Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters
Abstract
1. Introduction
2. Results
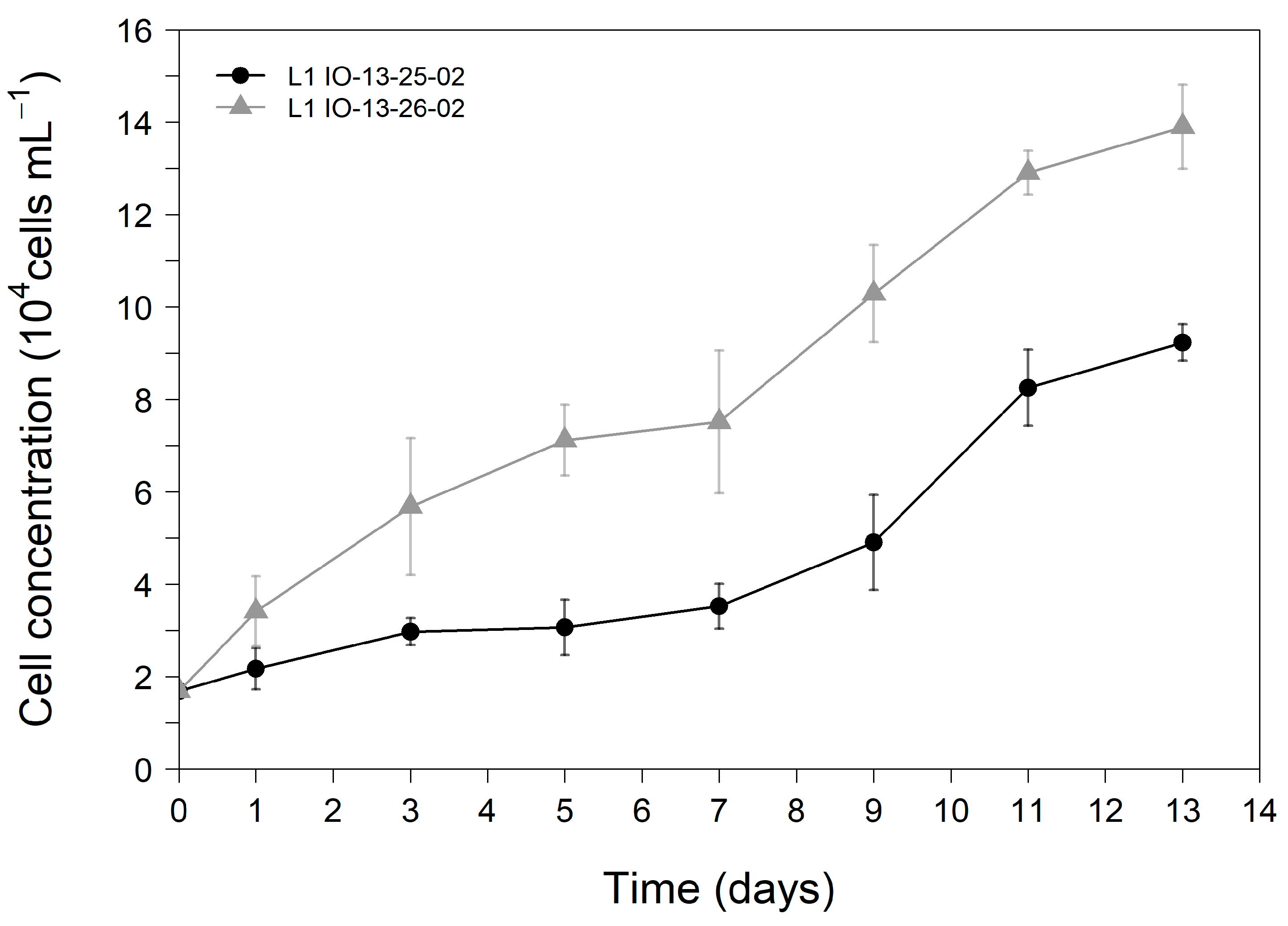
2.1. Growth Characterization of G. catenatum Cultures
2.2. Determination of PST Profile
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling and Isolation Procedures
5.2. Culture Growth Conditions
5.3. Determination of PST
5.3.1. Harvest and Extraction
5.3.2. Solid-Phase Extraction (SPE) Procedure
5.3.3. Analysis by HPLC-FLD with Pre-Column Oxidation
5.3.4. Toxins Identification and Quantification
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, J.; Kubota, T. Bioactive Metabolites from Marine Dinoflagellates. Compr. Nat. Prod. II Chem. Biol. 2010, 2, 263–325. [Google Scholar] [CrossRef]
- Assunção, J.; Guedes, A.; Malcata, F. Biotechnological and Pharmacological Applications of Biotoxins and Other Bioactive Molecules from Dinoflagellates. Mar. Drugs 2017, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Caeiro, M.F.; Costa, P.R.; Amorim, A. Gymnodinium Catenatum Graham Isolated from the Portuguese Coast: Toxin Content and Genetic Characterization. Harmful Algae 2015, 48, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gu, H.; Krock, B.; Luo, Z.; Zhang, Y. Toxic Dinoflagellate Blooms of Gymnodinium Catenatum and Their Cysts in Taiwan Strait and Their Relationship to Global Populations. Harmful Algae 2020, 97, 101868. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin Profile of Gymnodinium Catenatum (Dinophyceae) from the Portuguese Coast, as Determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Bustillos-Guzmán, J.; Morquecho, L.; Gárate-Lizárraga, I.; Alonso-Rodríguez, R.; Reyes-Salinas, A.; Erler, K.; Luckas, B. Variations of PSP Toxin Profiles during Different Growth Phases in Gymnodinium Catenatum (Dinophyceae) Strains Isolated from Three Locations in the Gulf of California, Mexico. J. Phycol. 2006, 42, 757–768. [Google Scholar] [CrossRef]
- Negri, A.P.; Bolch, C.J.S.; Geier, S.; Green, D.H.; Park, T.-G.; Blackburn, S.I. Widespread Presence of Hy-drophobic Paralytic Shellfish Toxins in Gymnodinium Catenatum. Harmful Algae 2007, 6, 774–780. [Google Scholar] [CrossRef]
- Negri, A.P.; Bolch, C.J.S.; Blackburn, S.I.; Dickman, M.; Llewellyn, L.E.; Mendez, S. Paralytic shellfish toxins in Gymnodinium catenatum strains from six countries. In Harmful Algal Blooms 2000, Proceedings of the Ninth International Conference on Harmful Algal Blooms, Hobart, Tasmania, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J.S., Lewis, R.J., Eds.; UNESCO: Paris, France, 2001; pp. 210–213. [Google Scholar]
- Ordás, M.C.; Santiago, F.; Franco, J.M.; Ordás, A.; Figueiras, A. Toxin and Molecular Analysis of Gym-nodinium Catenatum (Dinophyceae) Strains from Galicia (NW Spain) and Andalucia (S Spain). J. Plankton Res. 2004, 26, 341–349. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. An Unprecedented Analysis on Global Harmful Algal Blooms Launched by IOC. Available online: https://ioc.unesco.org/news/unprecedented-analysis-global-harmful-algal-blooms-launched-ioc (accessed on 9 November 2021).
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Dechraoui Bottein, M.-Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived Global Increase in Algal Blooms Is At-tributable to Intensified Monitoring and Emerging Bloom Impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Lagos, N.W.; Andrinolo, D. Paralytic Shellfish Poisoning (PSP): Toxicology and Kinetics. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; Botana, L.M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 203–215. ISBN 0-8247-8956-3. [Google Scholar]
- Gessner, B.D.; Bell, P.; Doucette, G.J.; Moczydlowski, E.; Poli, M.A.; van Dolah, F.; Hall, S. Hypertension and Identification of Toxin in Human Urine and Serum Following a Cluster of Mussel-Associated Para-lytic Shellfish Poisoning Outbreaks. Toxicon 1997, 35, 711–722. [Google Scholar] [CrossRef]
- García, C.; del Carmen Bravo, M.; Lagos, M.; Lagos, N. Paralytic Shellfish Poisoning: Post-Mortem Analysis of Tissue and Body Fluid Samples from Human Victims in the Patagonia Fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Montebruno, D. Paralytic Shellfish Poisoning in Chile. Med. Sci. Law 1993, 33, 243–246. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-32004R0853-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0853 (accessed on 28 October 2022).
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Strichartz, G.; Moczydlowski, E.; Ravindran, A.; Reichardt, P.B. The Saxitoxins-Sources, Chemistry, and Pharmacology. In Marine Toxins-Origin, Structure, and Molecular Pharmacology; Hall, S., Strichartz, G., Eds.; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 29–65. ISBN 0097-6156. [Google Scholar]
- Negri, A.; Stirling, D.; Quilliam, M.; Blackburn, S.; Bolch, C.; Burton, I.; Eaglesham, G.; Thomas, K.; Walter, J.; Willis, R. Three Novel Hydroxybenzoate Saxitoxin Analogues Isolated from the Dinoflagellate Gym-nodinium Catenatum. Chem. Res. Toxicol. 2003, 16, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Cristiano, M.L.S. Marine Paralytic Shellfish Toxins: Chemical Properties, Mode of Action, Newer Analogues, and Structure–Toxicity Relationship. Nat. Prod. Rep. 2022, 39, 33–57. [Google Scholar] [CrossRef]
- Llewellyn, L.; Negri, A.; Quilliam, M. High Affinity for the Rat Brain Sodium Channel of Newly Discovered Hydroxybenzoate Saxitoxin Analogues from the Dinoflagellate Gymnodinium Catenatum. Toxicon 2004, 43, 101–104. [Google Scholar] [CrossRef]
- Durán-Riveroll, L.M.; Cembella, A.D.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Correa-Basurto, J. Docking Simulation of the Binding Interactions of Saxitoxin Analogs Produced by the Marine Dinoflagellate Gymnodinium Catenatum to the Voltage-Gated Sodium Channel Nav1.4. Toxins 2016, 8, 129. [Google Scholar] [CrossRef]
- WHO Cyanobacterial Toxins: Saxitoxins; WHO: Geneva, Switzerland, 2020.
- Hallegraeff, G.M.; Blackburn, S.I.; Doblin, M.A.; Bolch, C.J.S. Global Toxicology, Ecophysiology and Popu-lation Relationships of the Chainforming PST Dinoflagellate Gymnodinium Catenatum. Harmful Algae 2012, 14, 130–143. [Google Scholar] [CrossRef]
- Graham, H.W. Gymnodinium Catenatum, a New Dinoflagellate from the Gulf of California. Trans. Am. Microsc. Soc. 1943, 62, 259. [Google Scholar] [CrossRef]
- Carvalho, I.L.d.; Pelerito, A.; Ribeiro, I.; Cordeiro, R.; Núncio, M.S.; Vale, P. Paralytic Shellfish Poisoning Due to Ingestion of Contaminated Mussels: A 2018 Case Report in Caparica (Portugal). Toxicon X 2019, 4, 100017. [Google Scholar] [CrossRef]
- Costa, P.R.; Braga, A.C.; Turner, A.D. Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus Galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium Catenatum. Toxins 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Franca, S.; Almeida, J.F. Paralytic Shellfish Poisons in Bivalve Molluscs on the Portugese Coast Caused by a Bloom of the Dinoflagellate Gymnodinium Catenatum. In Red Tides: Biology, Envionmental Science and Toxicology; Elsevier Science Publishers: New York, NY, USA, 1989; pp. 93–96. [Google Scholar]
- EFSA Marine Biotoxins in Shellfis –Saxitoxin Group. EFSA J. 2009, 1019, 1–76.
- Rodriguez-Navarro, A.J.M.D.; Lagos, N.P.D.; Lagos, M.M.D.; Braghetto, I.M.D.; Csendes, A.M.D.; Hamilton, J.M.D.; Figueroa, C.M.D.; Truan, D.M.S.; Garcia, C.M.S.; Rojas, A.M.D.; et al. Neosaxitoxin as a Local An-esthetic: Preliminary Observations from a First Human Trial. Anesthesiol. J. Am. Soc. Anesthesiol. 2007, 106, 339–345. [Google Scholar]
- Adams, H.J.; Blair, M.R.; Takman, B. The Local Anesthetic Activity of Saxitoxin Alone and with Vasocon-strictor and Local Anesthetic Agents. Arch. Int. Pharmacodyn. Ther. 1976, 224, 275–282. [Google Scholar]
- Kohane, D.S.; Lu, N.T.; Gökgöl-Kline, A.C.G.; Berde, C.B.; Shubina, M.; Kuang, Y.; Hall, S.; Strichartz, G.R. The Local Anesthetic Properties and Toxicity of Saxitonin Homologues for Rat Sciatic Nerve Block In Vivo. Reg. Anesth. Pain Med. 2000, 25, 52–59. [Google Scholar] [CrossRef]
- FAO/WHO Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bi-Valve Molluscs; FAO/WHO: Rome, Italy, 2016; Volume 108.
- Lin, Z.R.; Geng, H.X.; Zhang, Q.C.; Chen, Z.F.; Dai, L.; Yu, R.C. Toxin Production of Dinoflagellate Gym-nodinium Catenatum Isolated from the East China Sea. Harmful Algae 2022, 113, 102188. [Google Scholar] [CrossRef]
- Fernández-Herrera, L.J.; Band-Schmidt, C.J.; Zenteno-Savín, T.; Leyva-Valencia, I.; Hernández-Guerrero, C.J.; Muñoz-Ochoa, M. Cell Death and Metabolic Stress in Gymnodinium Catenatum Induced by Allelopathy. Toxins 2021, 13, 506. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, H.J.; Li, Z.; Youn, J.Y.; Kwak, K.Y.; Seo, M.H.; Hwang, J.; Lee, S.D.; Yun, S.M.; Oh, S.J.; et al. Effects of Different Nutrient and Trace Metal Concentrations on Growth of the Toxic Dinoflagellate Gym-nodinium Catenatum Isolated from Korean Coastal Waters. Sustainability 2020, 12, 4992. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J.; López-Cortés, D.J. Effect of Temperature on Growth and Paralytic Toxin Profiles in Isolates of Gymnodinium Catenatum (Dinophyceae) from the Pacific Coast of Mexico. Toxicon 2014, 90, 199–212. [Google Scholar] [CrossRef]
- Green, D.; Hart, M.; Blackburn, S.; Bolch, C. Bacterial Diversity of Gymnodinium Catenatum and Its Relationship to Dinoflagellate Toxicity. Aquat. Microb. Ecol. 2010, 61, 73–87. [Google Scholar] [CrossRef]
- Sako, Y.; Yoshida, T.; Uchida, A.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and Characterization of a Sulfotransferase Specific to N-21 of Saxitoxin and Gonyautoxin 2+3 from the Toxic Dinoflagellate Gymnodinium Catenatum (Dinophyceae). J. Phycol. 2001, 37, 1044–1051. [Google Scholar] [CrossRef]
- Yoshida, T.; Sako, Y.; Uchida, A.; Kakutani, T.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and Characterization of Sulfotransferase Specific to O-22 of 11-Hydroxy Saxitoxin from the Toxic Dinoflagel-late Gymnodinium Catenatum (Dinophyceae). Fish Sci. 2002, 68, 634–642. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Hong, H. A Sulfotransferase Specific to N-21 of Gonyautoxin 2/3 from Crude En-zyme Extraction of Toxic Dinoflagellate Alexandrium Tamarense CI01. Chin. J. Oceanol. Limnol. 2007, 25, 227–234. [Google Scholar] [CrossRef]
- Buzy, A.; Thibault, P.; Laycock, M. v Development of a Capillary Electrophoresis Method for the Charac-terization of Enzymatic Products Arising from the Carbamoylase Digestion of Paralytic Shellfish Poisoning Toxins. J. Chromatogr. A 1994, 688, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Cembella, A.D.; Ross, N.W. In Vitro Transformation of Paralytic Shellfish Toxins in the Clams Mya Arenaria and Protothaca Staminea. Harmful Algae 2006, 5, 79–90. [Google Scholar] [CrossRef]
- Lin, H.-P.; Cho, Y.; Yashiro, H.; Yamada, T.; Oshima, Y. Purification and Characterization of Paralytic Shellfish Toxin Transforming Enzyme from Mactra Chinensis. Toxicon 2004, 44, 657–668. [Google Scholar] [CrossRef]
- Oshima, Y.; Blackburn, S.I.; Hallegraeff, G.M. Comparative Study on Paralytic Shellfish Toxin Profiles of the Dinoflagellate Gymnodinium Catenatum from Three Different Countries. Mar. Biol. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Costa, P.R.; Pereira, P.; Guilherme, S.; Barata, M.; Nicolau, L.; Santos, M.A.; Pacheco, M.; Pousão-Ferreira, P. Bio-transformation Modulation and Genotoxicity in White Seabream upon Exposure to Paralytic Shellfish Toxins Produced by Gymnodinium Catenatum. Aquat. Toxicol. 2012, 106–107, 42–47. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis Immobilis Is a Diatom, Not a Chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, S.K.; Guldhe, A.; Rawat, I.; Bux, F. Microalgae Isolation and Basic Culturing Techniques. In Handbook of Marine Microalgae: Biotechnology Advances; Academic Press: Cambridge, MA, USA, 2015; pp. 43–54. [Google Scholar] [CrossRef]
- Wood, A.M.; Everroad, R.C.; Wingard, L.M. Measuring Growth Rates in Microalgal Cultures. In Algal Culturing Techniques; Elsevier: Burlington, MA, USA, 2005; pp. 269–285. [Google Scholar]
- Unknown AOAC, Paralytic Shellfish Poisoning Toxins in Shellfish. Prechromatographic and Liquid Chromatography with Fluorescence Detection. First Action, Official Method 2005.06. In AOAC Official Methods of Analysis; Horwitz, W., Latimer, G.W., Eds.; Gaithersburg AOAC International: Rockville, MD, USA, 2005; p. 83. [Google Scholar]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar]
- Leal, J.F.; Cristiano, M.L.S. Revisiting the HPLC-FLD Method to Quantify Paralytic Shellfish Toxins: C3,4 Quantification and the First Steps towards Validation. Toxins 2022, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.; Alfonso, A.; Botana, A.M.; Vieytes, M.R.; Botana, L.M. Comparative Analysis of Pre- and Post-Column Oxidation Methods for Detection of Paralytic Shellfish Toxins. Toxicon 2010, 56, 448–457. [Google Scholar] [CrossRef] [PubMed]

| Strain | IO13-25-02 | IO13-26-02 | |||
|---|---|---|---|---|---|
| Toxin | TEF (EFSA) | Concentration fmol Cell−1 | Molar Fraction (%) | Concentration fmol Cell−1 | Molar Fraction (%) |
| dcGTX2,3 | 0.4 | 1.0 ± 0.2 | 6 ± 1 | 0.57 ± 0.02 | 3.0 ± 0.1 |
| C1,2 | 0.1 | 5.6 ± 1.2 | 31 ± 4 | 2.8 ± 0.5 | 15 ± 1 |
| dcSTX | 1.0 | 6.8 ± 1.0 | 40 ± 8 | 9.5 ± 0.2 | 49 ± 3 |
| GTX5 (or B1) | 0.1 | 2.5 ± 0.4 | 14 ± 2 | 1.8 ± 0.2 | 9.8 ± 0.4 |
| GTX6 (or B2) | 0.1 | 3.4 ± 4.7 | 9 ± 16 | 4.2 ± 0.6 | 23 ± 2 |
| Region, Date | Strain | dcGTX2,3 | C1,2 | dcSTX | GTX5 | GTX2,3 | STX | GTX6 | C3,4 | dcNEO | GTX1,4 | NEO | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Portugal | Lisbon bay, 2018 | IO13-25-02 | 6 ± 1 | 31 ± 4 | 40 ± 8 | 14 ± 2 | nd | <LOQ | 9 ± 16 | <LOQ | nd | <LOQ | <LOQ | This study |
| Lisbon bay, 2018 | IO13-26-02 | 3.0 ± 0.1 | 15 ± 1 | 49 ± 3 | 9.8 ± 0.4 | nd | <LOQ | 23 ± 2 | <LOQ | nd | <LOQ | <LOQ | This study | |
| Lisbon bay, 2007 | 2 | 43 | 15 | 22 | nd | nd | 8 | 3 | 7 | --- | --- | [5] + | ||
| Lisbon bay, 2007 | C37/07 | 3.2 | 34.3 | 4.1 | 23.6 | --- | --- | 16.2 | 17.1 | 1.5 | --- | --- | [46] | |
| Espinho, 2005 | IO13-04 | 3 * | 67 * | 3 | 2 | 5 | nd | 2 | 14 * | 2 | 2 * | nd | [27] + | |
| Algarve, 2003 2008 | ||||||||||||||
| IO13-01 | --- | 22.1 | 1.4 | 41.4 | --- | --- | 15.5 | 19.6 | --- | --- | --- | [3] | ||
| IO13-17 ++ | --- | 44.8 | 35.4 | 19.8 | --- | --- | --- | --- | --- | --- | --- | |||
| Lisbon bay, 2003 2005 | IO13-02 IO13-06 ++ | --- --- | 13.0 41.4 | 2.2 35.1 | 23.1 23.5 | --- --- | --- --- | 27.4 --- | 34.3 --- | --- --- | --- --- | --- --- | [3] | |
| Aveiro, 2010 2011 | IO13-22 IO13-24 | --- --- | 9.0 14.6 | 1.5 0.9 | 24.0 13.0 | --- --- | --- --- | 30.8 39.0 | 34.7 32.5 | --- --- | --- --- | --- --- | [3] | |
| Aguda, 1989 | PT02 | 8.5 | 30.8 | 5.3 | 27.4 | 2.1 | 0.7 | 12.5 | 12.7 | --- | nd | --- | [7] + | |
| Portugal, unknown | 8.1 | 31.8 | 5.5 | 26.9 | 1.8 | 0.6 | 15.1 | 10.4 | --- | --- | --- | [8] | ||
| Spain | Ria de Vigo, 1985 | 5 strains | 1.0–7.7 | 13.5–26.3 | 3.3–4.6 | 18.5–37.1 | 0.3–3.3 | nd | 15.3–34.8 | 8.0–14.8 | --- | nd | --- | [7] + |
| Galicia, 1985-1993 | 5 strains | nd–1.8 (4) | 9.9–66.0 * (5) | 0.3–14.7 (5) | 18.4–27.1 (5) | nd–0.09 * (1) | 2.0–13.0 (5) | 2.9–48.4 (5) | nd–21.2 * (3) | --- | nd–1.8 (2) | nd–17.4 (1) | [9] +++ | |
| Andalucia, 1999 | 11 strains | 0.9–7.7 (11) | 12.5–97.4 * (11) | nd–3.7 (9) | nd–32.5 (9) | nd–4.9 * (3) | nd | nd–37.6 (8) | nd–34.6 * (9) | --- | nd | nd–57.8 (7) | [9] +++ | |
| Spain, unknown | 4.5 | 22.7 | 3.5 | 29.2 | 2,0 | nd | 22.8 | 15.3 | --- | --- | --- | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, J.F.; Bombo, G.; Pereira, H.; Vicente, B.; Amorim, A.; Cristiano, M.L.S. Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters. Toxins 2022, 14, 762. https://doi.org/10.3390/toxins14110762
Leal JF, Bombo G, Pereira H, Vicente B, Amorim A, Cristiano MLS. Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters. Toxins. 2022; 14(11):762. https://doi.org/10.3390/toxins14110762
Chicago/Turabian StyleLeal, Joana F., Gabriel Bombo, Hugo Pereira, Bernardo Vicente, Ana Amorim, and Maria L. S. Cristiano. 2022. "Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters" Toxins 14, no. 11: 762. https://doi.org/10.3390/toxins14110762
APA StyleLeal, J. F., Bombo, G., Pereira, H., Vicente, B., Amorim, A., & Cristiano, M. L. S. (2022). Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters. Toxins, 14(11), 762. https://doi.org/10.3390/toxins14110762







