An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy
Abstract
1. Introduction
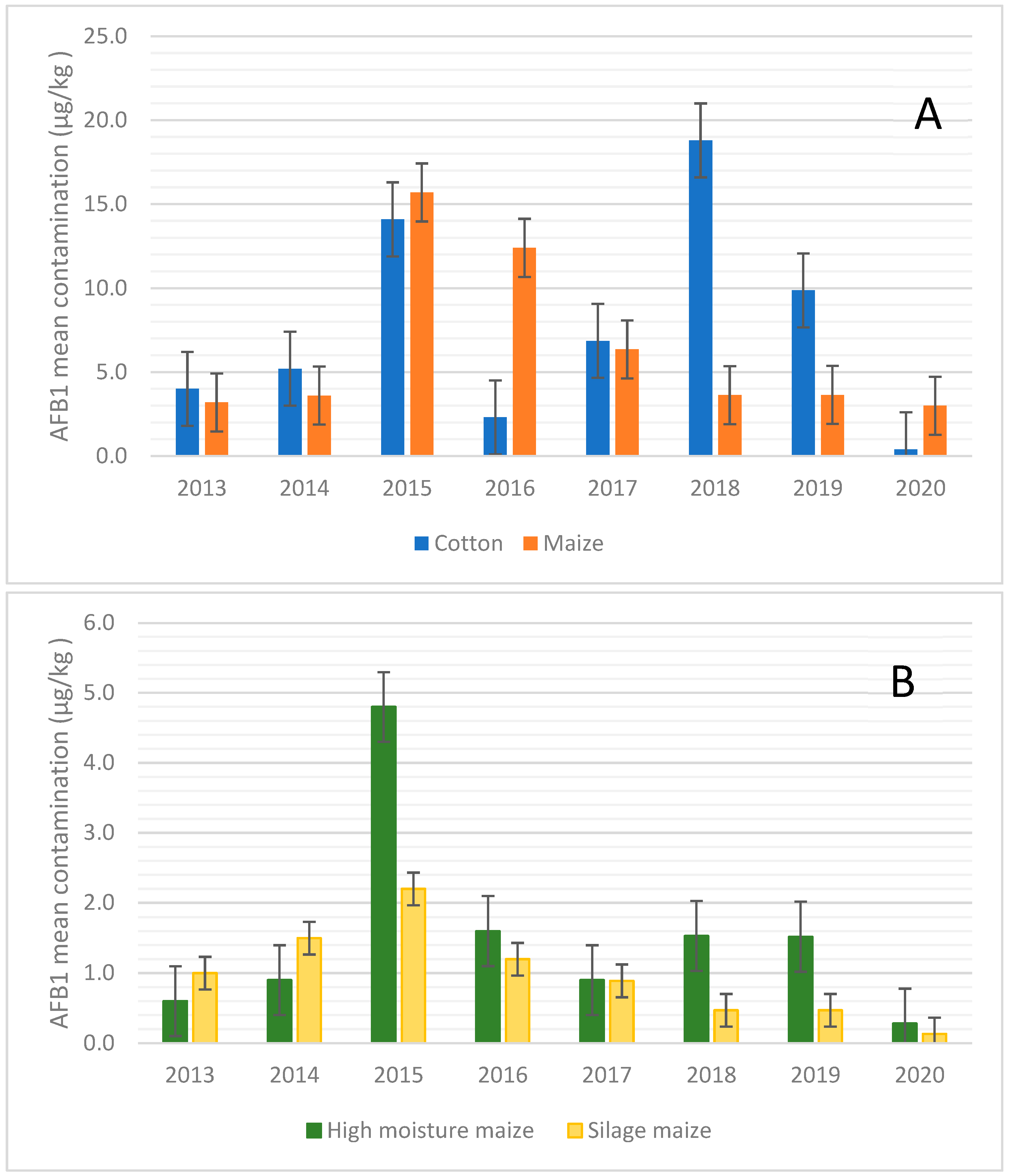
2. Results
3. Discussion
3.1. AFB1 Occurrence in Feed in Northern Italy
3.2. AFB1 Occurrence in Feed in Northern Italy: Year-to-Year Variation
3.2.1. Cotton
3.2.2. Maize, Maize Silage, and High-Moisture Maize
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Samples and Sample Preparation
5.3. Enzyme-Linked Immunosorbent Assay (ELISA) Procedure
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated mycotoxin management system in the feed supply chain: Innovative approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ferrari, L.; Rovere, N.; Fumagalli, F.; Mazzoleni, S.; Cheli, F. Advances in Understanding Key Contamination Risks in Animal Feed: Mycotoxins; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Cheli, F.; Fumagalli, F.; Ottoboni, M.; Pinotti, L. Safety of Cereals in the Mediterranean: An Update on EU Legislation. In Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Bailly, S.; El Mahgubi, A.; Carvajal-Campos, A.; Lorber, S.; Puel, O.; Oswald, I.P.; Bailly, J.D.; Orlando, B. Occurrence and Identification of Aspergillus Section Flavi in the Context of the Emergence of Aflatoxins in French Maize. Toxins 2018, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Improving Public Health through Mycotoxin Control; IARC Scientific Publication No. 158; IARC: Lyon, France, 2012. [Google Scholar]
- European Food Safety Authority (EFSA). Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040–6151. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Madhusudhanan, N.; Kavithalakshmi, S.N.; Shanmugasundaram, E.R.B.; Shanmugasundaram, K.R. Aflatoxin B1-Induced DNA Damage in Labeo rohita: Protective Effect of an Antioxidant Supplement, Amrita Bindu. Basic Clin. Pharmacol. Toxicol. 2006, 98, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Ross, R.K.; Yuan, J.M.; Yu, M.C.; Wogan, G.N.; Qian, G.S.; Tu, J.T.; Groopman, J.D.; Gao, Y.T.; Henderson, B.E. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992, 339, 943–946. [Google Scholar] [CrossRef]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef]
- Jiang, Y.; Jolly, P.E.; Ellis, W.O.; Wang, J.S.; Phillips, T.D.; Williams, J.H. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int. Immunol. 2005, 17, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Jurišić, N.; Schwartz-Zimmermann, H.E.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of aflatoxin biomarkers in excreta and ileal content of chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Bryden, W.L. Mycotoxin contamination of feed supply chain: Implication for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Diaz, G.J.; Calabrese, E.; Blain, R. Aflatoxicosis in Chickens (Gallus gallus): An Example of Hormesis? Poult. Sci. 2008, 87, 727–732. [Google Scholar] [CrossRef]
- Fouad, M.A.; Ruan, D.; El-Senousey, K.H.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin B1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef]
- Harvey, R.B.; Huff, W.E.; Kubena, L.F.; Corrier, D.E.; Phillips, T.D. Progression of aflatoxicosis in growing barrows. Am. J. Vet. Res. 1988, 49, 482–487. [Google Scholar]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Doerr, J.A. Mycotoxin interactions in poultry and swine. J. Anim. Sci. 1988, 66, 2351–2355. [Google Scholar] [CrossRef][Green Version]
- Southern, L.; Clawson, A. Effects of aflatoxins on finishing swine. J. Anim. Sci. 1979, 49, 1006–1011. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feed. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Britzi, M.; Friedman, S.; Miron, J.; Salomon, R.; Cuneah, O.; Shomisoni, J.A.; Soback, S.; Ashkenazi, R.; Armer, S.; Shlosberg, A. Carry-Over of Aflatoxin B1 to Aflatoxin M1 in High Yielding Israeli Cows in Mid- and Late-Lactation. Toxins 2013, 5, 173–183. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal By products. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Slizewska, K.; Biernasiak, J. Mycotoxin in cereal and soybean-based food and feed. In Soybean-Pest Resistance; El-Shemy, H.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 3565–3782. [Google Scholar]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, K.; Hu, X.; Liu, Z.; Wu, Y.; Huang, C. Genome-wide association analysis of forage quality in maize mature stalk. BMC Plant Biol. 2016, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, S.; Scarpino, V.; Lanzanova, C.; Romano, E.; Reyneri, A. Multi-mycotoxin long-term monitoring survey on North-italian maize over an 11-year period (2011–2021): The co-occurrence of regulated, masked and emerging mycotoxins and fungal metabolites. Toxins 2022, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- National Association of Italian Veterinary Doctors (ANMVI). Available online: https://www.anmvioggi.it/in-evidenza/63300-procedure-straordinarie-contro-il-rischio-aflatossine.html (accessed on 18 July 2022).
- Roila, R.; Branciari, R.; Verdini, E.; Ranucci, D.; Valiani, A.; Pelliccia, A.; Fioroni, L.; Pecorelli, I. A Study of the Occurrence of Aflatoxin M1 in Milk Supply Chain over a Seven-Year Period (2014–2020): Human Exposure Assessment and Risk Characterization in the Population of Central Italy. Foods 2021, 10, 1529. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Graziani, G.; Gaspari, A.; Ferrer, E.; Mañes, J.; Ritieni, A. Mycotoxin Occurrence and Risk Assessment in Gluten-Free Pasta through UHPLC-Q-Exactive Orbitrap MS. Toxins 2021, 13, 305. [Google Scholar] [CrossRef]
- Armorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Occurrence of aflatoxin B1 in conventional and organic flour in Italy and the role of sampling. Food Control 2015, 50, 858–863. [Google Scholar] [CrossRef]
- Canestrari, G.; Ricci, B.; Pizzamiglio, V.; Biancardi, A.; Piazza, P.; Merialdi, G.; Tosi, G.; Giacometti, F.; Nocetti, M.; Fustini, M.; et al. Aflatoxin B1 risk management in Parmigiano Reggiano dairy cow feed. Ital. J. Food Saf. 2016, 5, 5291–5293. [Google Scholar] [CrossRef]
- Decastelli, L.; Lai, J.; Gramaglia, M.; Monaco, A.; Nachtmann, C.; Oldano, F.; Ruffier, M.; Sezian, A.; Bandirola, C. Aflatoxins occurrence in milk and feed in Northern Italy during 2004–2005. Food Control 2007, 18, 1263–1266. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feestuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.; Logrieco, A.F.; Graeber, M.; Hirschberger, M.; Reichel, M.; Lippolis, V.; de Girolamo, A.; Lattanzio, V.M.T.; Slettengren, K. Aflatoxin Reduction in Maize by Industrial-Scale Cleaning Solutions. Toxins 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Prod. Process. Nutr. 2021, 3, 17–25. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Hassoun, P.; Brossard, L.; Bastianelli, D.; Lebas, F. Cotton Seeds. Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/742 (accessed on 27 September 2022).
- Jaime-Garcia, R.; Cotty, P.J. Aflatoxin Contamination of Commercial Cottonseed in South Texas. Phytopathology 2003, 93, 1190–1200. [Google Scholar] [CrossRef]
- Shar, Z.H.; Pirkash, O.; Shar, H.H.; Sherazi, S.T.H.; Mahesar, S.A. Aflatoxins in cotton seeds and cotton seed cake from Pakistan. Food Addit. Contam. Part B 2020, 13, 72–76. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/254292/global-corn-production-by-country (accessed on 18 July 2022).
- Dell’Orto, V.; Baldi, G.; Cheli, F. Mycotoxins in silage: Checkpoints for effective management and control. World Mycotoxin J. 2015, 8, 603–617. [Google Scholar] [CrossRef]
- Vandicke, J.; de Visschere, K.; Ameye, M.; Croubels, S.; de Saeger, S.; Audenaert, K.; Haesaert, G. Multi-mycotoxin contamination of maize silages in Flanders, Belgium: Monitoring mycotoxin levels from seed to feed. Toxins 2021, 13, 202. [Google Scholar] [CrossRef]
- Ognunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Segvić Klarić, M.; Cvetnić, Z.; Pepeljnjak, S.; Kosalec, I. Co-occurrence of aflatoxins, ochratoxin A, fumonisins, and zearalenone in cereals and feed, determined by competitive direct enzyme-linked immunosorbent assay and thin-layer chromatography. Arh. Hig. Rada Toksikol. 2009, 60, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twaruzek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Alvarado, A.M.; Zamora-Sanabria, R.; Granados-Chinchilla, F. A Focus on Aflatoxins in Feedstuffs: Levels of Contamination, Prevalence, Control Strategies, and Impacts on Animal Health. In Aflatoxin-Control, Analysis, Detection and Health Risks, 1st ed.; InTech Open: London, UK, 2017; p. 290. [Google Scholar]
- Battilani, P.; Toscano, P.; van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328–24334. [Google Scholar] [CrossRef]
- Piva, G.; Battilani, P.; Pietri, A. Emerging issues in Southern Europe: Aflatoxins in Italy. In The Mycotoxin Factbook. Food & Feed Topics; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 139–153. [Google Scholar]
- Diffenbaugh, N.S.; Giorgi, F.; Pal, J.S. Climate change hotspots in the United States. Geophys. Res. Lett. 2008, 35, L16709–L16713. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Barbano, C.; Scandolara, A.; Bertuzzi, T.; Marocco, A. Logistic regression modelling of cropping systems to predict fumonisin contamination in maize. J. Agric. Food Chem. 2008, 56, 10433–10438. [Google Scholar] [CrossRef]
- Medina, A.; González-Jartín, J.M.; Sainz, M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017, 18, 76–81. [Google Scholar] [CrossRef]
- Cheli, F. Mycotoxin Contamination Management Tools and Efficient Strategies in Feed Industry. Toxins 2020, 12, 480. [Google Scholar] [CrossRef]
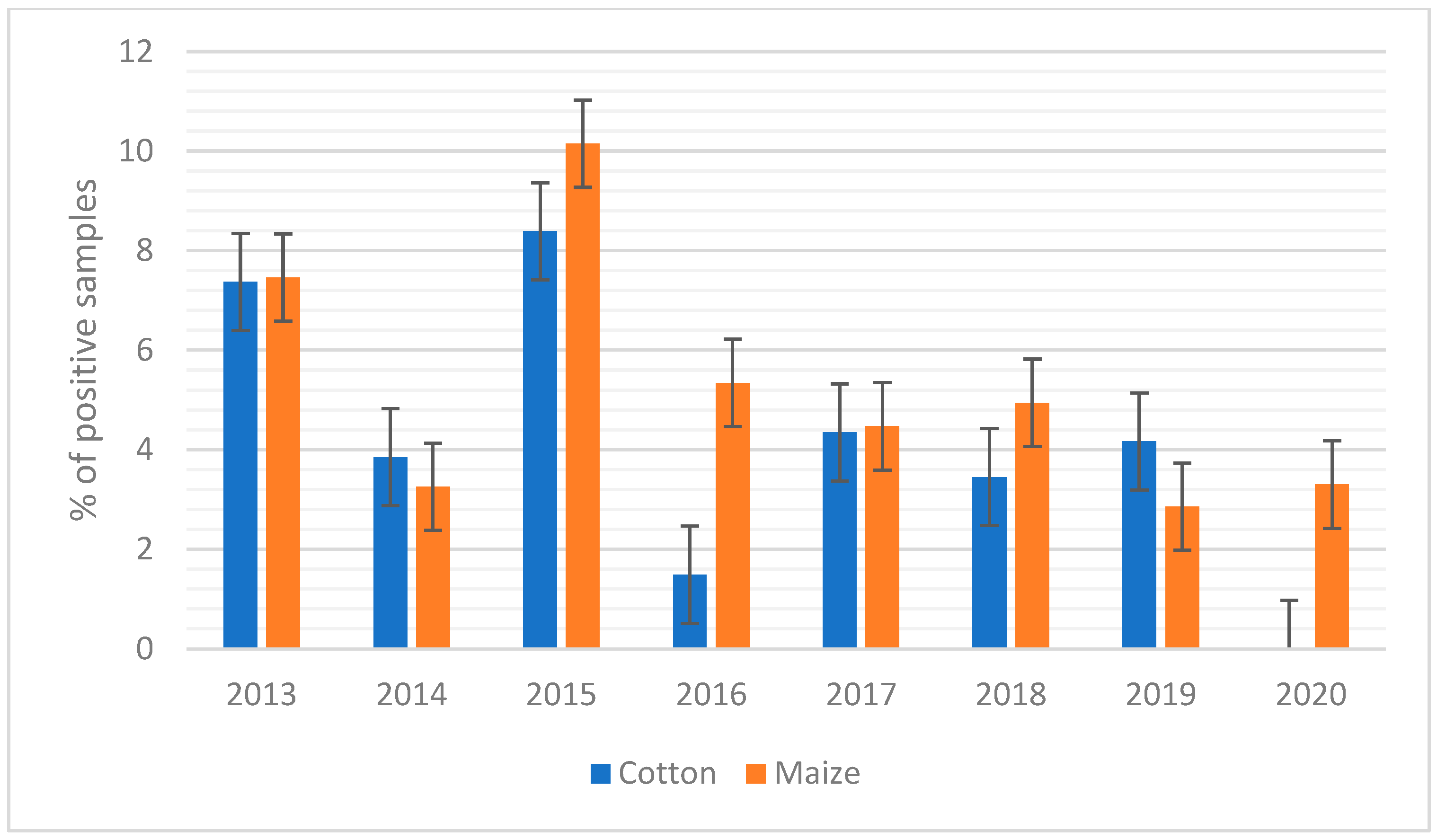
| Positive Samples | |||||||
|---|---|---|---|---|---|---|---|
| n 1 | n 2 | n 2 (%) | n 3 | n 3 (%) | Mean Contamination (µg/kg) | Maximum Contamination (µg/kg) | |
| Cotton | 480 | 55 | 11.5 | 26 | 5.4 | 8.3 | 728.7 |
| Wheat | 52 | 0 | 0 | 0 | 0 | <5 | / |
| Maize (flour and grain) | 5278 | 1256 | 23.8 | 534 | 10.1 | 9.1 | 1074 |
| Alfalfa hay | 19 | 1 | 5.3 | 0 | 0 | <5 | / |
| Silage maize | 2003 | 64 | 3.2 | 6 | 0.3 | <5 | 35.2 |
| High-moisture maize | 1364 | 122 | 8.9 | 19 | 1.4 | <5 | 200 |
| Soy | 64 | 3 | 4.7 | 1 | 1.6 | <5 | >20 |
| Unifeed | 293 | 7 | 2.4 | 1 | 0.3 | <5 | >20 |
| Cotton | Wheat | Maize | Alfalfa | HMM | SM | Unifeed | Soy |
|---|---|---|---|---|---|---|---|
| 480 | 52 | 5278 | 19 | 1364 | 2003 | 293 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vailati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins 2022, 14, 763. https://doi.org/10.3390/toxins14110763
Ferrari L, Fumagalli F, Rizzi N, Grandi E, Vailati S, Manoni M, Ottoboni M, Cheli F, Pinotti L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins. 2022; 14(11):763. https://doi.org/10.3390/toxins14110763
Chicago/Turabian StyleFerrari, Luca, Francesca Fumagalli, Nicoletta Rizzi, Elisa Grandi, Serena Vailati, Michele Manoni, Matteo Ottoboni, Federica Cheli, and Luciano Pinotti. 2022. "An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy" Toxins 14, no. 11: 763. https://doi.org/10.3390/toxins14110763
APA StyleFerrari, L., Fumagalli, F., Rizzi, N., Grandi, E., Vailati, S., Manoni, M., Ottoboni, M., Cheli, F., & Pinotti, L. (2022). An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins, 14(11), 763. https://doi.org/10.3390/toxins14110763







