Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia
Abstract
1. Introduction
“We must go deeper into greater pain; for it is not permitted that we stay”from “The Divine Comedy” by Dante Alighieri
2. Cylindrospermopsin (CYN)
3. Inhibitors of Protein Cleaving Enzymes
3.1. Protein Digestion of Daphnia
3.2. Carboxypeptidase (CXP) Inhibitors: Anabaenopeptins
3.3. Protease Inhibitors (PIs)
4. Anatoxin-a (ATX)
5. Microcystins (MCY)
6. Control of Toxic Cyanobacterial Blooms by Daphnia?
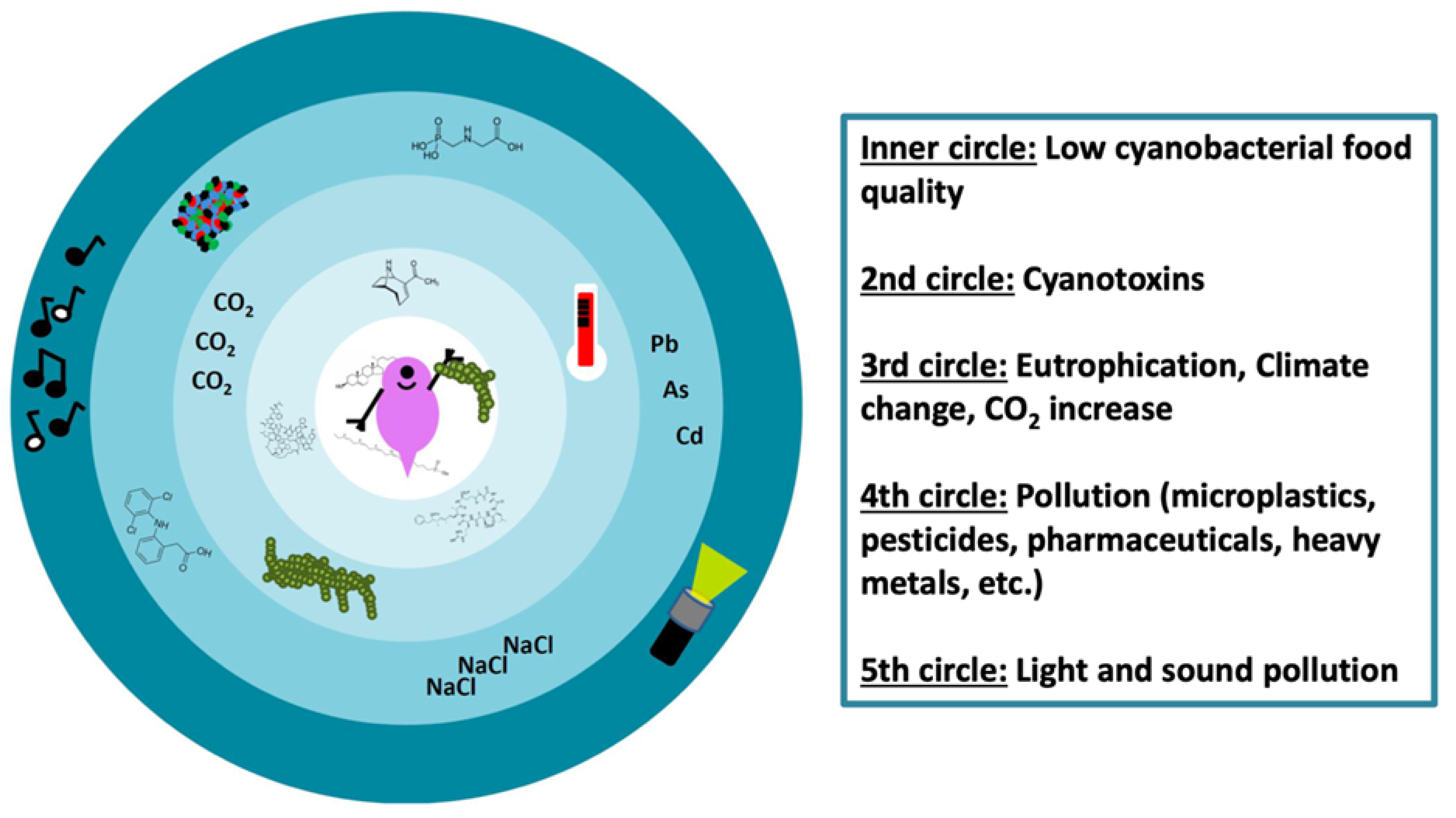
7. Outlook: Daphnia–Cyanobacteria Interactions in the Anthropocene
7.1. Anthropogenic Temperature Increase
7.2. Anthropogenic Light and Sound Pollution
7.3. Anthropogenic CO2 Increase
7.4. Anthropogenic Pollutants
7.5. Combined (Natural and/or Anthropogenic) Stressors
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mello, M.O.; Silva-Filho, M.C. Plant-insect interactions: An evolutionary arms race between two distinct defense mechanisms. Braz. J. Plant Physiol. 2002, 14, 71–81. [Google Scholar] [CrossRef]
- Liess, A.; Hillebrand, H. Invited review: Direct and indirect effects in herbivore-periphyton interactions. Arch. Hydrobiol. 2004, 159, 433–453. [Google Scholar] [CrossRef]
- Kerfoot, W.C.; Levitan, C.; DeMott, W.R. Daphnia-phytoplankton interactions: Density-dependent shifts in resource quality. Ecology 1988, 69, 1806–1825. [Google Scholar] [CrossRef]
- Peter, H.; Lampert, W. The effect of Daphnia body size on filtering rate inhibition in the presence of a filamentous cyanobacterium. Limnol. Oceanogr. 1989, 34, 1084–1089. [Google Scholar] [CrossRef]
- Bednarska, A.; Dawidowicz, P. Change in filter-screen morphology and depth selection: Uncoupled responses of Daphnia to the presence of filamentous cyanobacteria. Limnol. Oceanogr. 2007, 52, 2358–2363. [Google Scholar] [CrossRef]
- Arnold, D.E. Ingestion, assimilation, survival, and reproduction by Daphnia pulex fed seven species of blue-green algae1,2. Limnol. Oceanogr. 1971, 16, 906–920. [Google Scholar] [CrossRef]
- DeMott, W.R. Foraging strategies and growth inhibition in five daphnids feeding on mixtures of a toxic cyanobacterium and a green alga. Freshw. Biol. 1999, 42, 263–274. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Kuster, C.J.; Von Elert, E. Molecular mechanisms of tolerance to cyanobacterial protease inhibitors revealed by clonal differences in Daphnia magna. Mol. Ecol. 2012, 21, 4898–4911. [Google Scholar] [CrossRef]
- Lürling, M. Daphnia growth on microcystin-producing and microcystin-free Microcystis aeruginosa in different mixtures with the green alga Scenedesmus obliquus. Limnol. Oceanogr. 2003, 48, 2214–2220. [Google Scholar] [CrossRef]
- Lukas, M.; Wacker, A. Constraints by oxygen and food quality on carbon pathway regulation: A colimitation study with an aquatic key herbivore. Ecology 2014, 95, 3068–3079. [Google Scholar] [CrossRef]
- Threlkeld, S.T. The midsummer dynamics of two Daphnia species in Wintergreen Lake, Michigan. Ecology 1979, 60, 165–179. [Google Scholar] [CrossRef]
- Ghadouani, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshw. Biol. 2003, 48, 363–381. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Gustafsson, S.; Rengefors, K.; Bomark, L. Cyanobacterial chemical warfare affects zooplankton community composition. Freshw. Biol. 2007, 52, 1290–1301. [Google Scholar] [CrossRef]
- Baumann, H.I.; Jüttner, F. Inter-annual stability of oligopeptide patterns of Planktothrix rubescens blooms and mass mortality of Daphnia in Lake Hallwilersee. Limnologica 2008, 38, 350–359. [Google Scholar] [CrossRef]
- Porter, K.G.; McDonough, R. The energetic cost of response to blue-green algal filaments by cladocerans1. Limnol. Oceanogr. 1984, 29, 365–369. [Google Scholar] [CrossRef]
- Haney, J.F.; Forsyth, D.J.; James, M.R. Inhibition of zooplankton filtering rates by dissolved inhibitors produced by naturally occurring cyanobacteria. Arch. Hydrobiol. 1994, 132, 1–13. [Google Scholar] [CrossRef]
- Koch, U.; Martin-Creuzburg, D.; Grossart, H.-P.; Straile, D. Single dietary amino acids control resting egg production and affect population growth of a key freshwater herbivore. Oecologia 2011, 167, 981–989. [Google Scholar] [CrossRef]
- Fink, P.; Pflitsch, C.; Marin, K. Dietary essential amino acids affect the reproduction of the keystone herbivore Daphnia pulex. PLoS ONE 2011, 6, e28498. [Google Scholar] [CrossRef]
- Von Elert, E.; Wolffrom, T. Supplementation of cyanobacterial food with polyunsaturated fatty acids does not improve growth of Daphnia. Limnol. Oceanogr. 2001, 46, 1552–1558. [Google Scholar] [CrossRef]
- Von Elert, E.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. R. Soc. B Boil. Sci. 2003, 270, 1209–1214. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Von Elert, E. Impact of 10 dietary sterols on growth and reproduction of Daphnia galeata. J. Chem. Ecol. 2004, 30, 483–500. [Google Scholar] [CrossRef]
- DeMott, W.R.; Zhang, Q.-X.; Carmichael, W.W. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol. Oceanogr. 1991, 36, 1346–1357. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gademann, K.; Portmann, C. Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Kalinowska, R.; Skowroński, T. Cyanotoxin diversity and food web bioaccumulation in a reservoir with decreasing phosphorus concentrations and perennial cyanobacterial blooms. Harmful Algae 2013, 28, 118–125. [Google Scholar] [CrossRef]
- Park, H.K.; Jheong, W.H.; Kwon, O.S.; Ryu, J.K. Seasonal succession of toxic cyanobacteria and microcystins concentration in Paldang reservoir. Algae 2000, 15, 29–35. [Google Scholar]
- Messineo, V.; Melchiorre, S.; Di Corcia, A.; Gallo, P.; Bruno, M. Seasonal succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, Central Italy. Environ. Toxicol. 2009, 25, 18–27. [Google Scholar] [CrossRef]
- Sadler, T.; Kuster, C.; von Elert, E. Seasonal dynamics of chemotypes in a freshwater phytoplankton community—A metabolomic approach. Harmful Algae 2014, 39, 102–111. [Google Scholar] [CrossRef]
- Sidelev, S.I.; Korneva, L.; Solovyeva, V.V.; Zubishina, A.A.; Pligin, D.N. Molecular genetic identification and seasonal succession of toxigenic cyanobacteria in phytoplankton of the Rybinsk Reservoir (Russia). Inland Water Biol. 2016, 9, 368–374. [Google Scholar] [CrossRef]
- Woodhouse, J.N.; Kinsela, A.S.; Collins, R.; Bowling, L.C.; Honeyman, G.L.; Holliday, J.K.; Neilan, B.A. Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J. 2015, 10, 1337–1351. [Google Scholar] [CrossRef]
- Von Elert, E.; Oberer, L.; Merkel, P.; Huhn, T.; Blom, J.F. Cyanopeptolin 954, a chlorine-containing chymotrypsin inhibitor of Microcystis aeruginosa NIVA Cya 43. J. Nat. Prod. 2005, 68, 1324–1327. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Sadler, T.; Von Elert, E. Effect of nutrient limitation of cyanobacteria on protease inhibitor production and fitness of Daphnia magna. J. Exp. Biol. 2013, 216, 3649–3655. [Google Scholar] [CrossRef]
- Kohler, E.; Grundler, V.; Häussinger, D.; Kurmayer, R.; Gademann, K.; Pernthaler, J.; Blom, J.F. The toxicity and enzyme activity of a chlorine and sulfate containing aeruginosin isolated from a non-microcystin-producing Planktothrix strain. Harmful Algae 2014, 39, 154–160. [Google Scholar] [CrossRef]
- Entfellner, E.; Frei, M.; Christiansen, G.; Deng, L.; Blom, J.; Kurmayer, R. Evolution of anabaenopeptin peptide structural variability in the cyanobacterium Planktothrix. Front. Microbiol. 2017, 8, 219. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; De Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Hairston, N.G.; Lampert, W.; Cáceres, C.E.; Holtmeier, C.L.; Weider, L.J.; Gaedke, U.; Fischer, J.M.; Fox, J.A.; Post, D.M. Rapid evolution revealed by dormant eggs. Nature 1999, 401, 446. [Google Scholar] [CrossRef]
- Sarnelle, O.; Wilson, A.E. Local adaptation of Daphnia pulicaria to toxic cyanobacteria. Limnol. Oceanogr. 2005, 50, 1565–1570. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Experimental immunology First report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 4, 314–317. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Puerto, M.; Prieto, A.I.; Jos, Á.; Pichardo, S.; Vasconcelos, V.; Cameán, A.M. Protective role of dietary N-acetylcysteine on the oxidative stress induced by cylindrospermopsin in tilapia (Oreochromis niloticus). Environ. Toxicol. Chem. 2012, 31, 1548–1555. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I. Cylindrospermopsin genotoxicity and cytotoxicity: Role of Cytochrome P-450 and oxidative Stress. J. Toxicol. Environ. Health Part A 2005, 68, 739–753. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Chong, M.; Wong, B.; Lam, P.; Shaw, G.; Seawright, A. Toxicity and uptake mechanism of cylindrospermopsin and lophyrotomin in primary rat hepatocytes. Toxicon 2001, 40, 205–211. [Google Scholar] [CrossRef]
- Nogueira, I.C.; Lobo-Da-Cunha, A.; Vasconcelos, V.M. Effects of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum (cyanobacteria) ingestion on Daphnia magna midgut and associated diverticula epithelium. Aquat. Toxicol. 2006, 80, 194–203. [Google Scholar] [CrossRef]
- Nogueira, I.C.G.; Saker, M.L.; Pflugmacher, S.; Wiegand, C. Toxicity of the cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environ. Toxicol. 2004, 19, 453–459. [Google Scholar] [CrossRef]
- Hasler, A.D. The physiology of digestion of plankton crustacea, I: Some digestive enzymes of Daphnia. Biol. Bull. 1935, 68, 207–214. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing II. Digestive enzyme activity in Daphnia magna exposed to sublethal concentrations of cadmium, chromium and mercury. Chemosphere 1997, 35, 1053–1067. [Google Scholar] [CrossRef]
- Agrawal, M.K.; Zitt, A.; Bagchi, D.; Weckesser, J.; Bagchi, S.N.; von Elert, E. Characterization of proteases in guts of Daphnia magna and their inhibition by Microcystis aeruginosa PCC 7806. Environ. Toxicol. 2005, 20, 314–322. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Fink, P. Gene expression and activity of digestive enzymes of Daphnia pulex in response to food quality differences. Comp. Biochem. Physiol. Part B 2018, 218, 23–29. [Google Scholar] [CrossRef]
- Cremer, R.; Wacker, A.; Schwarzenberger, A. More light please: Daphnia benefit from light pollution by increased tolerance toward cyanobacterial chymotrypsin inhibitors. Front. Ecol. Evol. 2022, 10, 834422. [Google Scholar] [CrossRef]
- Von Elert, E.; Agrawal, M.K.; Gebauer, C.; Jaensch, H.; Bauer, U.; Zitt, A. Protease activity in gut of Daphnia magna: Evidence for trypsin and chymotrypsin enzymes. Comp. Biochem. Physiol. Part B 2004, 137, 287–296. [Google Scholar] [CrossRef]
- Dölling, R.; Becker, D.; Hawat, S.; Koch, M.; Schwarzenberger, A.; Zeis, B. Adjustments of serine proteases of Daphnia pulex in response to temperature changes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 194, 1–10. [Google Scholar] [CrossRef]
- Schwerin, S.; Zeis, B.; Lamkemeyer, T.; Paul, R.J.; Koch, M.; Madlung, J.; Fladerer, C.; Pirow, R. Acclimatory responses of the Daphnia pulex proteome to environmental changes. II. Chronic exposure to different temperatures (10 and 20 °C) mainly affects protein metabolism. BMC Physiol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Zitt, A.; Kroth, P.; Mueller, S.; Von Elert, E. Gene expression and activity of digestive proteases in Daphnia: Effects of cyanobacterial protease inhibitors. BMC Physiol. 2010, 10, 6. [Google Scholar] [CrossRef]
- Itou, Y.; Suzuki, S.; Ishida, K.; Murakami, M. Anabaenopeptins G and H, Potent Carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-595). Bioorg. Med. Chem. Lett. 1999, 9, 1243–1246. [Google Scholar] [CrossRef]
- Murakami, M.; Suzuki, S.; Itou, Y.; Kodani, S.; Ishida, K. New Anabaenopeptins, Potent Carboxypeptidase—A Inhibitors from the Cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000, 63, 1280–1282. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.-L. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Kurmayer, R.; Martin-Creuzburg, D. Toward disentangling the multiple nutritional constraints imposed by Planktothrix: The significance of harmful secondary metabolites and sterol limitation. Front. Microbiol. 2020, 11, 586120. [Google Scholar] [CrossRef]
- Köcher, S.; Resch, S.; Kessenbrock, T.; Schrapp, L.; Ehrmann, M.; Kaiser, M. From dolastatin 13 to cyanopeptolins, micropeptins, and lyngbyastatins: The chemical biology of Ahp-cyclodepsipeptides. Nat. Prod. Rep. 2019, 37, 163–174. [Google Scholar] [CrossRef]
- Jakobi, C.; Rinehart, K.L.; Neuber, R.; Mez, K.; Weckesser, J. Cyanopeptolin SS, a disulphated depsipeptide from a water bloom: Structural elucidation and biological activities. Phycologia 1996, 35, 111–116. [Google Scholar] [CrossRef]
- Carmichael, W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Agrawal, M.K.; Bagchi, D.; Bagchi, S.N. Acute inhibition of protease and suppression of growth in zooplankter, Moina macrocopa, by Microcystis blooms collected in Central India. Hydrobiologia 2001, 464, 37–44. [Google Scholar] [CrossRef]
- Czarnecki, O.; Henning, M.; Lippert, I.; Welker, M. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ. Microbiol. 2006, 8, 77–87. [Google Scholar] [CrossRef]
- Kuster, C.J.; Schwarzenberger, A.; von Elert, E. Seasonal dynamics of sestonic protease inhibition: Impact on Daphnia populations. Hydrobiologia 2012, 715, 37–50. [Google Scholar] [CrossRef]
- Weckesser, J.; Martin, C.; Jakobi, C. Cyanopeptolins, depsipeptides from cyanobacteria. Syst. Appl. Microbiol. 1996, 19, 133–138. [Google Scholar] [CrossRef]
- Martin, C.; Oberer, L.; Ino, T.; König, W.A.; Busch, M.; Weckesser, J. Cyanopeptolins, new depsipeptides from the cyanobacterium Microcystis sp. pcc 7806. J. Antibiot. 1993, 46, 1550–1556. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Friberg-Jensen, U. Frequency of inhibitors of daphnid trypsin in the widely distributed cyanobacterial genus Planktothrix. Environ. Microbiol. 2005, 7, 1667–1669. [Google Scholar] [CrossRef]
- Dittmann, E.; Neilan, B.A.; Erhard, M.; Von Döhren, H.; Börner, T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 1997, 26, 779–787. [Google Scholar] [CrossRef]
- Burberg, C.; Ilić, M.; Petzoldt, T.; von Elert, E. Nitrate determines growth and protease inhibitor content of the cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2018, 31, 1697–1707. [Google Scholar] [CrossRef]
- Von Elert, E.; Zitt, A.; Schwarzenberger, A. Inducible tolerance to dietary protease inhibitors in Daphnia magna. J. Exp. Biol. 2012, 215, 2051–2059. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; D’Hondt, S.; Vyverman, W.; von Elert, E. Seasonal succession of cyanobacterial protease inhibitors and Daphnia magna genotypes in a eutrophic Swedish lake. Aquat. Sci. 2013, 75, 433–445. [Google Scholar] [CrossRef]
- Asselman, J.; De Coninck, D.I.M.; Glaholt, S.; Colbourne, J.K.; Janssen, C.R.; Shaw, J.R.; De Schamphelaere, K.A.C. Identification of Pathways, gene networks, and paralogous gene families in Daphnia pulex responding to exposure to the toxic cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2012, 46, 8448–8457. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Sadler, T.; Motameny, S.; Ben-Khalifa, K.; Frommolt, P.; Altmüller, J.; Konrad, K.; Von Elert, E. Deciphering the genetic basis of microcystin tolerance. BMC Genom. 2014, 15, 776. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Von Elert, E. Cyanobacterial protease inhibitors lead to maternal transfer of increased protease gene expression in Daphnia. Oecologia 2012, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.F.; Baumann, H.I.; Codd, G.A.; Jüttner, F. Sensitivity and adaptation of aquatic organisms to oscillapeptin J and [D-Asp3,(E)-Dhb7]microcystin-RR. Arch. Hydrobiol. 2006, 167, 547–559. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Hasselmann, M.; Elert, E. Positive selection of digestive proteases in Daphnia: A mechanism for local adaptation to cyanobacterial protease inhibitors. Mol. Ecol. 2020, 29, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Keith, N.R.; Jackson, C.E.; Von Elert, E. Copy number variation of a protease gene of Daphnia: Its role in population tolerance. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2017, 327, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Ilić, M.; Von Elert, E. Daphnia populations are similar but not identical in tolerance to different protease inhibitors. Harmful Algae 2021, 106, 102062. [Google Scholar] [CrossRef]
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Kim, W. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. Breb. Int. Ver. Theor. Angew. Limnol. 1964, 15, 1964. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef]
- Rellán, S.; Osswald, J.; Saker, M.; Gago-Martinez, A.; Vasconcelos, V. First detection of anatoxin-a in human and animal dietary supplements containing cyanobacteria. Food Chem. Toxicol. 2009, 47, 2189–2195. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. 2001, 20, 1228–1235. [Google Scholar] [CrossRef]
- Fawell, J.K.; E Mitchell, R.; E Hill, R.; Everett, D.J. The toxicity of cyanobacterial toxins in the mouse: II Anatoxin-a. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef]
- Bumke-Vogt, C.; Mailahn, W.; Chorus, I. Anatoxin-a and neurotoxic cyanobacteria in German lakes and reservoirs. Environ. Toxicol. 1999, 14, 117–125. [Google Scholar] [CrossRef]
- Osswald, J.; Carvalho, A.; Claro, J.; Vasconcelos, V. Effects of cyanobacterial extracts containing anatoxin-a and of pure anatoxin-a on early developmental stages of carp. Ecotoxicol. Environ. Saf. 2009, 72, 473–478. [Google Scholar] [CrossRef]
- Cerasino, L.; Salmaso, N. Diversity and distribution of cyanobacterial toxins in the Italian subalpine lacustrine district. Oceanol. Hydrobiol. Stud. 2012, 41, 54–63. [Google Scholar] [CrossRef]
- Shams, S.; Capelli, C.; Cerasino, L.; Ballot, A.; Dietrich, D.; Sivonen, K.; Salmaso, N. Anatoxin-a producing Tychonema (Cyanobacteria) in European waterbodies. Water Res. 2015, 69, 68–79. [Google Scholar] [CrossRef]
- Sánchez, K.F.; Huntley, N.; Duffy, M.A.; Hunter, M.D. Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system. Proc. R. Soc. B Boil. Sci. 2019, 286, 20182231. [Google Scholar] [CrossRef]
- Claska, M.E.; Gilbert, J.J. The effect of temperature on the response of Daphnia to toxic cyanobacteria. Freshw. Biol. 1998, 39, 221–232. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Lentz, M.; Wiedner, C. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 2010, 56, 964–971. [Google Scholar] [CrossRef]
- Osswald, J.; Azevedo, J.; Vasconcelos, V.; Guilhermino, L. Experimental determination of the bioconcentration factors for anatoxin-a in juvenile rain-bow trout (Oncorhynchus mykiss). Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 77–86. [Google Scholar]
- Schwarzenberger, A.; Martin-Creuzburg, D. Daphnia’s adaptive molecular responses to the cyanobacterial neurotoxin anatoxin-α are maternally transferred. Toxins 2021, 13, 326. [Google Scholar] [CrossRef]
- De Abreu, F.Q.; Ferrão-Filho, A.D.S. Effects of an anatoxin-a(s)-producing strain of Anabaena spiroides (cyanobacteria) on the survivorship and somatic growth of two Daphnia similis clones. J. Environ. Prot. 2013, 4, 12–18. [Google Scholar] [CrossRef]
- Bownik, A.; Pawlik-Skowrońska, B. Early indicators of behavioral and physiological disturbances in Daphnia magna (Cladocera) induced by cyanobacterial neurotoxin anatoxin-a. Sci. Total Environ. 2019, 695, 133913. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, S.M.; Pflugmacher, S.; James, K.J.; Furey, A. Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat. Toxicol. 2004, 68, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 2021. [Google Scholar] [CrossRef]
- Rohrlack, T.; Dittmann, E.; Henning, M.; Börner, T.; Kohl, J.-G. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 1999, 65, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Ghadouani, A.; Pinel-Alloul, B.; Plath, K.; Codd, G.A.; Lampert, W. Effects of Microcystis aeruginosa and purified microcystin-LR on the feeding behavior of Daphnia pulicaria. Limnol. Oceanogr. 2004, 49, 666–679. [Google Scholar] [CrossRef]
- Hietala, J.; Reinikainen, M.; Walls, M. Variation in life history responses of Daphnia to toxic Microcystis aeruginosa. J. Plankton Res. 1995, 17, 2307–2318. [Google Scholar] [CrossRef]
- Semyalo, R.; Rohrlack, T.; Larsson, P. Growth and survival responses of a tropical Daphnia (Daphnia lumholtzi) to cell-bound microcystins. J. Plankton Res. 2009, 31, 827–835. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Dittmann, E.; Nogueira, I.; Vasconcelos, V.; Börner, T. Ingestion of microcystins by Daphnia: Intestinal uptake and toxic effects. Limnol. Oceanogr. 2005, 50, 440–448. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Bownik, A. Cyanobacterial anabaenopeptin-B, microcystins and their mixture cause toxic effects on the behavior of the freshwater crustacean Daphnia magna (Cladocera). Toxicon 2021, 198, 1–11. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. et Biophys. Acta (BBA) Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Chen, W.; Song, L.; Ou, D.; Gan, N. Chronic toxicity and responses of several important enzymes in Daphnia magna on exposure to sublethal microcystin-LR. Environ. Toxicol. 2005, 20, 323–330. [Google Scholar] [CrossRef]
- Gustafsson, S.; Rengefors, K.; Hansson, L.-A. Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 2005, 86, 2561–2567. [Google Scholar] [CrossRef]
- Radersma, R.; Hegg, A.; Noble, D.W.A.; Uller, T. Timing of maternal exposure to toxic cyanobacteria and offspring fitness in Daphnia magna: Implications for the evolution of anticipatory maternal effects. Ecol. Evol. 2018, 8, 12727–12736. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, X. Offspring tolerance to toxic Microcystis aeruginosa in Daphnia pulex shaped by maternal food availability and age. Fundam. Appl. Limnol. 2014, 185, 315–319. [Google Scholar] [CrossRef]
- Lemaire, V.; Brusciotti, S.; van Gremberghe, I.; Vyverman, W.; Vanoverbeke, J.; De Meester, L. Genotype × genotype interactions between the toxic cyanobacterium Microcystis and its grazer, the waterflea Daphnia. Evol. Appl. 2011, 5, 168–182. [Google Scholar] [CrossRef]
- Drugă, B.; Turko, P.; Spaak, P.; Pomati, F. Cyanobacteria affect fitness and genetic structure of experimental Daphnia populations. Environ. Sci. Technol. 2016, 50, 3416–3424. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Liang, H.; Li, Q.; Zhao, Y.; Chen, L.; Yang, W. Resistance variation within a Daphnia pulex population against toxic cyanobacteria. J. Plankton Res. 2013, 35, 1177–1181. [Google Scholar] [CrossRef]
- Wilson, A.E.; Sarnelle, O.; Tillmanns, A.R. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta-analyses of laboratory experiments. Limnol. Oceanogr. 2006, 51, 1915–1924. [Google Scholar] [CrossRef]
- Isanta-Navarro, J.; Hairston, N.G.; Beninde, J.; Meyer, A.; Straile, D.; Möst, M.; Martin-Creuzburg, D. Reversed evolution of grazer resistance to cyanobacteria. Nat. Commun. 2021, 12, 1945. [Google Scholar] [CrossRef]
- Lyu, K.; Gu, L.; Wang, H.; Zhu, X.; Zhang, L.; Sun, Y.; Huang, Y.; Yang, Z. Transcriptomic analysis dissects the mechanistic insight into the Daphnia clonal variation in tolerance to toxic Microcystis. Limnol. Oceanogr. 2018, 64, 272–283. [Google Scholar] [CrossRef]
- Lyu, K.; Meng, Q.; Zhu, X.; Dai, D.; Zhang, L.; Huang, Y.; Yang, Z. Changes in iTRAQ-based proteomic profiling of the cladoceran Daphnia magna exposed to microcystin-producing and microcystin-free Microcystis aeruginosa. Environ. Sci. Technol. 2016, 50, 4798–4807. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Simmons, D.B.; Sibley, P.K. Shotgun proteomics analysis reveals sub-lethal effects in Daphnia magna exposed to cell-bound microcystins produced by Microcystis aeruginosa. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100656. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Gu, L.; Li, B.; Lu, Y.; Wu, C.; Guan, H.; Yang, Z. Stress-responsive expression of a glutathione S-transferase (delta) gene in waterflea Daphnia magna challenged by microcystin-producing and microcystin-free Microcystis aeruginosa. Harmful Algae 2016, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, R.; Dao, T.S.; Wiegand, C. Transgenerational effects of microcystin-LR on Daphnia magna. J. Exp. Biol. 2012, 215, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.; von Elert, E. Dietary exposure of Daphnia to microcystins: No in vivo relevance of biotransformation. Aquat. Toxicol. 2014, 150, 73–82. [Google Scholar] [CrossRef]
- Asselman, J.; De Coninck, D.I.; Beert, E.; Janssen, C.R.; Orsini, L.; Pfrender, M.E.; Decaestecker, E.; De Schamphelaere, K.A. Bisulfite sequencing with Daphnia highlights a role for epigenetics in regulating stress response to Microcystis through preferential differential methylation of serine and threonine amino acids. Environ. Sci. Technol. 2016, 51, 924–931. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Courts, C.; Von Elert, E. Target gene approaches: Gene expression in Daphnia magna exposed to predator-borne kairomones or to microcystin-producing and microcystin-free Microcystis aeruginosa. BMC Genom. 2009, 10, 527. [Google Scholar] [CrossRef]
- Macke, E.; Callens, M.; De Meester, L.; Decaestecker, E. Host-genotype dependent gut microbiota drives zooplankton tolerance to toxic cyanobacteria. Nat. Commun. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Macke, E.; Callens, M.; Massol, F.; Vanoverberghe, I.; De Meester, L.; Decaestecker, E. Diet and genotype of an aquatic invertebrate affect the composition of free-living microbial communities. Front. Microbiol. 2020, 11, 380. [Google Scholar] [CrossRef]
- Sarnelle, O.; Gustafsson, S.; Hansson, L.-A. Effects of cyanobacteria on fitness components of the herbivore Daphnia. J. Plankton Res. 2010, 32, 471–477. [Google Scholar] [CrossRef]
- Chislock, M.F.; Sarnelle, O.; Olsen, B.K.; Doster, E.; Wilson, A.E. Large effects of consumer offense on ecosystem structure and function. Ecology 2013, 94, 2375–2380. [Google Scholar] [CrossRef]
- Paterson, M.J.; Findlay, D.L.; Salki, A.G.; Hendzel, L.L.; Hesslein, R.H. The effects of Daphnia on nutrient stoichiometry and filamentous cyanobacteria: A mesocosm experiment in a eutrophic lake. Freshw. Biol. 2002, 47, 1217–1233. [Google Scholar] [CrossRef]
- Vanni, M.J.; Temte, J. Seasonal patterns of grazing and nutrient limitation of phytoplankton in a eutrophic lake. Limnol. Oceanogr. 1990, 35, 697–709. [Google Scholar] [CrossRef]
- Gągała, I.; Izydorczyk, K.; Jurczak, T.; Pawełczyk, J.; Dziadek, J.; Wojtal-Frankiewicz, A.; Jóźwik, A.; Jaskulska, A.; Mankiewicz-Boczek, J. Role of environmental factors and toxic genotypes in the regulation of microcystins-producing cyanobacterial blooms. Microb. Ecol. 2013, 67, 465–479. [Google Scholar] [CrossRef]
- Sarnelle, O. Initial conditions mediate the interaction between Daphnia and bloom-forming cyanobacteria. Limnol. Oceanogr. 2007, 52, 2120–2127. [Google Scholar] [CrossRef]
- Christoffersen, K.; Riemann, B.; Klysner, A.; Søndergaard, M. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnol. Oceanogr. 1993, 38, 561–573. [Google Scholar] [CrossRef]
- Akbar, S.; Huang, J.; Zhou, Q.; Gu, L.; Sun, Y.; Zhang, L.; Lyu, K.; Yang, Z. Elevated temperature and toxic Microcystis reduce Daphnia fitness and modulate gut microbiota. Environ. Pollut. 2020, 271, 116409. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Effects of commercially available ultrasound on the zooplankton grazer Daphnia and consequent water greening in laboratory experiments. Water 2014, 6, 3247–3263. [Google Scholar] [CrossRef]
- Fu, F.; Place, A.; Garcia, N.; Hutchins, D. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010, 59, 55–65. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Bosker, T.; Olthof, G.; Vijver, M.G.; Baas, J.; Barmentlo, S.H. Significant decline of Daphnia magna population biomass due to microplastic exposure. Environ. Pollut. 2019, 250, 669–675. [Google Scholar] [CrossRef]
- Trotter, B.; Wilde, M.V.; Brehm, J.; Dafni, E.; Aliu, A.; Arnold, G.J.; Fröhlich, T.; Laforsch, C. Long-term exposure of Daphnia magna to polystyrene microplastic (PS-MP) leads to alterations of the proteome, morphology and life-history. Sci. Total Environ. 2021, 795, 148822. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, size, and polymer dependent effects of microplastics on Daphnia magna. J. Hazard. Mater. 2022, 426, 128136. [Google Scholar] [CrossRef] [PubMed]
- Hoffschröer, N.; Grassl, N.; Steinmetz, A.; Sziegoleit, L.; Koch, M.; Zeis, B. Microplastic burden in Daphnia is aggravated by elevated temperatures. Zoology 2020, 144, 125881. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.E.; Brunner, F.S.; Plaistow, S.J. Temperature and clone-dependent effects of microplastics on immunity and life history in Daphnia magna. Environ. Pollut. 2019, 255, 113178. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.A.; Arnott, S.E.; Fournier, I.B.; Schamp, B.S. Effects of chloride and nutrients on freshwater plankton communities. Limnol. Oceanogr. Lett. 2021; Early View. [Google Scholar] [CrossRef]
- Isanta-Navarro, J.; Arnott, S.E.; Klauschies, T.; Martin-Creuzburg, D. Dietary lipid quality mediates salt tolerance of a freshwater keystone herbivore. Sci. Total Environ. 2021, 769, 144657. [Google Scholar] [CrossRef]
- Coldsnow, K.D.; Relyea, R.A.; Hurley, J.M. Evolution to environmental contamination ablates the circadian clock of an aquatic sentinel species. Ecol. Evol. 2017, 7, 10339–10349. [Google Scholar] [CrossRef]
- Dietrich, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in Daphnia magna–A multigenerational study. Chemosphere 2010, 79, 60–66. [Google Scholar] [CrossRef]
- López-Valcárcel, M.E.; Parra, G.; del Arco, A. Environmental disturbance history undermines population responses to cope with anthropogenic and environmental stressors. Chemosphere 2020, 262, 128373. [Google Scholar] [CrossRef]
- Perry, T.; Heckel, D.G.; McKenzie, J.A.; Batterham, P. Mutations in Dα1 or Dβ2 nicotinic acetylcholine receptor subunits can confer resistance to neonicotinoids in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008, 38, 520–528. [Google Scholar] [CrossRef]
- Klauschies, T.; Isanta-Navarro, J. The joint effects of salt and 6PPD contamination on a freshwater herbivore. Sci. Total Environ. 2022, 829, 154675. [Google Scholar] [CrossRef]
- Martins, A.; da Silva, D.D.; Silva, R.; Carvalho, F.; Guilhermino, L. Warmer water, high light intensity, lithium and microplastics: Dangerous environmental combinations to zooplankton and Global Health? Sci. Total Environ. 2022, 854, 158649. [Google Scholar] [CrossRef]
- Fernandez-Figueroa, E.G.; Wilson, A.E. Local adaptation mediates direct and indirect effects of multiple stressors on consumer fitness. Oecologia 2022, 198, 483–492. [Google Scholar] [CrossRef]
- Orsini, L.; Gilbert, D.; Podicheti, R.; Jansen, M.; Brown, J.B.; Solari, O.S.; Spanier, K.I.; Colbourne, J.; Rusch, D.B.; Decaestecker, E.; et al. Daphnia magna transcriptome by RNA-Seq across 12 environmental stressors. Sci. Data 2016, 3, 160030. [Google Scholar] [CrossRef]
- De Coninck, D.I.M.; Asselman, J.; Glaholt, S.; Janssen, C.R.; Colbourne, J.K.; Shaw, J.R.; De Schamphelaere, K.A.C. Genome-Wide Transcription Profiles Reveal Genotype-Dependent Responses of Biological Pathways and Gene-Families in Daphnia Exposed to Single and Mixed Stressors. Environ. Sci. Technol. 2014, 48, 3513–3522. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total. Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
| Toxin Effects | Adaptations of Daphnia | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Growth | Fecundity | Ingestion | Oxygen Consumption | Heart Rate | Behaviour | Uptake in Tissues | Enzymes | Microbiome | Genes | Maternal Effects | Local Adaptation | |||
| Toxin types | Cylindro- spermopsin | x | x | x | x (changes in GST activity (51)) | ||||||||||
| Carboxypeptidase inhibitors | x (Inhibition of CXP) | x (changes in CXP expression (65)) | |||||||||||||
| Protease inhibitors | x | x | x | x | x (inhibition of proteases) | x (change in protease activity & band patterns (60, 77); protease isoforms with higher IC50 values (8)) | x (changes in gene expression (8, 60, 65, 79, 80); copy number variation (84); positive selection (83)) | x (81) | x (82–85) | ||||||
| Microcystins | x | x | x | x | x | x (inhibition of PPI and IIa) | x (effect on GST (124) and malate dehydrogenase (124)) | x (128) | x (expression changes in molecular pathways (79, 80, 120-122); epigenetic effects (126) change in GST (123), UBQ (127), oxidative stress (79) and transporter gene expression (65, 80, 125)) | x (112–114) | x (40, 119) | ||||
| Anatoxin-a | x | x | x | x | x | x | x (change in NAR gene expression (99)) | x (99) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarzenberger, A. Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia. Toxins 2022, 14, 770. https://doi.org/10.3390/toxins14110770
Schwarzenberger A. Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia. Toxins. 2022; 14(11):770. https://doi.org/10.3390/toxins14110770
Chicago/Turabian StyleSchwarzenberger, Anke. 2022. "Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia" Toxins 14, no. 11: 770. https://doi.org/10.3390/toxins14110770
APA StyleSchwarzenberger, A. (2022). Negative Effects of Cyanotoxins and Adaptative Responses of Daphnia. Toxins, 14(11), 770. https://doi.org/10.3390/toxins14110770





