Bilateral Simultaneous Optic Neuritis Following Envenomations by Indian Cobra and Common Krait
Abstract
1. Introduction
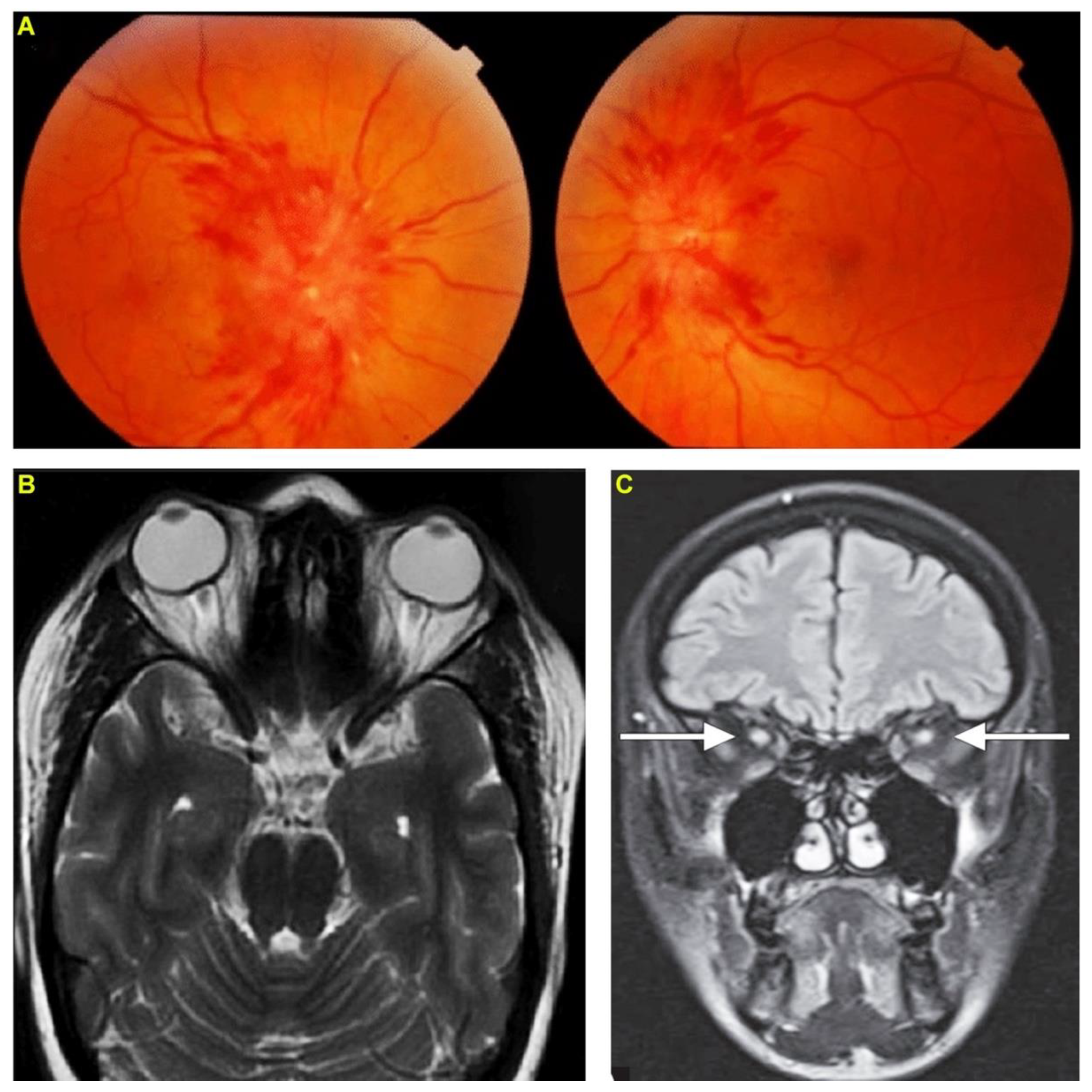
2. Case Report 1: Indian Cobra-Induced Optic Neuritis
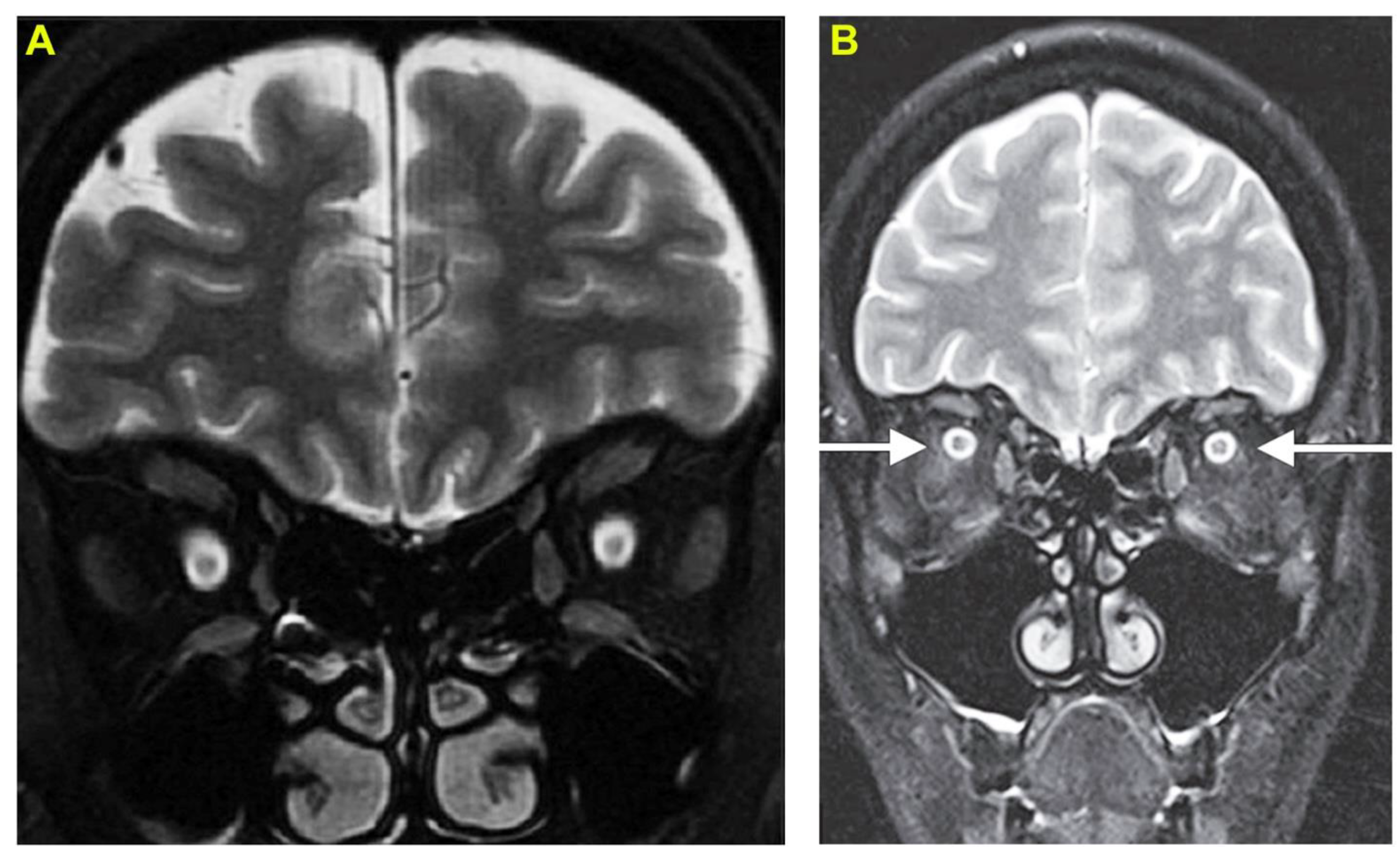
3. Case Report 2: Common Krait-Induced Optic Neuritis
4. Discussion
5. Conclusions
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; E Ray, S.; Ray, N.; A Warrell, D.; de Castañeda, R.R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Vaiyapuri, R.; Ashokan, R.; Ramasamy, K.; Nattamaisundar, K.; Jeyaraj, A.; Chandran, V.; Gajjeraman, P.; Baksh, M.F.; Gibbins, J.; et al. Snakebite and Its Socio-Economic Impact on the Rural Population of Tamil Nadu, India. PLoS ONE 2013, 8, e80090. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.F.; Vaiyapuri, R.; Gajjeraman, P.; Hutchinson, G.; Gibbins, J.M.; Bicknell, A.B.; Vaiyapuri, S. Challenges in diagnosing and treating snakebites in a rural population of Tamil Nadu, India: The views of clinicians. Toxicon 2017, 130, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, W.; Warrell, D.; Whitaker, R.; Menon, G.; Rodrigues, R.; Fu, S.H.; Begum, R.; Sati, P.; Piyasena, K.; Bhatia, M.; et al. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. eLife 2020, 9, e54076. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.P.; Chinnaraju, S.; Williams, H.F.; Pichamuthu, E.; Subharao, M.; Vaiyapuri, M.; Arumugam, S.; Vaiyapuri, R.; Baksh, M.F.; Patel, K.; et al. Venomous snakebites: Rapid action saves lives—A multifaceted community education programme increases awareness about snakes and snakebites among the rural population of Tamil Nadu, India. PLOS Negl. Trop. Dis. 2020, 14, e0008911. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Senthilkumaran, S.; Williams, H.F.; Patel, K.; Trim, S.A.; Thirumalaikolundusubramanian, P.; Vaiyapuri, S. Priapism following a juvenile Russell’s viper bite: An unusual case report. PLOS Negl. Trop. Dis. 2021, 15, e0009242. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, S.; Vijayakumar, P.; Savania, R.; Vaiyapuri, R.; Elangovan, N.; Patel, K.; Trim, S.A.; Thirumalaikolundusubramanian, P.; Vaiyapuri, S. Splenic rupture and subsequent splenectomy in a young healthy victim following Russell’s viper bite. Toxicon 2021, 204, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, S.; Miller, S.W.; Williams, H.F.; Savania, R.; Thirumalaikolundusubramanian, P.; Patel, K.; Vaiyapuri, S. Development of Wunderlich syndrome following a Russell’s viper bite. Toxicon 2022, 215, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, S.; Miller, S.W.; Williams, H.F.; Vaiyapuri, R.; Savania, R.; Elangovan, N.; Thirumalaikolundusubramanian, P.; Patel, K.; Vaiyapuri, S. Ultrasound-Guided Compression Method Effectively Counteracts Russell’s Viper Bite-Induced Pseudoaneurysm. Toxins 2022, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Arathisenthil, S.; Senthilkumaran, S.; Vijayakumar, P.; Savania, R.; Williams, H.F.; Elangovan, N.; Bicknell, A.B.; Patel, K.; Trim, S.A.; Thirumalaikolundusubramanian, P.; et al. Rapid development of a salivary calculus in submandibular gland and its potential causes in a young victim following Russell’s viper bite. Toxicon 2021, 206, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L. Optic Neuritis. Contin. Lifelong Learn. Neurol. 2019, 25, 1236–1264. [Google Scholar] [CrossRef]
- Chang, K.-C.; Huang, Y.-K.; Chen, Y.-W.; Chen, M.-H.; Tu, A.T.; Chen, Y.-C. Venom Ophthalmia and Ocular Complications Caused by Snake Venom. Toxins 2020, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Aye, M.T.H.; Naing, T.; Myint, K.T. Unusual ocular manifestations following viper bite. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.S.; Ranjan, R.; Manayath, G.J. Transient central retinal artery occlusion following viperine snake bite. Can. J. Ophthalmol. 2017, 52, e205–e208. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.R.; Rao, N.A. Anterior Segment Ischemia in Viper Bite. Ocul. Immunol. Inflamm. 2014, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Selhorst, J.B.; Chen, Y. The Optic Nerve. Skull Base 2009, 29, 029–035. [Google Scholar] [CrossRef]
- Osborne, B.J.; Volpe, N.J. Optic neuritis and risk of MS: Differential diagnosis and management. Clevel. Clin. J. Med. 2009, 76, 181–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of Snake Venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Tandon, R.; Sharma, T.; Gupta, A. Optic neuritis following snake bite. Indian J. Ophthalmol. 1997, 45, 236. [Google Scholar] [PubMed]
- Dhabhar, J.; Mehta, V.; Desai, N. Optic Neuritis After a Snakebite: A Diagnostic Dilemma. Ochsner J. 2020, 21, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Schwersenski, J.; Beatty, D.W. Unusual features in a case of snakebite, presumably due to a Cape cobra (Naja nigricollis). S. Afr. Med. J. 1982, 61, 597–598. [Google Scholar] [PubMed]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.; Johnston, C.; Siribaddana, S.; Isbister, G.K. Neuromuscular Effects of Common Krait (Bungarus caeruleus) Envenoming in Sri Lanka. PLOS Negl. Trop. Dis. 2016, 10, e0004368. [Google Scholar] [CrossRef] [PubMed]
- Troutman, W.G.; Wilson, L.E. Topical ophthalmic exposure to rattlesnake venom. Am. J. Emerg. Med. 1989, 7, 307–308. [Google Scholar] [CrossRef]
- Cantrell, F.L.; Barry, D.J.; Breckenridge, H. Ocular Exposure to Rattlesnake Venom. J. Toxicol. Clin. Toxicol. 2003, 41, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Aundhakar, S.C.; Mahajan, S.; Mane, M.B.; Arsekar, S.S. Bilateral optic neuritis following Ressell’s Viper’s bite-a rare complication. Indian J. Forensic Med. Toxicol. 2012, 6, 178–180. [Google Scholar]
- Guttmann-Friedmann, A. BLINDNESS AFTER SNAKE-BITE. Br. J. Ophthalmol. 1956, 40, 57–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.-C.; Yang, C.-M.; Hu, F.-R.; Lee, Y.-C. Penetrating Ocular Injury Caused by Venomous Snakebite. Am. J. Ophthalmol. 2005, 140, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Brandão, O.; De Bastos, H.C.; Nishioka, S.D.A.; Silveira, P.V.P. Lance-headed viper (Bothrops moojeni) bite wounding the eye. Revista do Inst. de Med. Trop. de São Paulo 1993, 35, 381–383. [Google Scholar] [CrossRef]
- Ryan, R.Y.M.; Seymour, J.; Loukas, A.; Lopez, J.A.; Ikonomopoulou, M.P.; Miles, J.J. Immunological Responses to Envenomation. Front. Immunol. 2021, 12, 661082. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA2, a Promising Target for Broad-Spectrum Antivenom Drug Development. BioMed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef]
- Sharma, S.; Ray, B.; Bhardwaj, D.; Dwivedi, A.K.; Roy, T.S. Age changes in the human oculomotor nerve—A stereological study. Ann. Anat.-Anat. Anz. 2009, 191, 260–266. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthilkumaran, S.; Miller, S.W.; Williams, H.F.; Thirumalaikolundusubramanian, P.; Patel, K.; Vaiyapuri, S. Bilateral Simultaneous Optic Neuritis Following Envenomations by Indian Cobra and Common Krait. Toxins 2022, 14, 805. https://doi.org/10.3390/toxins14110805
Senthilkumaran S, Miller SW, Williams HF, Thirumalaikolundusubramanian P, Patel K, Vaiyapuri S. Bilateral Simultaneous Optic Neuritis Following Envenomations by Indian Cobra and Common Krait. Toxins. 2022; 14(11):805. https://doi.org/10.3390/toxins14110805
Chicago/Turabian StyleSenthilkumaran, Subramanian, Stephen W. Miller, Harry F. Williams, Ponniah Thirumalaikolundusubramanian, Ketan Patel, and Sakthivel Vaiyapuri. 2022. "Bilateral Simultaneous Optic Neuritis Following Envenomations by Indian Cobra and Common Krait" Toxins 14, no. 11: 805. https://doi.org/10.3390/toxins14110805
APA StyleSenthilkumaran, S., Miller, S. W., Williams, H. F., Thirumalaikolundusubramanian, P., Patel, K., & Vaiyapuri, S. (2022). Bilateral Simultaneous Optic Neuritis Following Envenomations by Indian Cobra and Common Krait. Toxins, 14(11), 805. https://doi.org/10.3390/toxins14110805






