Effects of Four Isothiocyanates in Dissolved and Gaseous States on the Growth and Aflatoxin Production of Aspergillus flavus In Vitro
Abstract
1. Introduction
2. Results
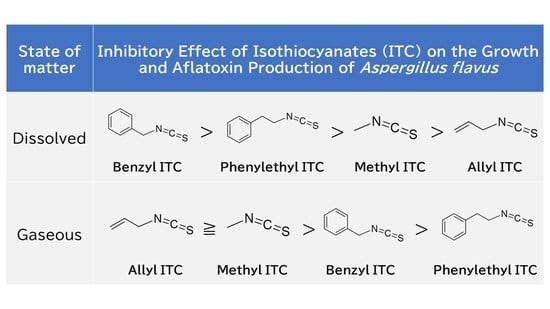
2.1. Antifungal Activity and Time-Course Action of Dissolved ITCs
2.2. Antifungal Activities of Gaseous ITCs
2.3. Effects of Short-Term ITC Exposure on the Growth of A. flavus
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. A. flavus Isolate
5.3. Antifungal Assay for Dissolved ITCs
5.4. Antifungal Assay for Gaseous ITCs
5.5. HPLC with Fluorescence Detection (HPLC-FL) for the Quantification of AFB1
5.6. Quantification of ITC Concentration in the Medium over Time
5.6.1. HPLC with Diode Array Detection for the Quantification of MITC and AITC
5.6.2. GC with Flame Ionization Detection (GC-FID) for the Quantification of BITC and PEITC
5.7. Effects of Short-Term ITC Exposure on the Germination and Growth of A. flavus
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical agents and related occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100f, 225–244. [Google Scholar]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Tabata, S.; Kamimura, H.; Ibe, A.; Hashimoto, H.; Tamura, Y.; Nishima, T. Fate of aflatoxins during cooking process and effect of food components on their stability. J. Food Hyg. Soc. Jpn 1992, 33, 150–156. [Google Scholar] [CrossRef]
- Lubulwa, A.S.G.; Davis, J.S. Estimating the social costs of the impacts of fungi and aflatoxins in maize and peanuts. In Stored Products Protection, Proceeding of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R.E., Eds.; University of Arizona Press: Wallingford, UK, 1994; Volume 2, pp. 1017–1042. [Google Scholar]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- Vázquez-Durán, A.; Nava-Ramírez, M.d.J.; Hernández-Patlán, D.; Solís-Cruz, B.; Hernández-Gómez, V.; Téllez-Isaías, G.; Méndez-Albores, A. Potential of Kale and Lettuce Residues as Natural Adsorbents of the Carcinogen Aflatoxin B1 in a Dynamic Gastrointestinal Tract-Simulated Model. Toxins 2021, 13, 771. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary Curcumin Alleviated Acute Ileum Damage of Ducks (Anas platyrhynchos) Induced by AFB1 through Regulating Nrf2-ARE and NF-κB Signaling Pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant bioactive compounds in pre- and postharvest management for aflatoxins reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Hareyama, Y.; Kushiro, M.; Fujii, Y. Comparative effect of allyl and methyl isothiocyanate on aflatoxin production and growth of Aspergillus flavus. JSM Mycotoxin. 2019, 69, 81–83. [Google Scholar] [CrossRef]
- Saladino, F.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Fernández-Franzón, M.; Meca, G. Shelf life improvement of the loaf bread using allyl, phenyl and benzyl isothiocyanates against Aspergillus parasiticus. LWT Food Sci. Technol. 2017, 78, 208–214. [Google Scholar] [CrossRef]
- Okano, K.; Ose, A.; Takai, M.; Kaneko, M.; Nishioka, C.; Ohzu, Y.; Odano, M.; Sekiyama, Y.; Mizukami, Y.; Nakamura, N.; et al. Inhibition of aflatoxin production and fungal growth on stored corn by allyl isothiocyanate vapor. Shokuhin Eiseigaku Zasshi 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Nishioka, C.; Iida, T.; Ozu, Y.; Kaneko, M.; Watanabe, Y.; Mizukami, Y.; Ichinoe, M. Inhibition of growth of seed-borne fungi and aflatoxin production on stored peanuts by allyl isothiocyanate vapor. Shokuhin Eiseigaku Zasshi 2018, 59, 45–50. [Google Scholar] [CrossRef]
- Cotty, P.J.; Jamie-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Ashiq, S.; Edwards, S.G.; Fatukasi, O.; Watson, A.; Back, M.A. In vitro activity of isothiocyanates against Fusarium graminearum. Plant Pathol. 2022, 71, 594–601. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.C.; Bi, Y.; Wang, T.L.; Dong, Y.P.; Yang, Q.; Zhang, T.T. 2-phenylethyl isothiocyanate exerts antifungal activity against Alternaria alternata by affecting Memmbrane integrity and mycotoxin production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Yang, C.X.; Wu, H.T.; Li, X.X.; Wu, H.Y.; Niu, T.X.; Wang, X.N.; Lian, R.; Zhang, G.L.; Hou, H.M. Comparison of the inhibitory potential of benzyl isothiocyanate and phenethyl isothiocyanate on Shiga toxin-producing and enterotoxigenic Escherichia coli. LWT Food Sci. Technol. 2020, 118. [Google Scholar] [CrossRef]
- Kurt, S.; Güneş, U.; Soylu, E.M. In vitro and in vivo antifungal activity of synthetic pure isothiocyanates against Sclerotinia sclerotiorum. Pest. Manag. Sci. 2011, 67, 869–875. [Google Scholar] [CrossRef]
- Azaiez, I.; Meca, G.; Manyes, L.; Fernández-Franzón, M. Antifungal activity of gaseous allyl, benzyl and phenyl isothiocyanate in vitro and their use for fumonisins reduction in bread. Food Control 2013, 32, 428–434. [Google Scholar] [CrossRef]
- Yang, B.L.; Li, L.; Geng, H.R.; Zhang, C.X.; Wang, G.; Yang, S.; Gao, S.; Zhao, Y.J.; Xing, F.G. Inhibitory effect of allyl and benzyl isothiocyanates on ochratoxin A producing fungi in grape and maize. Food Microbiol. 2021, 100, 103865. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Soylu, E.M. Assessment of glucosinolate-derived isothiocyanates as potential natural antifungal compounds against citrus sour rot disease agent Geotrichum citri-aurantii. J. Phytopathol. 2020, 168, 279–289. [Google Scholar] [CrossRef]
- Taylor, F.I.; Kenyon, D.; Rosser, S. Isothiocyanates inhibit fungal pathogens of potato in in vitro assays. Plant Soil 2014, 20, 281–289. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Furukawa, T.; Katayama, H.; Oikawa, A.; Negishi, L.; Ichikawa, T.; Suzuki, M.; Murase, K.; Takayama, S.; Sakuda, S. Dioctatin Activates ClpP to Degrade Mitochondrial Components and Inhibits Aflatoxin Production. Cell. Chem. Biol. 2020, 27, 1396–1409.e10. [Google Scholar] [CrossRef]
- Yabe, K.; Nakamura, H.; Ando, Y.; Terakado, N.; Nakajima, H.; Hamasaki, T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 1988, 54, 2096–2100. [Google Scholar] [CrossRef]
- Kushiro, M.; Hatabayashi, H.; Yabe, K.; Loladze, A. Detection of aflatoxigenic and atoxigenic Mexican Aspergillus Strains by the dichlorvos-ammonia (DV-AM) method. Toxins 2018, 10, 263. [Google Scholar] [CrossRef]
- Sekine, T.; Sugano, M.; Majid, A.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2123–2132. [Google Scholar] [CrossRef]
- Uchiyama, S.; Takeda, H.; Kobayashi, A.; Ito, S.; Sakurai, H.; Tada, Y.; Aoki, G.; Hosogai, T.; Yamanaka, T.; Maekawa, Y.; et al. Determination of methyl isothiocyanate in wine by GC and GC/MS. J. Food Hyg. Soc. Jpn 1992, 33, 603–608_1. [Google Scholar] [CrossRef][Green Version]
- Abe, M. Studies on the method for quantitative determination of isothiocyanates released from Brassic Leaves. Bull. Gakushuin Women’s Coll. 2014, 16, 53–61. [Google Scholar]




| ITCs | Concentration of Exposed ITCs (μg mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 10 | 20 | 40 | 60 | 80 | 100 | 200 | ||
| AITC | AFB1 | 27.2 ± 0.5 | 25.9 ± 1.1 | 20.8 ± 1.7 | 18.1 ± 2.0 | 12.9 ± 1.8 | 10.5 ± 0.4 | 7.4 ± 1.4 | 5.5 ± 0.7 | 2.0 ± 1.6 |
| Mycelial weight | 24.4 ± 2.6 | 25.0 ± 2.4 | 21.9 ± 3.6 | 22.5 ± 2.6 | 17.6 ± 2.3 | 15.2 ± 1.8 | 13.8 ± 1.5 | 10.7 ± 1.5 | <LOQ | |
| BITC | AFB1 | 31.2 ± 0.6 | 25.0 ± 2.9 | 9.9 ± 3.6 | 3.7 ± 1.6 | <LOQ | <LOQ | <LOD | <LOD | <LOD |
| Mycelial weight | 28.8 ± 0.6 | 24.9 ± 1.9 | 18.7 ± 3.0 | 13.8 ± 2.8 | <LOQ | <LOQ | <LOD | <LOD | <LOD | |
| MITC | AFB1 | 31.6 ± 0.5 | 27.0 ± 3.0 | 21.1 ± 2.5 | 15.5 ± 3.7 | 10.1 ± 2.9 | 4.4 ± 1.4 | 2.2 ± 1.2 | 0.8 ± 0.4 | <LOD |
| Mycelial weight | 28.3 ± 0.2 | 26.0 ± 0.4 | 24.6 ± 0.6 | 20.2 ± 2.1 | 17.1 ± 0.9 | 12.0 ± 1.2 | 8.7 ± 2.5 | <LOQ | <LOQ | |
| PEITC | AFB1 | 27.2 ± 0.5 | 23.0 ± 4.6 | 16.2 ± 1.1 | 8.0 ± 2.8 | 4.6 ± 2.4 | 0.9 ± 0.9 | <LOQ | <LOQ | <LOD |
| Mycelial weight | 24.4 ± 2.6 | 19.7 ± 3.1 | 19.0 ± 2.9 | 13.3 ± 1.8 | 7.9 ± 2.1 | <LOQ | <LOQ | <LOD | <LOD | |
| Hour | |||
|---|---|---|---|
| 0.5 | 24 | 48 | |
| AITC | 140.6 ± 3.4 | <LOD | <LOD |
| BITC | 153.6 ± 9.7 | 12.3 ± 4.3 | 7.9 ± 3.1 |
| MITC | 169.2 ± 13.0 | <LOD | <LOD |
| PEITC | 154.1 ± 15.7 | 10.8 ± 2.1 | 7.2 ± 3.6 |
| ITCs (μg mL−1) | Exposure Time (Hour) | |||
|---|---|---|---|---|
| 0 | 3 | 24 | ||
| AITC | 50 | +++ | +++ | + |
| 100 | +++ | +++ | + | |
| 150 | +++ | ++ | - | |
| 200 | +++ | ++ | - | |
| BITC | 50 | +++ | +++ | + |
| 100 | +++ | +++ | + | |
| 150 | +++ | ++ | + | |
| 200 | +++ | ++ | - | |
| MITC | 50 | +++ | +++ | + |
| 100 | +++ | ++ | + | |
| 150 | +++ | ++ | - | |
| 200 | +++ | ++ | - | |
| PEITC | 50 | +++ | +++ | + |
| 100 | +++ | +++ | + | |
| 150 | +++ | +++ | + | |
| 200 | +++ | +++ | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hareyama, Y.; Tarao, M.; Toyota, K.; Furukawa, T.; Fujii, Y.; Kushiro, M. Effects of Four Isothiocyanates in Dissolved and Gaseous States on the Growth and Aflatoxin Production of Aspergillus flavus In Vitro. Toxins 2022, 14, 756. https://doi.org/10.3390/toxins14110756
Hareyama Y, Tarao M, Toyota K, Furukawa T, Fujii Y, Kushiro M. Effects of Four Isothiocyanates in Dissolved and Gaseous States on the Growth and Aflatoxin Production of Aspergillus flavus In Vitro. Toxins. 2022; 14(11):756. https://doi.org/10.3390/toxins14110756
Chicago/Turabian StyleHareyama, Yohei, Mitsunori Tarao, Koki Toyota, Tomohiro Furukawa, Yoshiharu Fujii, and Masayo Kushiro. 2022. "Effects of Four Isothiocyanates in Dissolved and Gaseous States on the Growth and Aflatoxin Production of Aspergillus flavus In Vitro" Toxins 14, no. 11: 756. https://doi.org/10.3390/toxins14110756
APA StyleHareyama, Y., Tarao, M., Toyota, K., Furukawa, T., Fujii, Y., & Kushiro, M. (2022). Effects of Four Isothiocyanates in Dissolved and Gaseous States on the Growth and Aflatoxin Production of Aspergillus flavus In Vitro. Toxins, 14(11), 756. https://doi.org/10.3390/toxins14110756