decaestecker, C.; Van den Branden, C.; Verbeelen, D.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008, 73, 595–607. [Google Scholar] [CrossRef]
- Ni, Y.; Su, M.; Qiu, Y.; Chen, M.; Liu, Y.; Zhao, A.; Jia, W. Metabolic profiling using combined GC-MS and LC-MS provides a systems understanding of aristolochic acid-induced nephrotoxicity in rat. FEBS Lett. 2007, 581, 707–711. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Ishii, T.; Sugita, Y.; Bannai, S. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am. J. Physiol. 1992, 262, C50–C58. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]

| Metabolites | Chemical Shift (ppm) | AA vs. Ctrl | AA + NEB vs. AA |
|---|---|---|---|
| 1. Intracellular Extracts | |||
| Arginine | 3.76 | ↓ **** | - |
| Aspartate | 2.82 | ↑ * | - |
| Glutamate | 2.32 | ↓ * | - |
| Glutamine | 2.44 | ↓ * | - |
| Glutathione | 2.54 | ↓ **** | ↑ # |
| Glycerophosphocholine | 3.24 | ↑ * | ↓ # |
| UDP-N-acetylglucosamine | 5.96 | ↑ ** | - |
| 2. Extracellular Fluids | |||
| Glucose | 3.4 | ↑ * | - |
| Glutamate | 2.32 | ↓ *** | ↑ ## |
| Lactate | 1.32 | ↓ *** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoine, M.-H.; Husson, C.; Yankep, T.; Mahria, S.; Tagliatti, V.; Colet, J.-M.; Nortier, J. Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells. Toxins 2022, 14, 132. https://doi.org/10.3390/toxins14020132
Antoine M-H, Husson C, Yankep T, Mahria S, Tagliatti V, Colet J-M, Nortier J. Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells. Toxins. 2022; 14(2):132. https://doi.org/10.3390/toxins14020132
Chicago/Turabian StyleAntoine, Marie-Hélène, Cécile Husson, Tatiana Yankep, Souhaila Mahria, Vanessa Tagliatti, Jean-Marie Colet, and Joëlle Nortier. 2022. "Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells" Toxins 14, no. 2: 132. https://doi.org/10.3390/toxins14020132
APA StyleAntoine, M.-H., Husson, C., Yankep, T., Mahria, S., Tagliatti, V., Colet, J.-M., & Nortier, J. (2022). Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells. Toxins, 14(2), 132. https://doi.org/10.3390/toxins14020132






