Time- and Dose-Dependent Effects of Dietary Deoxynivalenol (DON) in Rainbow Trout (Oncorhynchus mykiss) at Organism and Tissue Level
Abstract
1. Introduction
2. Results
2.1. Performance
2.2. Health
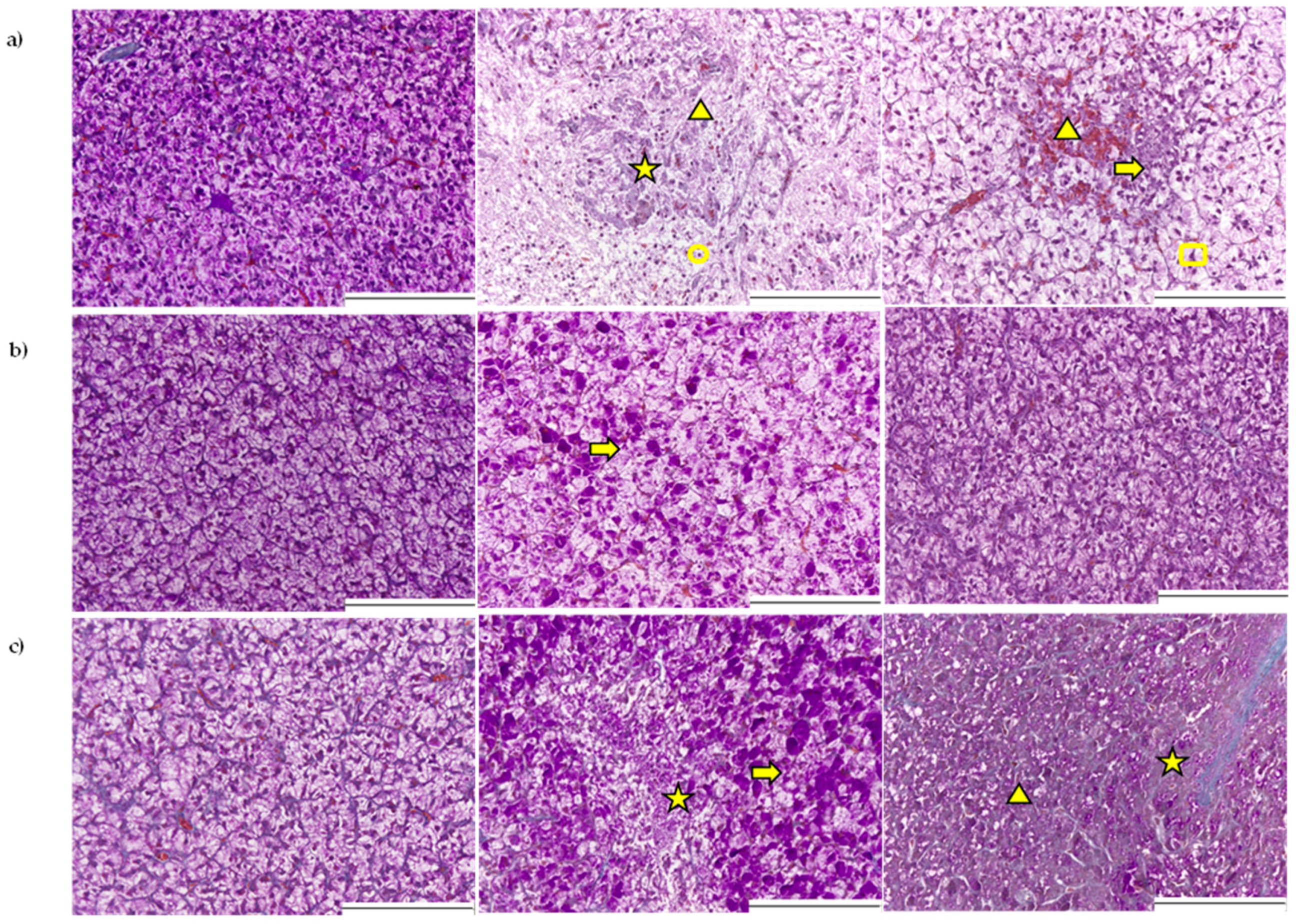
2.2.1. Histopathological Assessment of the Liver
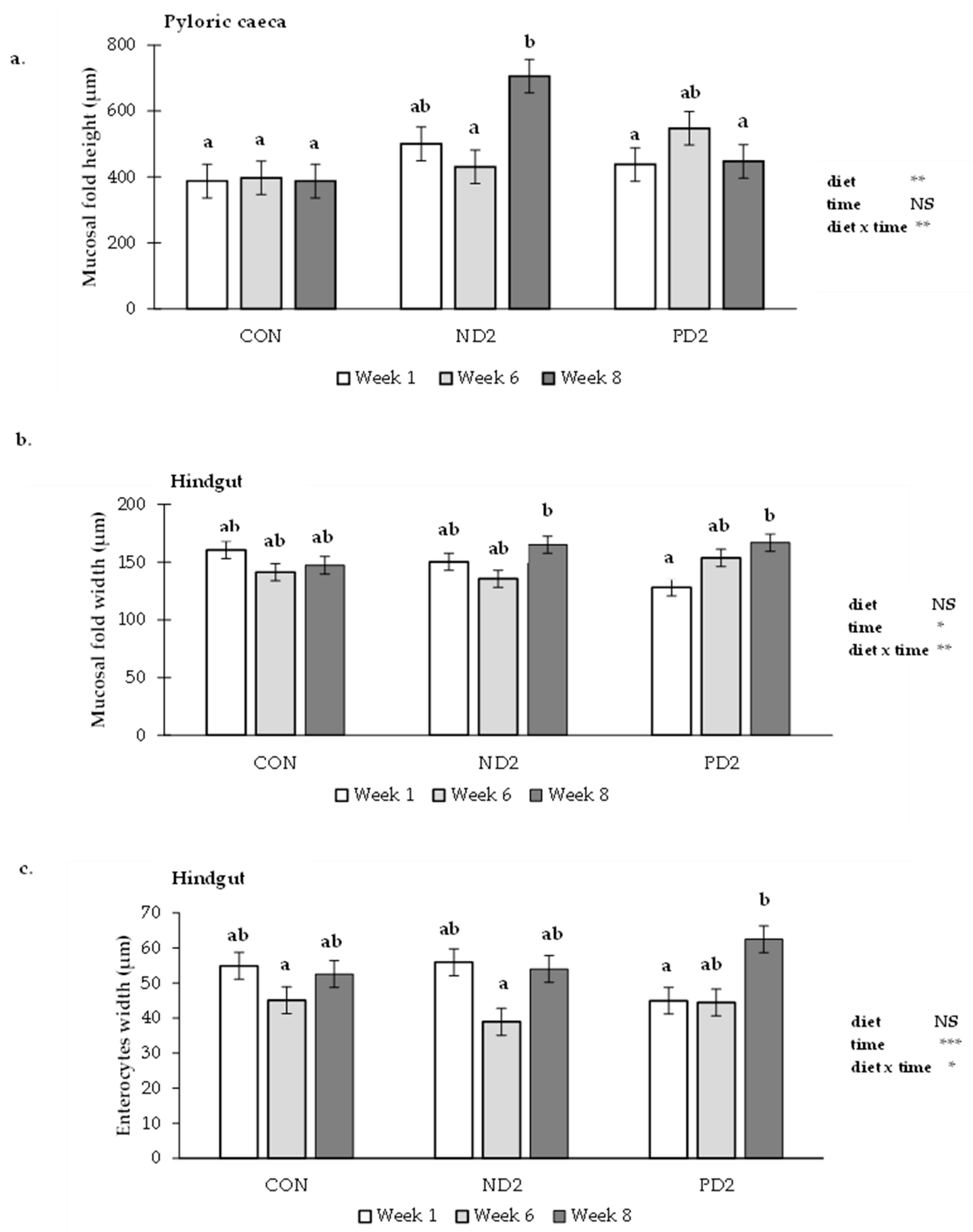
2.2.2. Histopathological Assessment of the Gastrointestinal Tract
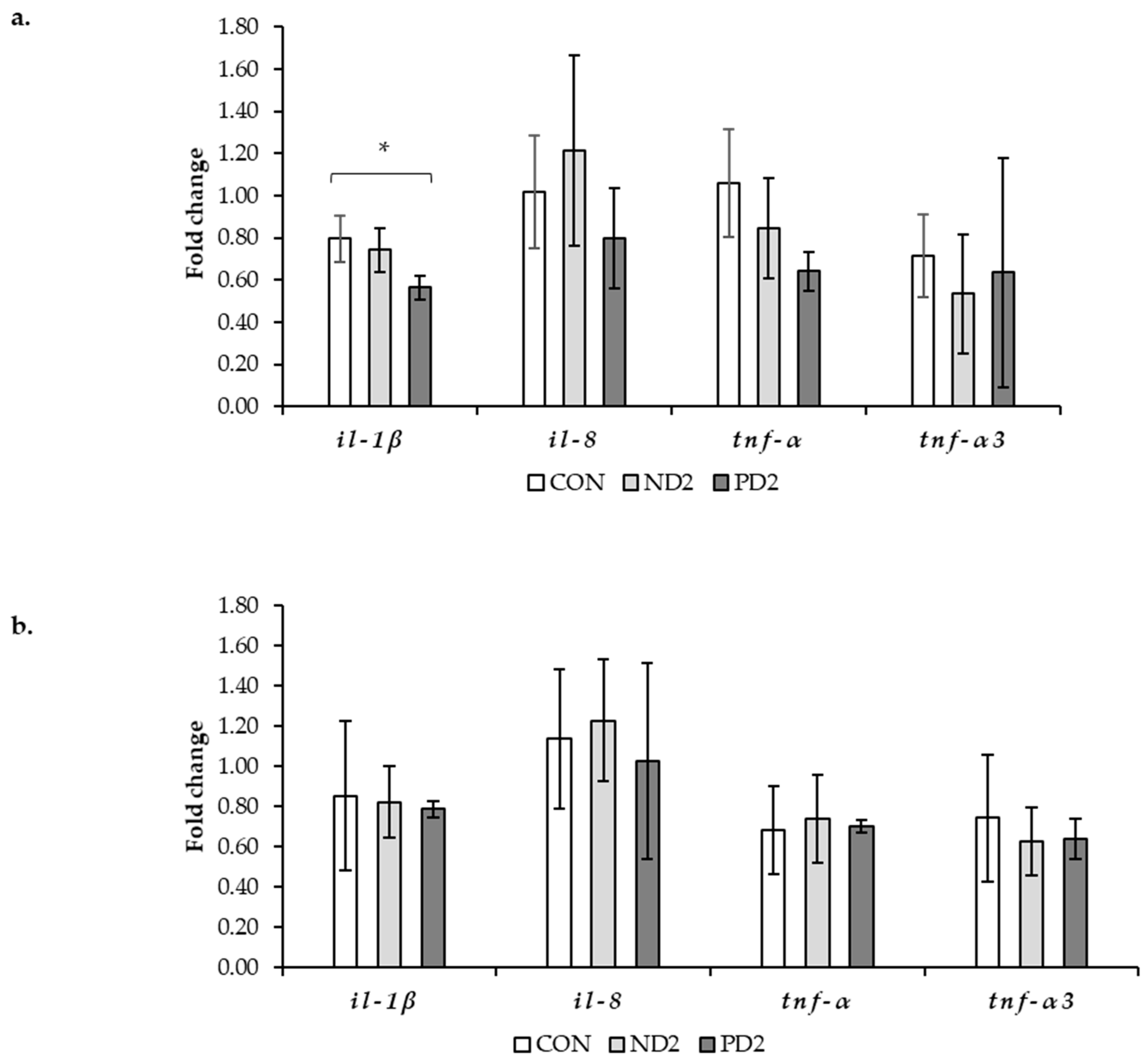
2.3. Assessment of Inflammation by Gene Expression
3. Discussion
3.1. Performance
3.2. Liver
3.3. Gastrointestinal Tract
4. Conclusions
5. Materials and Methods
5.1. In Vivo Experimental Procedure
5.2. Experimental Diets
5.3. Chemical Analysis of Feeds and Fish
5.4. Histopathological Examination of Liver and Gastrointestinal Tract
5.5. Gene Expression
5.6. Calculations
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koletsi, P.; Schrama, J.W.; Graat, E.A.M.; Wiegertjes, G.F.; Lyons, P.; Pietsch, C. The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of Deoxynivalenol (DON) on the health and growth of farmed fish species. Toxins 2021, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Rokvić, N.; Aksentijević, K.; Kureljušić, J.; Vasiljević, M.; Todorović, N.; Zdravković, N.; Stojanac, N. Occurrence and transfer of mycotoxins from ingredients to fish feed and fish meat of common carp (Cyprinus carpio) in Serbia. World Mycotoxin J. 2020, 13, 545–552. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Naehrer, K.; Santos, G.A. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev. Aquac. 2018, 10, 263–280. [Google Scholar] [CrossRef]
- Barbosa, T.S.; Pereyra, C.M.; Soleiro, C.A.; Dias, E.O.; Oliveira, A.A.; Keller, K.M.; Silva, P.P.O.; Cavaglieri, L.R.; Rosa, C.A.R. Mycobiota and mycotoxins present in finished fish feeds from farms in the Rio de Janeiro State, Brazil. Int. Aquat. Res. 2013, 5, 3. [Google Scholar] [CrossRef]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Manes, J.; Ferrer, E. Natural occurrence of emerging Fusarium mycotoxins in feed and fish from aquaculture. J. Agric. Food Chem. 2014, 62, 12462–12470. [Google Scholar] [CrossRef]
- Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic fungi and natural co-occurrence of mycotoxins in rainbow trout (Oncorhynchus mykiss) feeds. Toxins 2015, 7, 4595–4609. [Google Scholar] [CrossRef]
- Marijani, E.; Wainaina, J.M.; Charo-Karisa, H.; Nzayisenga, L.; Munguti, J.; Gnonlonfin, G.J.B.; Kigadye, E.; Okoth, S. Mycoflora and mycotoxins in finished fish feed and feed ingredients from smallholder farms in East Africa. Egypt. J. Aquat. Res. 2017, 43, 169–176. [Google Scholar] [CrossRef]
- Mwihia, E.W.; Lyche, J.L.; Mbuthia, P.G.; Ivanova, L.; Uhlig, S.; Gathumbi, J.K.; Maina, J.G.; Eshitera, E.E.; Eriksen, G.S. Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya. Toxins 2020, 12, 627. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed. Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Thielecke, F.; Nugent, A.P. Contaminants in Grain-A Major Risk for Whole Grain Safety? Nutrients 2018, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to fork risk assessment of mycotoxins under climate change influences-recent developments. Trends Food Sci. Technol. 2021, 126, 126–141. [Google Scholar] [CrossRef]
- Richard-Forget, F.; Atanasova, V.; Chéreau, S. Using metabolomics to guide strategies to tackle the issue of the contamination of food and feed with mycotoxins: A review of the literature with specific focus on Fusarium mycotoxins. Food Control 2021, 121, 107610. [Google Scholar] [CrossRef]
- Wu, Q.; Kuča, K.; Humpf, H.-U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef]
- Hooft, J.M.; Elmor, A.E.H.I.; Encarnação, P.; Bureau, D.P. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture 2011, 311, 224–232. [Google Scholar] [CrossRef]
- Ryerse, I.A.; Hooft, J.M.; Bureau, D.P.; Hayes, M.A.; Lumsden, J.S. Purified deoxynivalenol or feed restriction reduces mortality in rainbow trout, Oncorhynchus mykiss (Walbaum), with experimental bacterial coldwater disease but biologically relevant concentrations of deoxynivalenol do not impair the growth of Flavobacterium psychrophilum. J. Fish Dis. 2015, 38, 809–819. [Google Scholar]
- Hooft, J.; Bureau, D. Evaluation of the efficacy of a commercial feed additive against the adverse effects of feed-borne deoxynivalenol (DON) on the performance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2017, 473, 237–245. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Navarro-Guillén, C.; Gilannejad, N.; Dias, J.; Schatzmayr, D.; Bichl, G.; Czabany, T.; Moyano, F.J.; Rema, P.; Yúfera, M.; et al. Impact of deoxynivalenol on rainbow trout: Growth performance, digestibility, key gene expression regulation and metabolism. Aquaculture 2018, 490, 362–372. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Menanteau-Ledouble, S.; Schöller, M.; Eder, A.; Schmidt-Posthaus, H.; Mackenzie, S.; El-Matbouli, M. Effects of deoxynivalenol exposure time and contamination levels on rainbow trout. J. World Aquac. Soc. 2019, 50, 137–154. [Google Scholar] [CrossRef]
- Hooft, J.M.; Ferreira, C.; Lumsden, J.S.; Sulyok, M.; Krska, R.; Bureau, D.P. The effects of naturally occurring or purified deoxynivalenol (DON) on growth performance, nutrient utilization and histopathology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 505, 319–332. [Google Scholar] [CrossRef]
- Hooft, J.M.; Wu, P.; Powell, C.D.; Lou, Y.; Squires, E.J.; Cant, J.P.; Sulyok, M.; Krska, R.; Bureau, D.P. A comparative investigation of the effects of feed-borne deoxynivalenol (DON) on growth performance, nutrient utilization and metabolism of detoxification in rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus) fed diets containing different levels of digestible carbohydrates. Aquaculture 2019, 505, 306–318. [Google Scholar]
- Hooft, J.M.; Bureau, D.P. Deoxynivalenol: Mechanisms of action and its effects on various terrestrial and aquatic species. Food Chem. Toxicol. 2021, 157, 112616. [Google Scholar] [CrossRef] [PubMed]
- Ryerse, I.A.; Hooft, J.M.; Bureau, D.P.; Anthony Hayes, M.; Lumsden, J.S. Diets containing corn naturally contaminated with deoxynivalenol reduces the susceptibility of rainbow trout (Oncorhynchus mykiss) to experimental Flavobacterium psychrophilum infection. Aquac. Res. 2016, 47, 787–796. [Google Scholar] [CrossRef]
- Pietsch, C.; Katzenback, B.A.; Garcia-Garcia, E.; Schulz, C.; Belosevic, M.; Burkhardt-Holm, P. Acute and subchronic effects on immune responses of carp (Cyprinus carpio L.) after exposure to deoxynivalenol (DON) in feed. Mycotoxin Res. 2015, 31, 151–164. [Google Scholar] [CrossRef]
- Pietsch, C.; Burkhardt-Holm, P. Feed-borne exposure to deoxynivalenol leads to acute and chronic effects on liver enzymes and histology in carp. World Mycotoxin J. 2015, 8, 619–627. [Google Scholar] [CrossRef]
- Serviento, A.M.; Brossard, L.; Renaudeau, D. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behavior in finishing pigs1. J. Anim. Sci. 2018, 96, 5209–5221. [Google Scholar] [CrossRef]
- Wellington, M.O.; Bosompem, M.A.; Petracek, R.; Nagl, V.; Columbus, D.A. Effect of long-term feeding of graded levels of deoxynivalenol (DON) on growth performance, nutrient utilization, and organ health in finishing pigs and DON content in biological samples. J. Anim. Sci. 2020, 98, skaa378. [Google Scholar] [CrossRef]
- Woodward, B.; Young, L.G.; Lun, A.K. Vomitoxin in diets for rainbow trout (Salmo gairdneri). Aquaculture 1983, 35, 93–101. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Beck, A.P. Fundamental concepts for semiquantitative tissue scoring in translational research. ILAR J. 2018, 59, 13–17. [Google Scholar] [CrossRef]
- Pietsch, C.; Schulz, C.; Rovira, P.; Kloas, W.; Burkhardt-Holm, P. Organ damage and hepatic lipid accumulation in carp (Cyprinus carpio L.) after feed-borne exposure to the Mycotoxin, Deoxynivalenol (DON). Toxins 2014, 6, 756–778. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Küchenmeister, U.; Jonas, L.; Kohlschein, P.; Pöhland, R.; Dänicke, S. Influence of diets with cereal grains contaminated by graded levels of two Fusarium toxins on selected enzymatic and histological parameters of liver in gilts. Food Chem. Toxicol. 2006, 44, 1228–1235. [Google Scholar] [CrossRef]
- Moldal, T.; Bernhoft, A.; Rosenlund, G.; Kaldhusdal, M.; Koppang, E.O. Dietary Deoxynivalenol (DON) may impair the epithelial barrier and modulate the cytokine signaling in the intestine of Atlantic Salmon (Salmo salar). Toxins 2018, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Girgis, G.N.; Barta, J.R.; Brash, M.; Smith, T.K. Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010, 54, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Hogasen, H.R.; Rosenlund, G.; Ivanova, L.; Berntssen, M.H.G.; Alexander, J.; Eriksen, G.S.; Faeste, C.K. Tissue distribution and elimination of deoxynivalenol and ochratoxin A in dietary-exposed Atlantic salmon (Salmo salar). Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1211–1224. [Google Scholar] [CrossRef]
- Matejova, I.; Vicenova, M.; Vojtek, L.; Kudlackova, H.; Nedbalcova, K.; Martin, F.; Šišperová, E.; Modrá, H.; Svobodova, Z. Effect of the mycotoxin deoxynivalenol on the immune responses of rainbow trout (Oncorhynchus mykiss). Veterinární Med. 2015, 60, 515–521. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Urán, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Obach, A.; Jensen, L.; Koppe, W.; Verreth, J.A.J. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac. Nutr. 2008, 14, 324–330. [Google Scholar] [CrossRef]
- Uran, P.A.; Goncalves, A.A.; Taverne-Thiele, J.J.; Schrama, J.W.; Verreth, J.A.; Rombout, J.H. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zheng, B. Summarizing the goodness of fit of generalized linear models for longitudinal data. Stat. Med. 2000, 19, 1265–1275. [Google Scholar] [CrossRef]
| Experimental Diets | |||||||
|---|---|---|---|---|---|---|---|
| Performance Parameters | CON | ND1 | ND2 | PD1 | PD2 | SEM | p-Value |
| Initial BW (g) | 8.0 | 8.0 | 7.4 | 8.2 | 8.1 | 0.21 | NS |
| Final BW (g) | 36.5 | 36.1 | 35.5 | 36.8 | 35.5 | 0.31 | NS |
| Growth (g/d) | 0.71 | 0.70 | 0.70 | 0.72 | 0.68 | 0.008 | NS |
| SGR (% BW/d) | 3.80 | 3.78 | 3.91 | 3.76 | 3.69 | 0.069 | NS |
| FCR | 0.68 | 0.70 | 0.70 | 0.69 | 0.71 | 0.008 | NS |
| HSI (%) | 4.4 | 4.4 | 4.7 | 4.2 | 3.8 | 0.36 | NS |
| Condition factor (K) | 2.1 | 2.0 | 1.8 | 2.0 | 1.9 | 0.09 | NS |
| Retained protein (g/fish) | 4.2 b | 4.0 ab | 3.9 a | 4.1 b | 3.8 a | 0.05 | ** |
| Protein retention efficiency (%) | 51.0 c | 49.1 ac | 48.1 ab | 50.4 bc | 47.1 a | 0.59 | ** |
| Retained energy (MJ/fish) | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.002 | NS |
| Energy retention efficiency (%) | 45.9 | 45.8 | 44.3 | 45.8 | 44.6 | 0.58 | NS |
| Experimental Diets | |||||||
|---|---|---|---|---|---|---|---|
| Performance Parameters | CON | ND1 | ND2 | PD1 | PD2 | SEM | p-Value |
| Initial BW (g) | 36.6 | 36.1 | 35.5 | 36.6 | 36.1 | 0.47 | NS |
| Final BW (g) | 67.8 a | 64.2 ab | 61.9 b | 65.1 ab | 63.1 ab | 1.06 | * |
| Feed intake (g/fish/d) | 1.71 | 1.72 | 1.58 | 1.71 | 1.67 | 0.045 | NS |
| Feed intake (g/kg0.8/d) | 18.9 | 19.5 | 18.9 | 19.2 | 19.2 | 0.48 | NS |
| Growth (g/d) | 2.09 a | 1.88 ab | 1.76 b | 1.90 ab | 1.80 b | 0.048 | ** |
| SGR (% BW/d) | 4.12 a | 3.84 ab | 3.70 b | 3.83 ab | 3.73 b | 0.064 | ** |
| FCR | 0.79 a | 0.86 ab | 0.85 ab | 0.84 ab | 0.89 b | 0.017 | * |
| HSI (%) | 3.9 | 3.4 | 3.8 | 3.0 | 3.0 | 0.40 | NS |
| Condition factor (K) | 2.0 | 1.9 | 1.9 | 1.9 | 2.0 | 0.07 | NS |
| Pathological Indicators | Experimental Diets | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| +Week | CON | ND2 | PD2 | SEM | Diet | Time | Diet × Time | R2 (Fish Effect) ‡ | |
| Vacuolization Score | |||||||||
| Glycogen # | 1 | 2.5 | 2.3 | 1.8 y | |||||
| 6 | 2.0 | 1.9 | 1.9 y | 0.19 | *** | NS | * | 0.42 (NA) | |
| 8 | 2.4 b | 2.0 b | 1.0 a,x | ||||||
| Lipid # | 1 | 1.6 | 1.8 | 1.9 | |||||
| 6 | 2.0 | 1.8 | 1.9 | 0.13 | NS | NS | NS | 0.16 (NA) | |
| 8 | 1.9 | 2.0 | 2.0 | ||||||
| Nuclei characteristics ++ | |||||||||
| Pyknotic (%) | 1 | 55 a | 95 b | 93 b | |||||
| 6 | 33 | 87 | 78 | *** | NS | NS | 0.36 (0.50) | ||
| 8 | 23 a | 72 b | 15 a | ||||||
| Pleomorphic (%) | 1 | 35 a | 82 b | 82 b | |||||
| 6 | 25 | 65 | 68 | * | NS | NS | 0.29 (0.51) | ||
| 8 | 18 a | 83 b | 17 a | ||||||
| Other indicators | |||||||||
| Necrosis (%) | 1 | 28 | 62 y | 37 x | |||||
| 6 | 25 | 22 x | 39 x | NS | NS | ** | 0.21 (0.22) | ||
| 8 | 18 a | 45 a, x, y | 93 b, y | ||||||
| Necrosis score # | 1 | 0.3 | 1.2 | 0.5 x | |||||
| 6 | 0.4 | 0.5 | 0.5 x | 0.20 | * | NS | * | 0.35 (NA) | |
| 8 | 0.2 a | 0.7 ab | 1.1 b y | ||||||
| Haemorrhage (%) | 1 | 12 a | 50 b y | 13 a | |||||
| 6 | 5 | 5 x | 12 | *** | NS | *** | 0.15 (0.04) | ||
| 8 | 3 a | 15 b x | 30 b | ||||||
| Inflammation (%) | 1 | 0 a | 2 a x | 13 b | |||||
| 6 | 3 a | 45 b y | 9 a | * | NS | * | 0.17 (0.16) | ||
| 8 | 5 | 22 y | 5 | ||||||
| Experimental Diets | |||||
|---|---|---|---|---|---|
| Ingredient (%) | CON | ND1 | ND2 | PD1 | PD2 |
| Wheat (no DON) | 40.00 | 18.00 | - | 40.00 | 40.00 |
| Wheat (DON contaminated) | - | 22.00 | 40.00 | - | - |
| Pure DON | - | - | - | 0.00009 | 0.00016 |
| LT fishmeal | 49.02 | 49.02 | 49.02 | 49.02 | 49.02 |
| Fish oil | 9.90 | 9.90 | 9.90 | 9.90 | 9.90 |
| Mineral and vitamin premix 1 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 |
| Analysed nutrient composition 2 (%) | |||||
| Dry Matter | 96.5 | 94.3 | 94.0 | 93.3 | 95.7 |
| Protein | 41.8 | 41.5 | 41.3 | 41.7 | 41.6 |
| Fat | 15.8 | 16.3 | 16.2 | 16.3 | 16.2 |
| Ash | 9.7 | 9.9 | 9.8 | 9.8 | 9.5 |
| Gross Energy (MJ/kg) | 21.62 | 21.60 | 21.64 | 21.62 | 21.57 |
| Mycotoxins concentration (µg/kg) 2 | |||||
| DON 3 | 70 | 679 | 1192 | 781 | 1566 |
| Enniatin A/A1 4 | 1.2 | 12.1 | 12.9 | - | - |
| Enniatin B/B1 4 | - | 8.6 | 19.9 | - | - |
| T2 Toxin | - | - | 3.8 | - | - |
| Ergotamin(in)e | 2.7 | 3.4 | - | 2.1 | - |
| Ergocryptin(in)e | 4.1 | - | - | - | - |
| Target Gene | Accession Number | Forward Primer Sequence 5’-3’ | Reverse Primer Sequence 5’-3’ |
|---|---|---|---|
| elf-1α | AF498320.1 | TCTACAAAATCGGCGGTA | CCTCAGTGGTGACATTAGC |
| il-1β | AJ557021.2 | CACCACCACCACCAAT | AAGAGGAAGCGAACCG |
| il-8 + | NM001124362.1 | TGTCAGCCAGCCTTG | ACATCCAGACAAATCTCCT |
| tnf-α | AJ277604.2 | GGCTGTGTGGGGTC | GCTTCAATGTATGGTGGG |
| tnf-α3 | HE798146.1 | TACCAAGAAACAAGATCACA | TCTGTCCACTCCACTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koletsi, P.; Wiegertjes, G.F.; Graat, E.A.M.; Lyons, P.; Schrama, J. Time- and Dose-Dependent Effects of Dietary Deoxynivalenol (DON) in Rainbow Trout (Oncorhynchus mykiss) at Organism and Tissue Level. Toxins 2022, 14, 810. https://doi.org/10.3390/toxins14110810
Koletsi P, Wiegertjes GF, Graat EAM, Lyons P, Schrama J. Time- and Dose-Dependent Effects of Dietary Deoxynivalenol (DON) in Rainbow Trout (Oncorhynchus mykiss) at Organism and Tissue Level. Toxins. 2022; 14(11):810. https://doi.org/10.3390/toxins14110810
Chicago/Turabian StyleKoletsi, Paraskevi, Geert F. Wiegertjes, Elisabeth A. M. Graat, Philip Lyons, and Johan Schrama. 2022. "Time- and Dose-Dependent Effects of Dietary Deoxynivalenol (DON) in Rainbow Trout (Oncorhynchus mykiss) at Organism and Tissue Level" Toxins 14, no. 11: 810. https://doi.org/10.3390/toxins14110810
APA StyleKoletsi, P., Wiegertjes, G. F., Graat, E. A. M., Lyons, P., & Schrama, J. (2022). Time- and Dose-Dependent Effects of Dietary Deoxynivalenol (DON) in Rainbow Trout (Oncorhynchus mykiss) at Organism and Tissue Level. Toxins, 14(11), 810. https://doi.org/10.3390/toxins14110810






