Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study
Abstract
1. Introduction
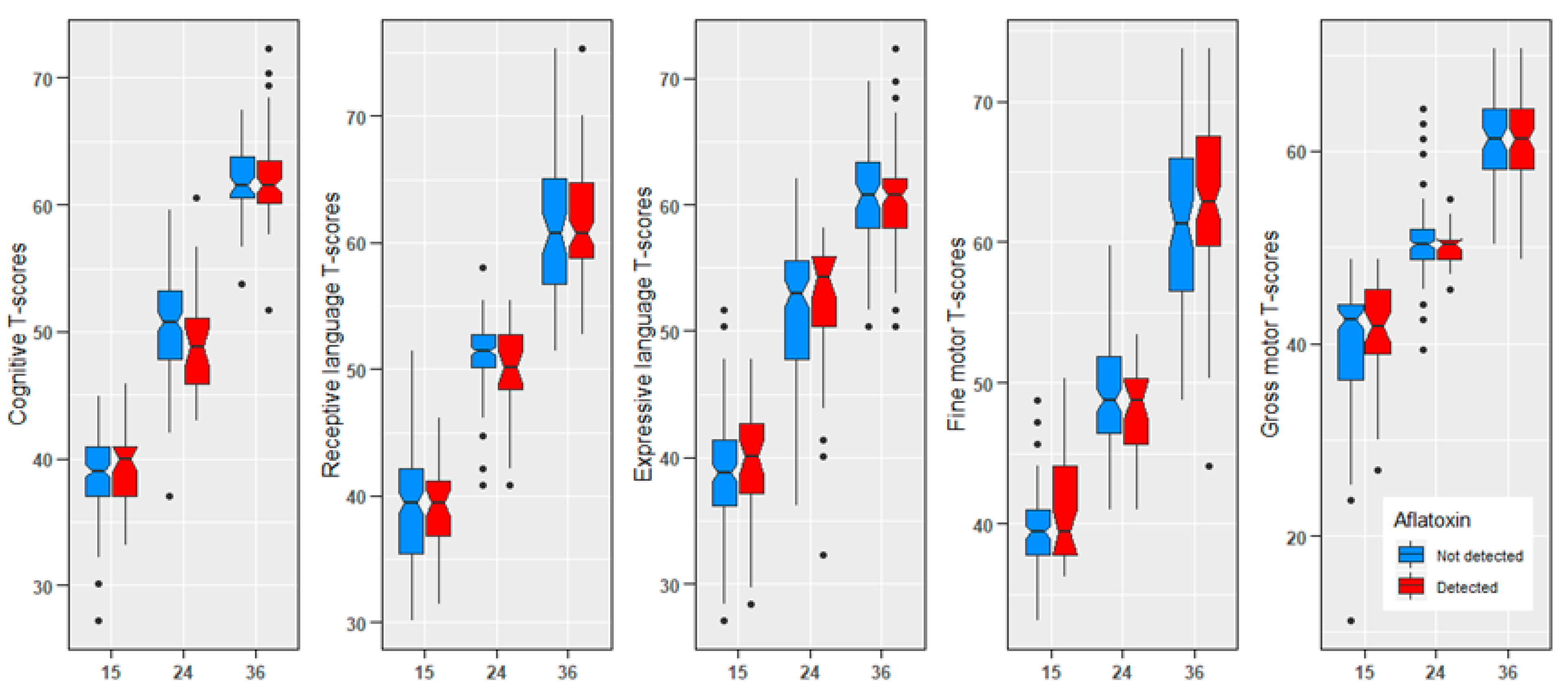
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Study Site
4.3. Data Collection
4.4. Biological Sample Collection
4.5. Aflatoxin Plasma Biomarker Assay
4.6. Child Development
4.7. Nutritional Status
4.8. Home Environment
4.9. Maternal Measures
4.10. Maternal Intelligence
4.11. Family Characteristics
4.12. Statistical Analysis
4.13. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnussen, A.; Parsi, M.A. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. WJG 2013, 19, 1508. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.; Alink, G.; Andersen, R.; Braesco, V.; Holst, B. Mycotoxins–General. Nutrition 2001, 86, S5–S35. [Google Scholar]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef]
- Turner, P.C.; Sylla, A.; Diallo, M.S.; Castegnaro, J.J.; Hall, A.J.; Wild, C.P. The role of aflatoxins and hepatitis viruses in the etiopathogenesis of hepatocellular carcinoma: A basis for primary prevention in Guinea–Conakry, West Africa. J. Gastroenterol. Hepatol. 2002, 17, S441–S448. [Google Scholar] [CrossRef]
- Mahfuz, M.; Hasan, S.T.; Alam, M.A.; Das, S.; Fahim, S.M.; Islam, M.M.; Gazi, M.A.; Hossain, M.; Egner, P.A.; Groopman, J.D. Aflatoxin exposure was not associated with childhood stunting: Results from a birth cohort study in a resource-poor setting of Dhaka, Bangladesh. Public Health Nutr. 2021, 24, 3361–3370. [Google Scholar] [CrossRef]
- Mahfuz, M.; Alam, M.A.; Fahim, S.M.; Gazi, M.A.; Raihan, M.J.; Hossain, M.; Egner, P.A.; Bessong, P.O.; Petri, W.A.; Groopman, J.D. Aflatoxin exposure in children living in Mirpur, Dhaka: Data from MAL-ED companion study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 655–662. [Google Scholar] [CrossRef]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Riley, R.T.; Egner, P.A.; Groopman, J.D.; Wu, F. Chronic aflatoxin exposure in children living in Bhaktapur, Nepal: Extension of the MAL-ED study. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 106–111. [Google Scholar] [CrossRef]
- Yard, E.E.; Daniel, J.H.; Lewis, L.S.; Rybak, M.E.; Paliakov, E.M.; Kim, A.A.; Montgomery, J.M.; Bunnell, R.; Abudo, M.U.; Akhwale, W. Human aflatoxin exposure in Kenya, 2007: A cross-sectional study. Food Addit. Contam. Part A 2013, 30, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 2011, 7, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Walker, S.P.; Fernald, L.C.; Andersen, C.T.; DiGirolamo, A.M.; Lu, C.; McCoy, D.C.; Fink, G.; Shawar, Y.R.; Shiffman, J. Early childhood development coming of age: Science through the life course. Lancet 2017, 389, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.P.; Wong, J.J. Pharmacokinetics and excretion of aflatoxins. Toxicol. Aflatoxins Hum. Health Vet. Agric. Significance 1994, 4, 73–88. [Google Scholar]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the biological and health effects of aflatoxins on body organs and body systems. Aflatoxins-Recent Adv. Future Prospect. 2013, 12, 239–265. [Google Scholar]
- Bbosa, G.S.; Kitya, D.; Ogwal-Okeng, J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health 2013, 5, 10A. [Google Scholar] [CrossRef]
- Qureshi, H.; Hamid, S.S.; Ali, S.S.; Anwar, J.; Siddiqui, A.A.; Khan, N.A. Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood-brain barrier. Med. Mycol. 2015, 53, 409–416. [Google Scholar] [CrossRef]
- Smith, L.E.; Stoltzfus, R.J.; Prendergast, A. Food chain mycotoxin exposure, gut health, and impaired growth: A conceptual framework. Adv. Nutr. 2012, 3, 526–531. [Google Scholar] [CrossRef]
- Bahey, N.G.; Abd Elaziz, H.O.; Gadalla, K.K.E.S. Toxic effect of aflatoxin B1 and the role of recovery on the rat cerebral cortex and hippocampus. Tissue Cell 2015, 47, 559–566. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R. Evaluation of neuroprotective effects of quercetin against aflatoxin B1-intoxicated mice. Animals 2020, 10, 898. [Google Scholar] [CrossRef]
- Laag, E.M.; Abd Elaziz, H.O. Effect of aflatoxin-B1 on rat cerebellar cortex: Light and electron microscopic study. Egypt. J. Histol. 2013, 36, 601–610. [Google Scholar] [CrossRef]
- Trebak, F.; Alaoui, A.; Alexandre, D.; El Ouezzani, S.; Anouar, Y.; Chartrel, N.; Magoul, R. Impact of aflatoxin B1 on hypothalamic neuropeptides regulating feeding behavior. Neurotoxicology 2015, 49, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Oyelami, O.; Maxwell, S.; Adelusola, K.; Aladekoma, T.; Oyelese, A. Aflatoxins in the autopsy brain tissue of children in Nigeria. Mycopathologia 1995, 132, 35–38. [Google Scholar] [CrossRef]
- Sania, A.; Sudfeld, C.R.; Danaei, G.; Fink, G.; McCoy, D.C.; Zhu, Z.; Fawzi, M.C.S.; Akman, M.; Arifeen, S.E.; Barros, A.J. Early life risk factors of motor, cognitive and language development: A pooled analysis of studies from low/middle-income countries. BMJ Open 2019, 9, e026449. [Google Scholar] [CrossRef]
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120 (Suppl. S1), S28–S48. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Hsu, H.-H.; Chandyo, R.K.; Shrestha, B.; Bodhidatta, L.; Tu, Y.-K.; Gong, Y.-Y.; Egner, P.A.; Ulak, M.; Groopman, J.D. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: An extension of the MAL-ED study. PLoS ONE 2017, 12, e0172124. [Google Scholar] [CrossRef] [PubMed]
- Peet, E.D.; McCoy, D.C.; Danaei, G.; Ezzati, M.; Fawzi, W.; Jarvelin, M.-R.; Pillas, D.; Fink, G. Early childhood development and schooling attainment: Longitudinal evidence from British, Finnish and Philippine birth cohorts. PLoS ONE 2015, 10, e0137219. [Google Scholar] [CrossRef]
- Case, A.; Paxson, C. Stature and status: Height, ability, and labor market outcomes. J. Political Econ. 2008, 116, 499–532. [Google Scholar] [CrossRef]
- Engle, P.L.; Black, M.M.; Behrman, J.R.; De Mello, M.C.; Gertler, P.J.; Kapiriri, L.; Martorell, R.; Young, M.E.; International Child Development Steering Group. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet 2007, 369, 229–242. [Google Scholar] [CrossRef]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B.; Group, I.C.D.S. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Fink, G.; Peet, E.; Danaei, G.; Andrews, K.; McCoy, D.C.; Sudfeld, C.R.; Smith Fawzi, M.C.; Ezzati, M.; Fawzi, W.W. Schooling and wage income losses due to early-childhood growth faltering in developing countries: National, regional, and global estimates. Am. J. Clin. Nutr. 2016, 104, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.X.; Kjaerulf, F.; Turner, S.; Cohen, L.; Donnelly, P.D.; Muggah, R.; Davis, R.; Realini, A.; Kieselbach, B.; MacGregor, L.S. Transforming our world: Implementing the 2030 agenda through sustainable development goal indicators. J. Public Health Policy 2016, 37, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Mahfuz, M.; Islam, M.M.; Mondal, D.; Hossain, M.I.; Ahmed, A.S.; Tofail, F.; Gaffar, S.A.; Haque, R.; Guerrant, R.L. The MAL-ED cohort study in Mirpur, Bangladesh. Clin. Infect. Dis. 2014, 59 (Suppl. S4), S280–S286. [Google Scholar] [CrossRef]
- Groopman, J.D.; Egner, P.A.; Schulze, K.J.; Wu, L.S.-F.; Merrill, R.; Mehra, S.; Shamim, A.A.; Ali, H.; Shaikh, S.; Gernand, A. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1-lysine albumin biomarkers. Food Chem. Toxicol. 2014, 74, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.A.; Grieve, A.J. Bayley Scales of Infant and Toddler Development; Sage Publications Inc.: Thousand Oaks, CA, USA, 2007. [Google Scholar]
- Nahar, B.; Hamadani, J.D.; Ahmed, T.; Tofail, F.; Rahman, A.; Huda, S.; Grantham-McGregor, S. Effects of psychosocial stimulation on growth and development of severely malnourished children in a nutrition unit in Bangladesh. Eur. J. Clin. Nutr. 2009, 63, 725–731. [Google Scholar] [CrossRef]
- Nahar, B.; Hossain, M.; Hamadani, J.; Ahmed, T.; Huda, S.; Grantham-McGregor, S.; Persson, L. Effects of a community-based approach of food and psychosocial stimulation on growth and development of severely malnourished children in Bangladesh: A randomised trial. Eur. J. Clin. Nutr. 2012, 66, 701–709. [Google Scholar] [CrossRef]
- Aboud, F.E.; Singla, D.R.; Nahil, M.I.; Borisova, I. Effectiveness of a parenting program in Bangladesh to address early childhood health, growth and development. Soc. Sci. Med. 2013, 97, 250–258. [Google Scholar] [CrossRef]
- World Health Organization; World Health Organization, Nutrition for Health. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Caldwell, B.M.; Bradley, R.H. Home Observation for Measurement of the Environment; University of Arkansas: Little Rock, AR, USA, 1979. [Google Scholar]
- Beusenberg, M.; Orley, J.H.; World Health Organization. A User’s Guide to the Self Reporting Questionnaire (SRQ); World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Harding, T.W.; De Arango, V.; Baltazar, J.; Climent, C.; Ibrahim, H.; Ladrido-Ignacio, L.; Wig, N. Mental disorders in primary health care: A study of their frequency and diagnosis in four developing countries. Psychol. Med. 1980, 10, 231–241. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Rasmussen, Z.A.; Scharf, R.J.; Rasheed, M.A.; Svensen, E.; Seidman, J.C.; Tofail, F.; Koshy, B.; Shrestha, R.; Maphula, A. The MAL-ED cohort study: Methods and lessons learned when assessing early child development and caregiving mediators in infants and young children in 8 low-and middle-income countries. Clin. Infect. Dis. 2014, 59 (Suppl. S4), S261–S272. [Google Scholar] [CrossRef]
- Raven, J. Manual for the Coloured Progressive Matrices (Revised); NFRE-Nelson: Windsor, UK, 1984. [Google Scholar]
| Indicator | Unit | n (%), Mean (SD), or Median (IQR) |
|---|---|---|
| Female sex, n (%) | % | 106 (51) |
| Birth weight, mean (SD) | kg | 2.82 (0.41) |
| Birth order, median (IQR) | order | 2 (1, 2) |
| Mother age, median | year | 25 (20, 28) |
| Maternal education, median (IQR) | year | 5 (2, 7) |
| Maternal height, mean (SD) | cm | 148.91 (5.21) |
| Maternal raven’s factor, median (IQR) | score unit | 13 (9, 22) |
| HOME emotional and verbal responsivity score, mean (SD) | score unit | 11.16 (1.3) |
| HOME environmental safety score, mean (SD) | score unit | 3.19 (0.65) |
| HOME cleanliness score, mean (SD) | score unit | 3.98 (0.11) |
| Monthly household income, median (IQR) | USD | 100 (72, 138) |
| Incidence of diarrhoea, mean (SD) | Number of new cases in a year | 3.29 (2.12) |
| Incidence of ALRI, mean (SD) | Number of new cases in a year | 0.40 (0.62) |
| Indicator | 15 Months (n = 194) | 24 Months (n = 167) | 36 Months (n = 163) |
|---|---|---|---|
| Aflatoxin detected, n (%) | 40 (20.6%) | 28 (16.8%) | 99 (60.7%) |
| LAZ, mean (SD) | −1.80 (0.930) | −2.03 (0.96) | −1.99 (0.84) |
| Haemoglobin, mean (SD) | 11.3 (1.48) | 11.7 (1.51) | 12.3 (1.33) |
| Cognitive T-scores, mean (SD) | 39.0 (3.14) | 50.4 (3.98) | 61.9 (3.05) |
| Receptive T-scores, mean (SD) | 39.1 (3.79) | 50.8 (3.10) | 61.4 (4.56) |
| Expressive T-scores, mean (SD) | 39.0 (4.42) | 51.9 (5.71) | 60.2 (3.75) |
| Fine motor T-scores, mean (SD) | 40.2 (2.90) | 48.7 (3.52) | 62.2 (5.68) |
| Gross motor T-scores, mean (SD) | 39.7 (6.55) | 50.5 (3.43) | 60.7 (4.28) |
| Variable | Cognitive | Receptive | Expressive | Fine Motor | Gross Motor | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | p-Value | Beta | p-Value | Beta | p-Value | Beta | p-Value | Beta | p-Value | |
| Aflatoxin detected | −0.702 | 0.036 | −0.844 | 0.021 | −0.927 | 0.056 | 0.696 | 0.078 | −0.075 | 0.872 |
| Birth weight in kg | 0.977 | 0.022 | 1.529 | 0.003 | 1.863 | 0.002 | 1.206 | 0.02 | 1.988 | 0.003 |
| Birth order | −0.063 | 0.676 | −0.216 | 0.234 | −0.418 | 0.052 | −0.087 | 0.634 | −0.091 | 0.699 |
| Length for age z score | 0.415 | 0.022 | 0.45 | 0.035 | 0.591 | 0.021 | 0.239 | 0.279 | 1.501 | <0.001 |
| Haemoglobin | 0.112 | 0.308 | 0.012 | 0.922 | 0.331 | 0.036 | 0.07 | 0.592 | 0.421 | 0.008 |
| Incidence of diarrhoea | −0.071 | 0.377 | −0.193 | 0.046 | −0.217 | 0.059 | −0.111 | 0.257 | −0.181 | 0.151 |
| Incidence of ALRI | −0.045 | 0.875 | −0.105 | 0.76 | −0.494 | 0.227 | 0.007 | 0.983 | 0.597 | 0.179 |
| Soluble TfR | 0.023 | 0.687 | −0.045 | 0.501 | −0.082 | 0.301 | −0.054 | 0.417 | −0.037 | 0.675 |
| Maternal age (y) | −0.038 | 0.271 | −0.076 | 0.068 | −0.072 | 0.148 | −0.034 | 0.416 | −0.036 | 0.5 |
| Maternal education (y) | 0.169 | 0.002 | 0.263 | <0.001 | 0.343 | <0.001 | 0.191 | 0.004 | 0.223 | 0.008 |
| Maternal height (cm) | 0.073 | 0.041 | 0.081 | 0.06 | 0.135 | 0.007 | 0.079 | 0.068 | 0.144 | 0.009 |
| Raven’s factor | 0.07 | <0.001 | 0.123 | <0.001 | 0.091 | 0.001 | 0.083 | 0.001 | 0.115 | <0.001 |
| HOME emotional and verbal responsivity score | 0.161 | 0.239 | 0.203 | 0.211 | 0.263 | 0.174 | −0.093 | 0.575 | 0.226 | 0.277 |
| HOME environmental safety score | 0.458 | 0.091 | 0.622 | 0.054 | 0.913 | 0.017 | 0.345 | 0.297 | 0.908 | 0.027 |
| HOME cleanliness score | 2.703 | 0.135 | 1.042 | 0.627 | 1.87 | 0.468 | 4.354 | 0.047 | 1 | 0.715 |
| SRQ-20 score | −0.019 | 0.577 | 0.01 | 0.802 | −0.036 | 0.461 | −0.006 | 0.868 | 0.018 | 0.719 |
| Monthly household income (USD) | 0.004 | 0.018 | 0.005 | 0.025 | 0.008 | <0.001 | 0.002 | 0.327 | 0.004 | 0.156 |
| Cognitive | Receptive | Expressive | Fine Motor | Gross Motor | |
|---|---|---|---|---|---|
| Aflatoxin detected | −0.69 (−1.36, −0.02) * | −0.90 (−1.62, −0.17) * | −1.01 (−1.96, −0.05) * | 0.59 (−0.19, 1.37) | −0.15 (−1.06, 0.77) |
| Age in months | 1.10 (1.06, 1.13) * | 1.07 (1.03, 1.11) * | 1.01 (0.96, 1.06) * | 1.04 (1.00, 1.08) * | 0.98 (0.93, 1.03) * |
| Female sex | 0.17 (−0.57, 0.91) | 0.27 (−0.58, 1.12) | 0.75 (−0.24, 1.73) | 0.75 (−0.15, 1.64) | −0.81 (−1.89, 0.28) |
| Birth weight in kg | 0.68 (−0.27, 1.63) | 1.17 (0.07, 2.27) * | 1.82 (0.54, 3.09) * | 0.76 (−0.33, 1.86) | 0.24 (−1.13, 1.62) |
| Birth order | −0.49 (−1.06, 0.09) | ||||
| Length for age z score | 0.07 (−0.37, 0.51) | −0.17 (−0.67, 0.34) | −0.13 (−0.74, 0.48) | 1.07 (0.43, 1.70) * | |
| Haemoglobin | 0.23 (−0.08, 0.54) | 0.29 (−0.02, 0.60) | |||
| Incidence of diarrhoea | −0.15 (−0.35, 0.04) | −0.16 (−0.38, 0.07) | −0.13 (−0.38, 0.12) | ||
| Incidence of ALRI | 0.88 (−0.03, 1.80) | ||||
| Maternal age (y) | −0.06 (−0.15, 0.02) | 0.03 (−0.11, 0.16) | |||
| Maternal education (y) | 0.06 (−0.07, 0.19) | 0.03 (−0.13, 0.18) | 0.16 (−0.02, 0.33) | 0.07 (−0.08, 0.23) | −0.04 (−0.23, 0.15) |
| Maternal height (cm) | 0.03 (−0.05, 0.10) | 0.02 (−0.07, 0.10) | 0.05 (−0.06, 0.15) | 0.03 (−0.05, 0.12) | 0.04 (−0.07, 0.16) |
| Raven’s factor | 0.05 (0.00, 0.10) * | 0.11 (0.05, 0.16) * | 0.02 (−0.04, 0.09) | 0.06 (−0.00, 0.11) | 0.07 (0.00, 0.14) * |
| HOME emotional and verbal responsivity score | 0.36 (−0.04, 0.76) | ||||
| HOME environmental safety score | 0.19 (−0.40, 0.78) | 0.20 (−0.48, 0.88) | 0.52 (−0.29, 1.32) | 0.28 (−0.58, 1.14) | |
| HOME cleanliness score | 2.99 (−1.05, 7.04) | 5.11 (0.30, 9.92) * | |||
| Monthly household income (USD) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfuz, M.; Hossain, M.S.; Alam, M.A.; Gazi, M.A.; Fahim, S.M.; Nahar, B.; Ahmed, T. Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study. Toxins 2022, 14, 855. https://doi.org/10.3390/toxins14120855
Mahfuz M, Hossain MS, Alam MA, Gazi MA, Fahim SM, Nahar B, Ahmed T. Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study. Toxins. 2022; 14(12):855. https://doi.org/10.3390/toxins14120855
Chicago/Turabian StyleMahfuz, Mustafa, Md. Shabab Hossain, Md. Ashraful Alam, Md. Amran Gazi, Shah Mohammad Fahim, Baitun Nahar, and Tahmeed Ahmed. 2022. "Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study" Toxins 14, no. 12: 855. https://doi.org/10.3390/toxins14120855
APA StyleMahfuz, M., Hossain, M. S., Alam, M. A., Gazi, M. A., Fahim, S. M., Nahar, B., & Ahmed, T. (2022). Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study. Toxins, 14(12), 855. https://doi.org/10.3390/toxins14120855





