A Pilot Study of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Subtype A2 for Post-Stroke Lower Limb Spasticity: Comparison with OnabotulinumtoxinA
Abstract
1. Introduction
2. Results
2.1. Patients Breakdown
2.2. Primary Outcomes
2.3. Secondary Outcomes
3. Discussion
4. Future Perspectives
5. Conclusions
6. Subjects and Methods
6.1. BoNT Preparations
6.2. Entry Criteria
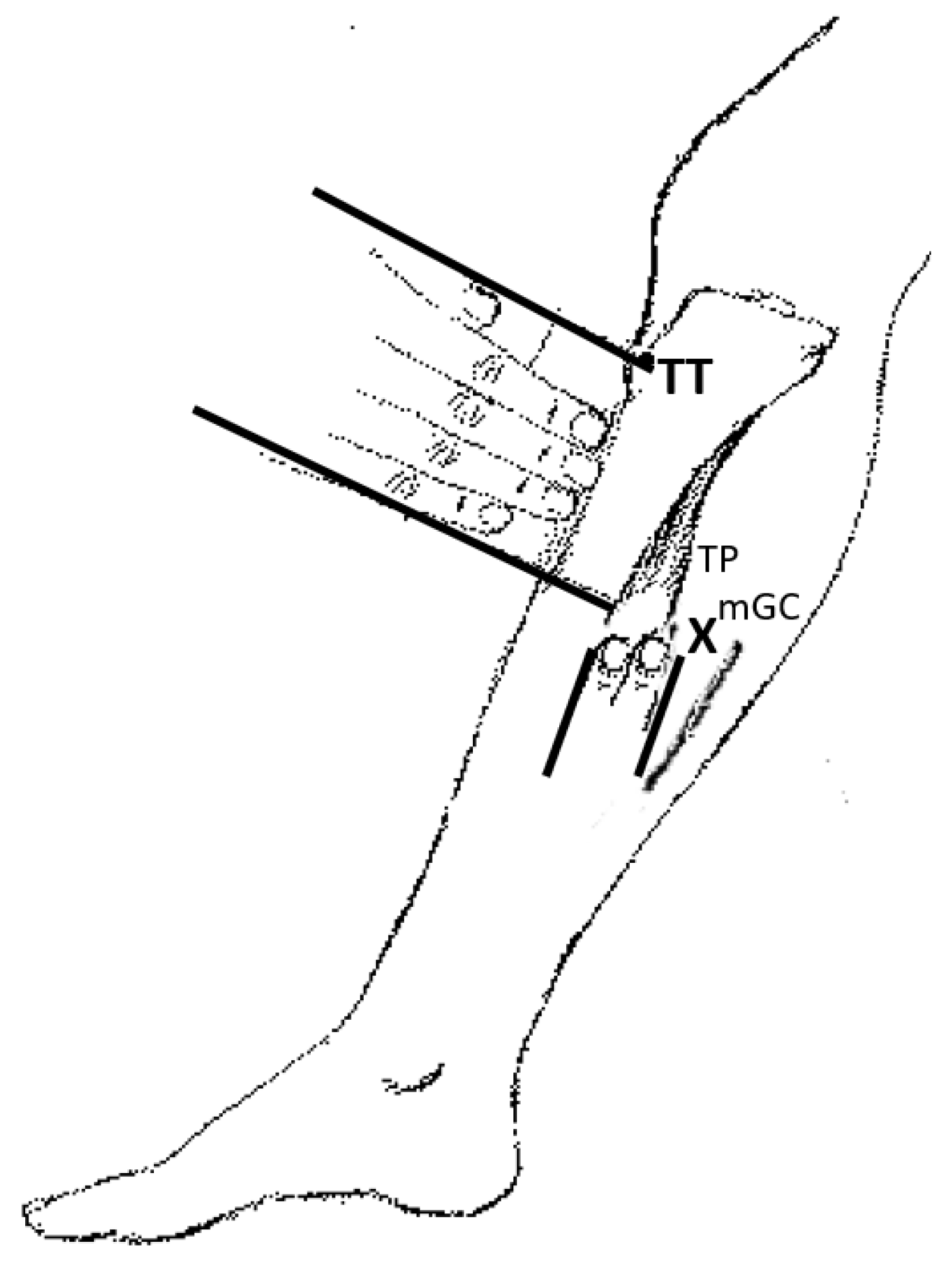
6.3. BoNT Injection
6.4. Outcome Measures
6.4.1. Modified Ashworth Scale (MAS)
6.4.2. Functional Independence Measure (FIM)
6.4.3. 10 m Walking Time
6.4.4. Manual Muscle Testing and Grip Strength
6.4.5. Blood Sampling
6.5. Schedule of Visits
6.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OnaA | onabotulintoxinA (BOTOX®) |
| A2NTX | low-molecular-weight subtype A2 botulinum toxin |
| BoNT | botulinum neurotoxin or its preparations |
| FAS | full analysis set |
| ITT | intention to treat |
References
- Nukina, M.; Mochida, Y.; Sakaguchi, S.; Sakaguchi, G. Purification of Clostridium botulinum type G progenitor toxin. Zent. Für Bakteriol. Mikrobiol. Und Hyg. 1988, 268, 220–227. [Google Scholar] [CrossRef]
- Lebeda, F.J.; Cer, R.Z.; Mudunuri, U.; Stephens, R.; Singh, B.R.; Adler, M. The zinc-dependent protease activity of the botulinum neurotoxins. Toxins 2010, 2, 978–997. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L.; Pullman, S.L. Botulinum toxins in neurological disease. Muscle Nerve 2004, 29, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; Folli, A.; D’Abrosca, F.; Grana, E.; Baricich, A.; Carda, S.; Invernizzi, M. Multidimensional Effectiveness of Botulinum Toxin in Neuropathic Pain: A Systematic Review of Randomized Clinical Trials. Toxins 2022, 14, 308. [Google Scholar] [CrossRef]
- Morineaux, V.; Mazuet, C.; Hilaire, D.; Enche, J.; Popoff, M.R. Characterization of botulinum neurotoxin type A subtypes by immunocapture enrichment and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5559–5570. [Google Scholar] [CrossRef]
- Pellett, S.; Bradshaw, M.; Tepp, W.H.; Pier, C.L.; Whitemarsh, R.C.M.; Chen, C.; Barbieri, J.T.; Johnson, E.A. The Light Chain Defines the Duration of Action of Botulinum Toxin Serotype A Subtypes. mBio 2018, 9, e00089-18. [Google Scholar] [CrossRef]
- Fonfria, E.; Maignel, J.; Lezmi, S.; Martin, V.; Splevins, A.; Shubber, S.; Kalinichev, M.; Foster, K.; Picaut, P.; Krupp, J. The Expanding Therapeutic Utility of Botulinum Neurotoxins. Toxins 2018, 10, 208. [Google Scholar] [CrossRef]
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Snyder, D.; Foster, K. AbobotulinumtoxinA (Dysport((R))), OnabotulinumtoxinA (Botox((R))), and IncobotulinumtoxinA (Xeomin((R))) Neurotoxin Content and Potential Implications for Duration of Response in Patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef]
- Fujii, N. Structure and function of botulinum toxin. Hokkaido J. Med Sci. 1995, 70, 19–28. [Google Scholar]
- Dressler, D.; Pan, L.; Adib Saberi, F.; Bigalke, H. Do complexing proteins provide mechanical protection for botulinum neurotoxins? J. Neural Transm. 2019, 126, 1047–1050. [Google Scholar] [CrossRef]
- Hefter, H.; Brauns, R.; Urer, B.; Rosenthal, D.; Albrecht, P. Effective long-term treatment with incobotulinumtoxin (Xeomin(R)) without neutralizing antibody induction: A monocentric, cross-sectional study. J. Neurol. 2020, 267, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beylot, C. Different botulinum toxins and their specifications. Ann. Dermatol. Venereol. 2009, 136 (Suppl. S4), S77–S85. [Google Scholar] [CrossRef]
- Takeuchi, T.; Okuno, T.; Miyashiro, A.; Kohda, T.; Miyamoto, R.; Izumi, Y.; Kozaki, S.; Kaji, R. Clinical Safety and Tolerability of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Botulinum Neurotoxin Subtype A2, in Comparison with Subtype A1 Toxins. Toxins 2021, 13, 824. [Google Scholar] [CrossRef]
- Akaike, N.; Ito, Y.; Shin, M.C.; Nonaka, K.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kozaki, S.; Kaji, R. Effects of A2 type botulinum toxin on spontaneous miniature and evoked transmitter release from the rat spinal excitatory and inhibitory synapses. Toxicon 2010, 56, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, S.; Nakaue, S.; Kamata, Y. Immunological characterization of the neurotoxin produced by Clostridium botulinum type A associated with infant botulism in Japan. Microbiol. Immunol. 1995, 39, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Pier, C.L.; Chen, C.; Tepp, W.H.; Lin, G.; Janda, K.D.; Barbieri, J.T.; Pellett, S.; Johnson, E.A. Botulinum neurotoxin subtype A2 enters neuronal cells faster than subtype A1. FEBS Lett. 2011, 585, 199–206. [Google Scholar] [CrossRef]
- Torii, Y.; Kiyota, N.; Sugimoto, N.; Mori, Y.; Goto, Y.; Harakawa, T.; Nakahira, S.; Kaji, R.; Kozaki, S.; Ginnaga, A. Comparison of effects of botulinum toxin subtype A1 and A2 using twitch tension assay and rat grip strength test. Toxicon 2011, 57, 93–99. [Google Scholar] [CrossRef]
- Torii, Y.; Sasaki, M.; Shin, M.C.; Akaike, N.; Kaji, R. Comparison of efficacy and toxicity between botulinum toxin subtypes A1 and A2 in cynomolgus macaques. Toxicon 2018, 153, 114–119. [Google Scholar] [CrossRef]
- Mukai, Y.; Shimatani, Y.; Sako, W.; Asanuma, K.; Nodera, H.; Sakamoto, T.; Izumi, Y.; Kohda, T.; Kozaki, S.; Kaji, R. Comparison between botulinum neurotoxin type A2 and type A1 by electrophysiological study in healthy individuals. Toxicon 2014, 81, 32–36. [Google Scholar] [CrossRef]
- Kaji, R.; Miyashiro, A.; Sato, N.; Takeuchi, T.; Kaji, S.; Furumoto, T. Randomised double-blind clinical trial of botulinum toxin subtype A2 (A2NTX) in comparison with subtype A1 (onabotulinumtoxinA) (ClinicalTrials.gov NCT01910363) (P4.333). Neurology 2015, 14, 84. [Google Scholar]
- Kaji, R.; Miyashiro, A.; Kaji, S.; Takeuchi, T. Comparative study of spread of A1 and A2 subtypes of botulinum toxin preparations for blepharospasm: Proof-ofconcept randomized controlled trial. Mov. Disord. 2015, 30, 510. [Google Scholar]
- Kaji, R. Clinical differences between A1 and A2 botulinum toxin subtypes. Toxicon 2015, 107, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Masakado, Y.; Kagaya, H.; Kondo, K.; Otaka, Y.; Dekundy, A.; Hanschmann, A.; Geister, T.L.; Kaji, R. Efficacy and Safety of IncobotulinumtoxinA in the Treatment of Lower Limb Spasticity in Japanese Subjects. Front. Neurol. 2022, 13, 832937. [Google Scholar] [CrossRef]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; Group, G.S.K.S.S. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; Group, G.S.K.S.S. Botulinum toxin type A in post-stroke lower limb spasticity: A multicenter, double-blind, placebo-controlled trial. J. Neurol. 2010, 257, 1330–1337. [Google Scholar] [CrossRef]
- Koizumi, H.; Goto, S.; Okita, S.; Morigaki, R.; Akaike, N.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kaji, R. Spinal Central Effects of Peripherally Applied Botulinum Neurotoxin A in Comparison between Its Subtypes A1 and A2. Front. Neurol. 2014, 5, 98. [Google Scholar] [CrossRef]
- Castelao, M.; Marques, R.E.; Duarte, G.S.; Rodrigues, F.B.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2017, 12, CD003633. [Google Scholar] [CrossRef]
- Apkon, S.D.; Cassidy, D. Safety considerations in the use of botulinum toxins in children with cerebral palsy. PM R 2010, 2, 282–284. [Google Scholar] [CrossRef]
- Walker, F.O.; Hunt, V.P. The lethal botulinum toxic shot syndrome. JAMA 1992, 267, 3149. [Google Scholar] [CrossRef]
- Torii, Y.; Akaike, N.; Harakawa, T.; Kato, K.; Sugimoto, N.; Goto, Y.; Nakahira, S.; Kohda, T.; Kozaki, S.; Kaji, R.; et al. Type A1 but not type A2 botulinum toxin decreases the grip strength of the contralateral foreleg through axonal transport from the toxin-treated foreleg of rats. J. Pharmacol. Sci. 2011, 117, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.A.; Swope, D.M.; Grimes, D. Diffusion of botulinum toxins. Tremor Other Hyperkinetic Mov. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Yu, P.; Zhai, H.; Li, Z.; Dong, R.; Wu, T.; Li, Y.; Di, Z.; Sun, Y.; Long, X.; Yu, N. Pivotal role of injection volume on sunken cheek prevention in masseter muscle BoNT-A injection: A cadaver study. J. Cosmet. Dermatol. 2022, 21, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Davis, T. Botulinum toxin injection, dilution confusion: The impact of toxin diffusion on clinical practice. J. Pediatr. Rehabilitation Med. 2020, 13, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Camoes-Barbosa, A.; Ribeiro, I.M.; Medeiros, L. Contralateral Upper Limb Weakness Following Botulinum Toxin A Injection for Poststroke Spasticity. Acta Med. Port. 2020, 33, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Akaike, N.; Shin, M.C.; Wakita, M.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kato, K.; Kaji, R.; Kozaki, S. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J. Physiol. 2013, 591, 1031–1043. [Google Scholar] [CrossRef]
- Cai, B.B.; Francis, J.; Brin, M.F.; Broide, R.S. Botulinum neurotoxin type A-cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience 2017, 352, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Dashtipour, K. Abo-, inco-, ona-, and rima-botulinum toxins in clinical therapy: A primer. Pharmacotherapy 2013, 33, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Kroken, A.R.; Blum, F.C.; Zuverink, M.; Barbieri, J.T. Entry of Botulinum Neurotoxin Subtypes A1 and A2 into Neurons. Infect. Immun. 2017, 85, e00795-16. [Google Scholar] [CrossRef]
- Sakaguchi, G.; Ohishi, I.; Kozaki, S. Purification and Oral Toxicities of Clostridium botulinum Progenitor Toxins. In Biomedical Aspects of Botulism.; Lewis, G.E., Ed.; Academic Press: New York, NY, USA, 1981; pp. 21–34. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Dodds, T.A.; Martin, D.P.; Stolov, W.C.; Deyo, R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993, 74, 531–536. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hara, Y.; Akaboshi, K.; Chino, N. Relationship between shoulder muscle strength and functional independence measure (FIM) score among C6 tetraplegics. Spinal Cord 1999, 37, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Flansbjer, U.B.; Holmback, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Hallett, M.; Cohen, L.G. Double-blind trial of botulinum toxin for treatment of focal hand dystonia. Mov. Disord. 1995, 10, 466–471. [Google Scholar] [CrossRef] [PubMed]




| OnaA n = 16 | A2NTX n = 15 | Test | p | |
|---|---|---|---|---|
| Sex (male/female) | 15/1 | 10/5 | Fisher’s exact | 0.00014 * |
| Age (years) | 65.8 ± 8.3 (52~78) | 65.5 ± 9.2 (42~78) | t | 0.88 |
| Duration of illness (months) | 98.1 ± 59.1 (22~240) | 88.9 ± 66.0 (32~241.5) | t | 0.58 |
| Side of Paresis R/L | 5/11 | 8/7 | Fisher’s exact | 0.23 |
| Cause Bleeding/Infarct | 12/4 | 9/6 | Fisher’s exact | 0.02 * |
| MAS (Ankle Flexion) | 2.53 ± 0.85 (1~3) | 2.53 ± 0.92 (1~4) | t | 0.99 |
| MAS (Ankle Extension) | 0.94 ± 0.44 (0~2) | 1.00 ± 0.93 (0~3) | t | 0.81 |
| Hand Grip Power (kg) | 33.1 ± 8.2 (16.4~49.8) | 27.2 ± 8.2 (15.5~38.1) | t | 0.09 |
| 10 m Gait Time (s) | 29.2 ± 24.5 (12.0~119.0) | 32.5 ± 28.8 (9.3~114.2) | t | 0.58 |
| FIM (Full: 35) | 26.9 ± 5.7 (17~33) | 25.2 ± 4.8 (17~34) | t | 0.41 |
| MMT (Tibialis Anterior) | 2.59 ± 1.38 (0–4) | 2.40 ± 1.40 (0–4) | t | 0.35 |
| OnaA n = 13 | A2NTX n = 11 | Test | p | |
|---|---|---|---|---|
| Sex (male/female) | 12/1 | 8/3 | Fisher’s exact | 0.08 |
| Age (years) | 64.1 ± 8.0 (52~78) | 63.2 ± 10.3 (42~78) | t | 0.88 |
| Duration of illness (months) | 88.7 ± 47.8 (22~192) | 90.1 ± 53.5 (32~179) | t | 0.94 |
| Side of Paresis R/L | 5/8 | 8/3 | Fisher’s exact | 0.54 |
| Cause Bleeding/Infarct | 10/3 | 8/3 | Fisher’s exact | 0.12 |
| MAS (Ankle Flexion) | 2.85 ± 0.55 (1~3) | 2.73 ± 0.90 (1~4) | t | 0.99 |
| MAS (Ankle Extension) | 1.08 ± 0.28 (1~2) | 1.36 ± 0.81 (1~3) | t | 0.81 |
| Hand Grip Power (kg) | 33.5 ± 10.4 (16.4~49.8) | 28.0 ±8.1 (15.5~41.2) | t | 0.09 |
| 10 m Gait Time (sec) | 27.9 ± 27.3 (12.0~112.0) | 37.5 ± 32.4 (9.3~114.2) | t | 0.58 |
| FIM (Full: 35) | 26.7 ± 6.0 (16~33) | 26.0 ± 4.8 (17~34) | t | 0.41 |
| MMT (Tibialis Anterior) | 2.53 ± 1.39 (0–4) | 2.45 ± 1.21 (1–4) | t | 0.44 |
| A2NTX | OnaA | p Value | ||
|---|---|---|---|---|
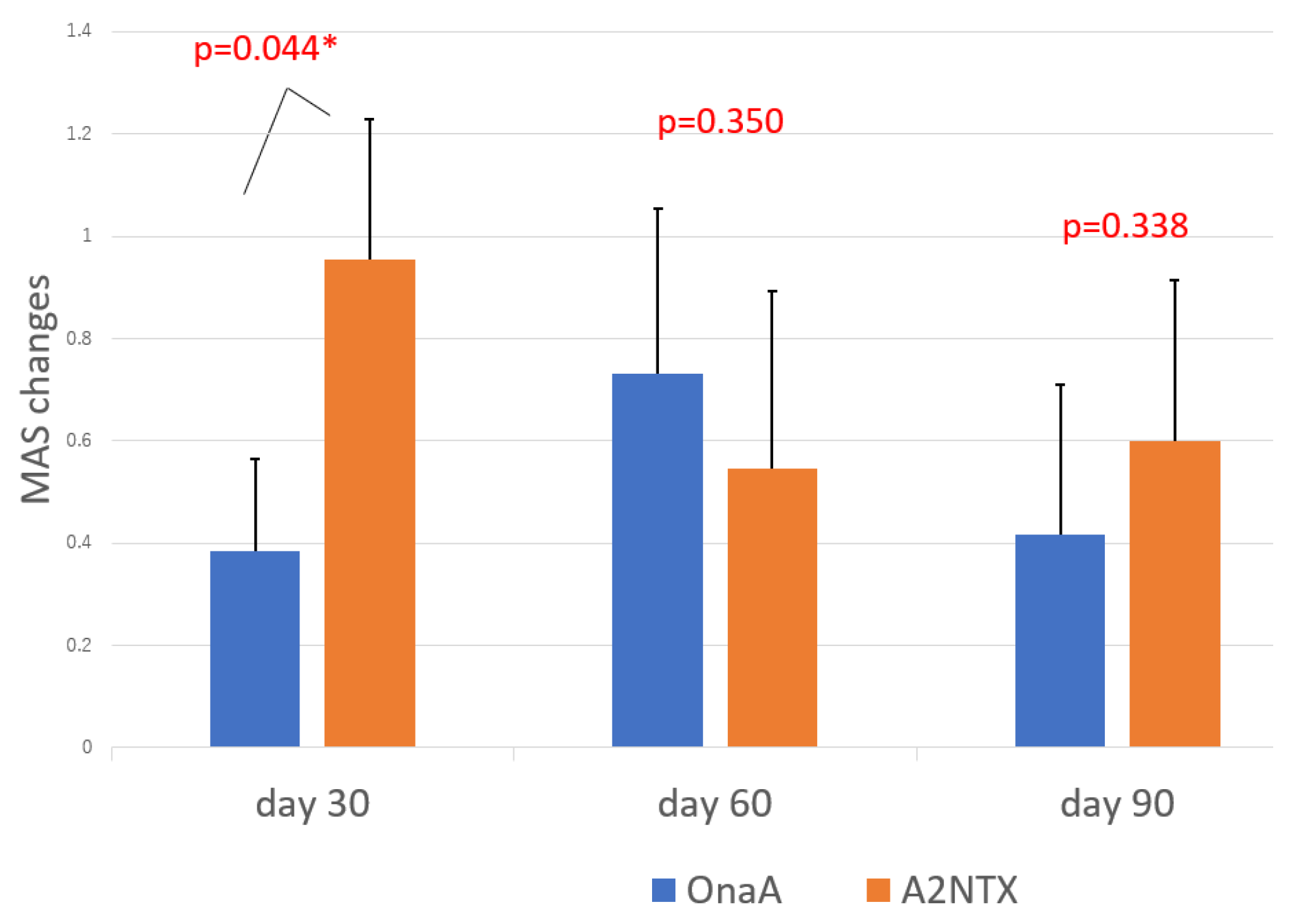
| MAS changes | Day 30 | 0.95 ± 0.27 (n = 11) | 0.38 ± 0.18 (n = 13) | 0.044 * |
| Day 60 | 0.55 ± 0.27 (n = 11) | 0.73 ± 0.32 (n = 13) | 0.350 | |
| Day 90 | 0.60 ± 0.31(n = 10) | 0.42 ± 0.29 (n = 12) | 0.338 | |
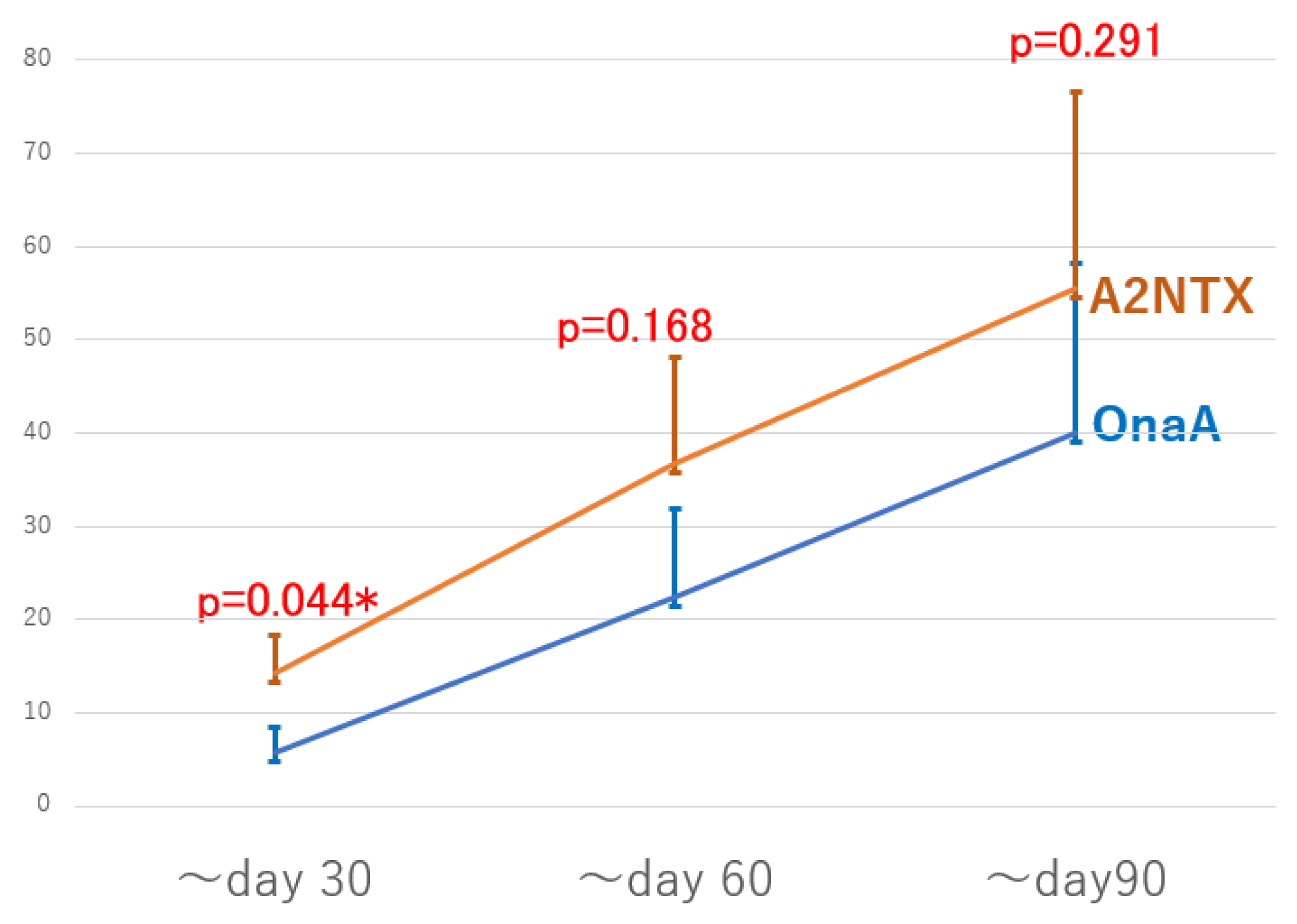
| MAS changes/AUC * | ~Day 30 | 14.32 ± 4.10 (n = 11) | 5.77 ± 2.71 (n = 13) | 0.044 * |
| ~Day 60 | 36.82 ± 11.37 (n = 11) | 22.5 ± 9.30 (n = 13) | 0.168 | |
| ~Day 90 | 55.50 ± 21.07 (n = 10) | 40.0 ± 18.24 (n = 12) | 0.291 |
| Efficacy | A2NTX | p | OnaA | p |
|---|---|---|---|---|
| FIM (Day 0) | 25.1 ± 1.3 (n = 14) | 26.7 ± 1.5 (n = 15) | ||
| FIM (Day 30) | 26.0 ± 1.2 (n = 14) | 0.17 | 27.6 ± 1.2(n = 15) | 0.22 |
| FIM (Day 60) | 27.9 ± 0.9 (n = 14) | 0.005 * | 28.3 ± 1.1 (n = 15) | 0.09 |
| 10 m walking (Day 0) | 31.6 ± 8.5 (n = 15) | 26.4 ± 7.9 (n = 14) | ||
| 10 m walking (Day 30) | 28.2 ± 7.1 (n = 15) | 0.09 | 25.7 ± 7.0 (n = 14) | 0.26 |
| 10 m walking (Day 60) | 27.7 ± 6.4 (n = 15) | 0.06 | 24.9 ± 7.3 (n = 14) | 0.07 |
| Safety | ||||
| Hand Grip (Day 0) | 27.2 ± 2.1 (n = 15) | 33.2 ± 2.5 (n = 16) | ||
| Hand Grip (Day 30) | 26.5 ± 2.1 (n = 15) | 0.14 | 31.9 ± 2.3 (n = 16) | 0.05 * |
| Hand Grip (Day 60) | 28.4 ± 2.2 (n = 14) | 0.77 | 30.7 ± 2.7 (n = 15) | 0.002 * |
| ΔMMT (TA: Day 30) | −0.23 ± 0.19 (n = 15) | −0.56 ± 0.28 (n = 16) | 0.17 ‡ | |
| ΔMMT (TA: Day 60) | 0.30 ± 0.26 (n = 15) | −0.31 ± 0.4 (n = 16) | 0.11 ‡ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaji, R.; Miyashiro, A.; Sato, N.; Furumoto, T.; Takeuchi, T.; Miyamoto, R.; Kohda, T.; Izumi, Y.; Kozaki, S. A Pilot Study of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Subtype A2 for Post-Stroke Lower Limb Spasticity: Comparison with OnabotulinumtoxinA. Toxins 2022, 14, 739. https://doi.org/10.3390/toxins14110739
Kaji R, Miyashiro A, Sato N, Furumoto T, Takeuchi T, Miyamoto R, Kohda T, Izumi Y, Kozaki S. A Pilot Study of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Subtype A2 for Post-Stroke Lower Limb Spasticity: Comparison with OnabotulinumtoxinA. Toxins. 2022; 14(11):739. https://doi.org/10.3390/toxins14110739
Chicago/Turabian StyleKaji, Ryuji, Ai Miyashiro, Nori Sato, Taiki Furumoto, Toshiaki Takeuchi, Ryosuke Miyamoto, Tomoko Kohda, Yuishin Izumi, and Shunji Kozaki. 2022. "A Pilot Study of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Subtype A2 for Post-Stroke Lower Limb Spasticity: Comparison with OnabotulinumtoxinA" Toxins 14, no. 11: 739. https://doi.org/10.3390/toxins14110739
APA StyleKaji, R., Miyashiro, A., Sato, N., Furumoto, T., Takeuchi, T., Miyamoto, R., Kohda, T., Izumi, Y., & Kozaki, S. (2022). A Pilot Study of A2NTX, a Novel Low-Molecular-Weight Neurotoxin Derived from Subtype A2 for Post-Stroke Lower Limb Spasticity: Comparison with OnabotulinumtoxinA. Toxins, 14(11), 739. https://doi.org/10.3390/toxins14110739







