Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. flavus Infection and Aflatoxin Accumulation in Maize
Abstract
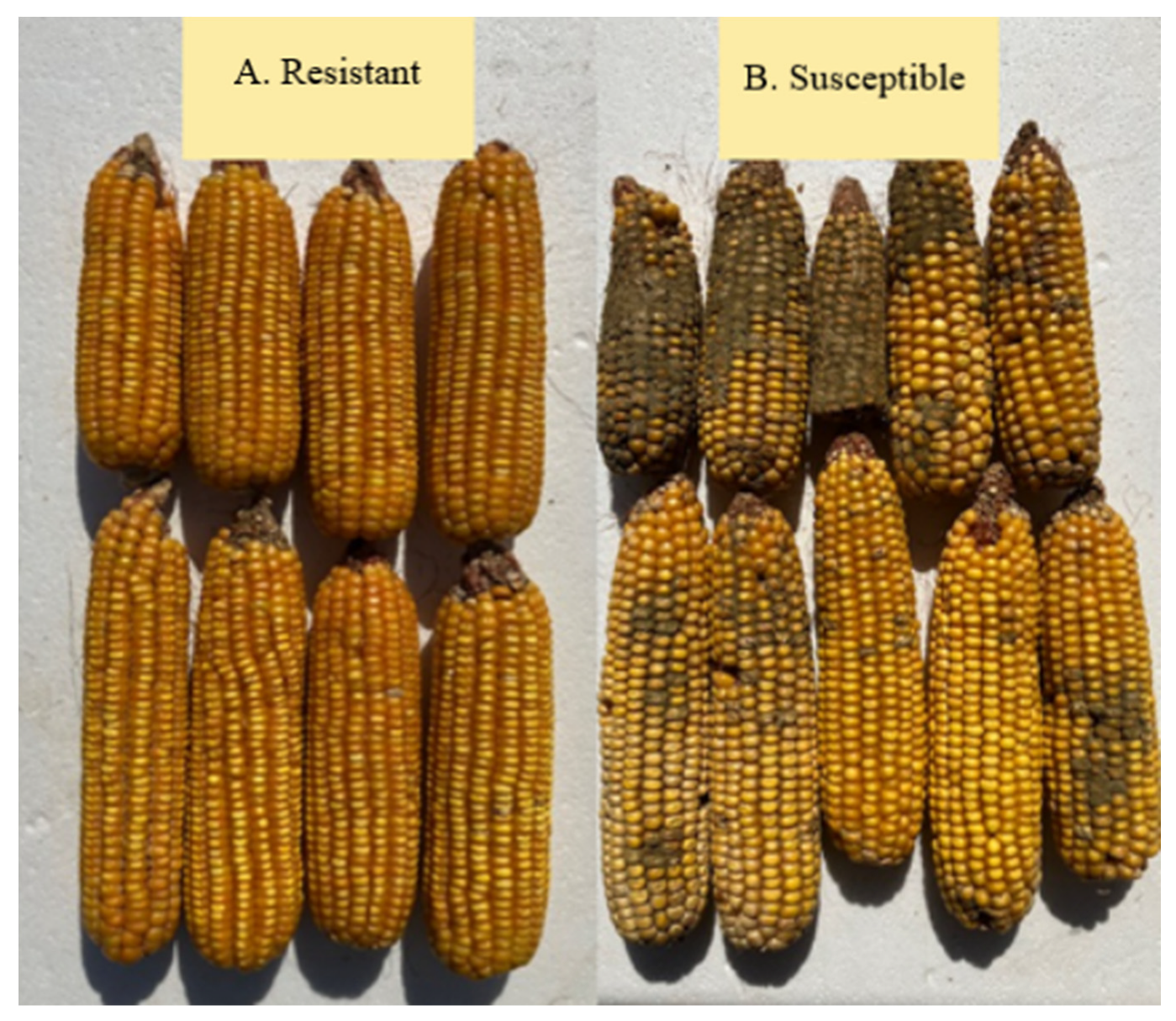
1. Introduction
2. Results & Discussion
2.1. Plant Signaling
2.2. Structural Components
2.3. Defense Compounds
2.4. Other Pathways
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Novakova, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenar, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins, and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Kale, S.; Yu, J. Aflatoxins: Background, toxicology, and molecular biology. In Foodborne Diseases; Simjee, S., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 355–373. [Google Scholar]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, K.A.; Payne, G.A. Unlocking the secrets behind secondary metabolism: A review of Aspergillus flavus from pathogenicity to functional genomics. Toxin Rev. 2003, 22, 423–459. [Google Scholar] [CrossRef]
- Klich, M. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Sherif, S.O.; Salama, E.E.; Abdel-Wahhab, M.A. Mycotoxins and child health: The need for health risk assessment. Int. J. Hyg. Environ. Health 2009, 212, 347–368. [Google Scholar] [CrossRef]
- Asiki, G.; Seeley, J.; Srey, C.; Baisley, K.; Lightfoot, T.; Archileo, K.; Agol, D.; Abaasa, A.; Wakeham, K.; Routledge, M.N.; et al. A pilot study to evaluate aflatoxin exposure in a rural Ugandan population. Trop. Med. Int. Health 2014, 19, 592–599. [Google Scholar] [CrossRef]
- Castegnaro, M.; McGregor, D. Carcinogenic risk assessment of mycotoxins. Rev. Med. Vet. 1998, 149, 671–678. [Google Scholar]
- Jackson, P.E.; Groopman, J.D. Aflatoxin and liver cancer. Best Pract. Res. Clin. Gastroenterol. 1999, 13, 545–555. [Google Scholar] [CrossRef]
- Lillehoj, E.B. The aflatoxin-in-maize problem: The historical perspective. In Aflatoxin in Maize, Proceedings of the Workshop, El Batan, Mexico, 7–11 April 1986; Zuber, M.S., Lillehoj, E.B., Renfro, B.L., Eds.; CIMMYT: Texcoco, Mexico, 1987; pp. 152–163. [Google Scholar]
- Kpodo, K.A.; Bankole, S.A. Mycotoxin contamination in foods in West and Central Africa. In Mycotoxins. Detection Methods, Management, Public Health and Agricultural Trade; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; CABI International: Wallingford, UK, 2008; pp. 103–116. [Google Scholar]
- Doehlemann, G.; Wahl, R.; Horst, R.J.; Voll, L.M.; Poree, F.; Stitt, M.; Sonnewald, U.; Kahmann, R. Reprogramming a maize plant: Transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008, 56, 181–195. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Okoth, S.; Nyongesa, B.; Joutsjoki, V.; Korhonen, H.; Ayugi, V.; Kang’ethe, E. Sclerotia formation and toxin production in large sclerotial Aspergillus flavus isolates from Kenya. Adv. Microbiol. 2016, 6, 47–56. [Google Scholar] [CrossRef][Green Version]
- Roze, L.V.; Chanda, A.; Laivenieks, M.; Beaudry, R.M.; Artymovich, K.A.; Koptina, A.V.; Awad, D.W.; Valeeva, D.; Jones, A.D.; Linz, J.E. Volatile profiling reveals intracellular metabolic changes in Aspergillus parasiticus: VeA regulates branched chain amino acid and ethanol metabolism. BMC Biochem. 2010, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Warburton, M.L.; Brooks, T.D.; Windham, G.L.; Williams, W.P. Identification of novel QTL contributing resistance to aflatoxin accumulation in maize. Mol. Breed. 2011, 27, 491–499. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Ogunola, O.F.; Smith, J.S.; Xu, W.; Bhattramakki, D.; Jeffers, D.; Williams, W.P.; Warburton, M.L. Characterization of a source of resistance to aflatoxin accumulation in maize. Agrosyst. Geosci. Environ. 2021, 4, e20203. [Google Scholar] [CrossRef]
- Smith, J.S.; Williams, W.P.; Windham, G.L.; Xu, W.; Warburton, M.L.; Bhattramakki, D. Identification of quantitative trait loci contributing resistance to aflatoxin accumulation in maize inbred Mp715. Mol. Breed. 2019, 39, 91. [Google Scholar] [CrossRef]
- Warburton, M.L.; Williams, W.P.; Windham, G.L.; Murray, S.C.; Xu, W.; Hawkins, L.K.; Duran, J.F. Phenotypic and genetic characterization of a maize association mapping panel developed for the identification of new sources of resistance to Aspergillus flavus and aflatoxin accumulation. Crop Sci. 2013, 53, 2374–2382. [Google Scholar] [CrossRef]
- Bengosi Bertagna, F.A.; Kuki, M.C.; Tessmann, D.J.; Barth Pinto, R.J.; Scapim, C.A.; Williams, W.P.; Warburton, M.L. Association mapping and pathway analysis of ear rot disease caused by Aspergillus flavus in a panel of tropical maize germplasm. Crop Sci. 2021, 61, 4128–4138. [Google Scholar] [CrossRef]
- Barendse, W.; Reverter, A.; Bunch, R.J.; Harrison, B.E.; Barris, W.; Thomas, M.B. A validated whole-genome association study of efficient food conversion in cattle. Genetics 2007, 176, 1893–1905. [Google Scholar] [CrossRef]
- Freebern, E.; Santos, D.J.; Fang, L.; Jiang, J.; Parker Gaddis, K.L.; Liu, G.E.; VanRaden, P.M.; Maltecca, C.; Cole, J.B.; Ma, L. GWAS and fine-mapping of livability and six disease traits in Holstein cattle. BMC Genom. 2020, 21, 41. [Google Scholar] [CrossRef]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Cano-Gamez, E.; Trynka, G. From GWAS to function: Using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef]
- Thrash, A.; Tang, J.D.; DeOrnellis, M.; Peterson, D.G.; Warburton, M.L. PAST: The Pathway Association Studies Tool to infer biological meaning from GWAS datasets. Plants 2020, 9, 58. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Bucan, M. Pathway-based approaches for analysis of genomewide association studies. Am. J. Hum. Genet. 2007, 81, 1278–1283. [Google Scholar] [CrossRef]
- Tang, J.D.; Perkins, A.; Williams, W.P.; Warburton, M.L. Using genome-wide associations to identify metabolic pathways involved in maize aflatoxin accumulation resistance. BMC Genom. 2015, 16, 673. [Google Scholar] [CrossRef]
- Warburton, M.L.; Tang, J.; Windham, G.L.; Hawkins, L.K.; Murray, S.C.; Xu, W.; Boykin, D.; Perkins, A.; Williams, W.P. Genome wide association mapping of Aspergillus flavus and aflatoxin accumulation resistance in maize. Crop Sci. 2015, 55, 1857–1867. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Fu, J.; Zhou, J.; Wu, X. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 2015, 388, 99–117. [Google Scholar] [CrossRef]
- Sun, C.X.; Li, M.Q.; Gao, X.; Liu, L.N.; Wu, X.F.; Zhou, J.H. Metabolic response of maize plants to multi-factorial abiotic stresses. Plant Biol. 2015, 18, 120–129. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.; Stanley, P.D.; Holmes, E. Application of NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Cheng, F.; Hu, C.; Quan, S.; Lin, H.; Wang, J.; Chen, G.; Zhao, X.; Alexander, D.; Guo, L.; et al. Metabolic map of mature maize kernels. Metabolomics 2014, 10, 775–787. [Google Scholar] [CrossRef]
- Malysheva, S.V.; Arroyo-Manzanares, N.; Cary, J.W.; Ehrlich, K.C.; Vanden Bussche, J.; Vanhaecke, L.; Bhatnagar, D.; Di Mavungu, J.D.; De Saeger, S. Identification of novel metabolites from Aspergillus flavus by high resolution and multiple stage mass spectrometry. Food Addit. Contam. 2014, 31, 111–120. [Google Scholar] [CrossRef]
- Forseth, R.; Amaike, S.; Schwenk, D.; Affeldt, K.J.; Hoffmeister, D.; Schroeder, F.; Keller, N.P. Homologous NRPS-like gene clusters mediate redundant small molecule biosynthesis in Aspergillus flavus. Angew. Chem. Int. Ed. 2013, 52, 1590–1594. [Google Scholar] [CrossRef]
- Li, H.; Thrash, A.; Tang, J.D.; He, L.; Yan, J.; Warburton, M.L. Leveraging GWAS data to identify metabolic pathways and networks involved in maize lipid biosynthesis. Plant J. 2019, 98, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Maxwell, S.; Feng, T.; Zhu, X.; Elston, R.C.; Koyutürk, M.; Chance, M.R. Gene, pathway and network frameworks to identify epistatic interactions of single nucleotide polymorphisms derived from GWAS data. BMC Syst. Biol. 2012, 6, S15. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Kaplan, F.; Huffaker, A.; Dafoe, N.J.; Vaughan, M.M.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Nat. Acad. Sci. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef]
- Shivaji, R.; Camas, A.; Ankala, A.; Engelberth, J.; Tumlinson, J.H.; Williams, W.P.; Wilkinson, J.R.; Luthe, D.S. Plants on constant alert: Elevated levels of jasmonic acid and jasmonate-induced transcripts in caterpillar-resistant maize. J. Chem. Ecol. 2010, 36, 179–191. [Google Scholar] [CrossRef]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef]
- Christensen, S.A.; Sims, J.; Vaughan, M.M.; Hunter, C.; Block, A.; Willett, D.; Alborn, H.T.; Huffaker, A.; Schmelz, E.A. Commercial hybrids and mutant genotypes reveal complex protective roles for inducible terpenoid defenses in maize. J. Exp. Bot. 2018, 69, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Suwarno, W.B.; Hannok, P.; Palacios-Rojas, N.; Windham, G.; Crossa, J.; Pixley, K.V. Provitamin A carotenoids in grain reduce aflatoxin contamination of maize while combating vitamin A deficiency. Front. Plant Sci. 2019, 20, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.K.; Mylroie, J.E.; Oliveira, D.A.; Smith, J.S.; Ozkan, S.; Windham, G.L.; Williams, W.P.; Warburton, M.L. Characterization of the maize chitinase genes and their effect on Aspergillus flavus and aflatoxin accumulation resistance. PLoS ONE 2015, 10, e0126185. [Google Scholar] [CrossRef]
- Gembeh, S.V.; Brown, R.L.; Grimm, C.; Cleveland, T.E. Identification of chemical components of corn kernel pericarp wax associated with resistance to Aspergillus flavus infection and aflatoxin production. J. Agric. Food Chem. 2001, 49, 4635–4641. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Brown, R.L.; Russin, J.S.; Lax, A.R.; Cleveland, T.E. A corn trypsin inhibitor with antifungal activity inhibits Aspergillus flavus α-amylase. Phytopathology 1999, 89, 902–907. [Google Scholar] [CrossRef]
- Woloshuk, C.P.; Cavaletto, J.R.; Cleveland, T.E. Inducers of aflatoxin biosynthesis from colonized maize kernels are generated by amylase activity from Aspergillus flavus. Phytopathology 1997, 87, 164–169. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Brown, R.L.; Damann, K.E.; Cleveland, T.E. PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol. Plant Pathol. 2010, 11, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A.; et al. The maize lipoxygenase, Zm LOX 10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Gobel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol. Plant-Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef]
- Ogunola, O.F.; Hawkins, L.K.; Mylroie, E.; Kolomiets, M.V.; Borrego, E.; Tang, J.D.; Williams, W.P.; Warburton, M.L. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS ONE 2017, 12, e0181265. [Google Scholar] [CrossRef]
- Warburton, M.L.; Rauf, S.; Marek, L.; Hussain, M.; Ogunola, O.; de Jesus Sanchez Gonzalez, J. The use of crop wild relatives in maize and sunflower breeding. Crop Sci. 2017, 57, 1227–1240. [Google Scholar] [CrossRef]
- Agostini, R.B.; Postigo, A.; Rius, S.P.; Rech, G.E.; Campos-Bermudez, V.A.; Vargas, W.A. Long-lasting primed state in maize plants: Salicylic acid and steroid signaling pathways as key players in the early activation of immune responses in silks. Mol. Plant-Microbe Interact. 2019, 32, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Septiani, P.; Lanubile, A.; Stagnati, L.; Busconi, M.; Nelissen, H.; Pè, M.E.; Dell’Acqua, M.; Marocco, A. Unravelling the genetic basis of Fusarium seedling rot resistance in the MAGIC maize population: Novel targets for breeding. Sci. Rep. 2019, 9, 5665. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; De Leonardis, S.; Battilani, P.; Paciolla, C.; Marocco, A. Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol. Plant-Microbe Interact. 2015, 28, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Pechan, T. Maize-pathogen interactions: An ongoing combat from a proteomics perspective. Int. J. Mol. Sci. 2015, 16, 28429–28448. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827. [Google Scholar] [CrossRef]
- Du Fall, L.A.; Solomon, P.S. Role of cereal secondary metabolites involved in mediating the outcome of plant-pathogen interactions. Metabolites 2011, 1, 64–78. [Google Scholar] [CrossRef]
- Tan, J.; Bednarek, P.; Liu, J.; Schneider, B.; Svatos, A.; Hahlbrock, K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 2004, 65, 691–699. [Google Scholar] [CrossRef]
- Förster, C.; Gershenzon, J.; Köllner, T.G. Evolution of DIMBOA-Glc O-Methyltransferases from Flavonoid O-Methyltransferases in the grasses. Molecules 2022, 27, 1007. [Google Scholar] [CrossRef]
- Förster, C.; Handrick, V.; Ding, Y.; Nakamura, Y.; Paetz, C.; Schneider, B.; Castro-Falcón, G.; Hughes, C.C.; Luck, K.; Poosapati, S.; et al. Biosynthesis and antifungal activity of fungus-induced O-methylated flavonoids in maize. Plant Physiol. 2022, 188, 167–190. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Stefen, D.L.V.; Leolato, L.S.; Gindri, D.M.; Nerling, D. Revisiting carotenoids and their role in plant stress responses: From biosynthesis to plant signaling mechanisms during stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, K.D., Palma, M.J., Corpas, J.F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 207–232. [Google Scholar]
- Castano-Duque, L.; Gilbert, M.K.; Mack, B.M.; Lebar, M.D.; Carter-Wientjes, C.H.; Sickler, C.M.; Cary, J.W.; Rajasekaran, K. Flavonoids modulate the accumulation of toxins from Aspergillus flavus in maize kernels. Front. Plant Sci. 2021, 12, 761446. [Google Scholar] [CrossRef] [PubMed]
- Aquije, G.M.F.V.; Zorzal, P.B.; Buss, D.S.; Ventura, J.A.; Fernandes, P.M.B.; Fernandes, A.A.R. Cell wall alterations in the leaves of fusariosis-resistant and susceptible pineapple cultivars. Plant Cell Rep. 2010, 29, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L.; Glauser, G.; Erb, M.; Flors, V.; Frey, M.; et al. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Singla, P.; Bhardwaj, R.D.; Kaur, S.; Kaur, J.; Grewal, S.K. Metabolic adjustments during compatible interaction between barley genotypes and stripe rust pathogen. Plant Physiol. Biochem. 2020, 147, 295–302. [Google Scholar] [CrossRef]
- Huby, E.; Napier, J.A.; Baillieul, F.; Michaelson, L.V.; Dhondt-Cordelier, S. Sphingolipids: Towards an integrated view of metabolism during the plant stress response. New Phytol. 2020, 225, 659–670. [Google Scholar] [CrossRef]
- Glenz, R.; Kaiping, A.; Göpfert, D.; Weber, H.; Lambour, B.; Sylvester, M.; Fröschel, C.; Mueller, M.J.; Osman, M.; Waller, F. The major plant sphingolipid long chain base phytosphingosine inhibits growth of bacterial and fungal plant pathogens. Sci. Rep. 2022, 12, 1081. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Yadav, M.P.; Moreau, R.A.; Hicks, K.B. Phenolic acids, lipids, and proteins associated with purified corn fiber arabinoxylans. J. Agric. Food Chem. 2007, 55, 943–947. [Google Scholar] [CrossRef]
- Butts-Wilmsmeyer, C.J.; Mumm, R.H.; Bohn, M.O. Concentration of beneficial phytochemicals in harvested grain of US yellow dent maize (Zea mays L.) germplasm. J. Agric. Food Chem. 2017, 65, 8311–8318. [Google Scholar] [CrossRef]
- Butts-Wilmsmeyer, C.J.; Mumm, R.H.; Bohn, M.O. Quantitative genetic analysis of hydroxycinnamic acids in maize (Zea mays L.) for plant improvement and production of health-promoting compounds. J. Agric. Food Chem. 2020, 68, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Bily, A.C.; Reid, L.M.; Taylor, J.H.; Johnston, D.; Malouin, C.; Burt, A.J.; Bakan, B.; Regnault-Roger, C.; Pauls, K.P.; Arnason, J.T.; et al. Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: Resistance factors to Fusarium graminearum. Phytopathology 2003, 93, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; McNulty, N.P.; Rodionov, D.A.; Khoroshkin, M.S.; Griffin, N.W.; Cheng, J.; Latreille, P.; Kerstetter, R.A.; Terrapon, N.; Henrissat, B.; et al. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 2015, 350, aac5992. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.K.; West, S.I.; Hornostaj, A.R.; Lawson, D.M.; Fairhurst, S.A.; Sanchez, R.O.; Hough, P.; Robinson, B.H.; Casey, R. Probing a novel potato lipoxygenase with dual positional specifcity reveals primary determinants of substrate binding and requirements for a surface hydrophobic loop and has implications for the role of lipoxygenases in tubers. Biochem. J. 2001, 353, 345–355. [Google Scholar] [CrossRef]
- Kolomiets, M.; Battilani, P.; Borrego, E.; Reverberi, M.; Lanubile, A.; Scala, V.; Falavigna, C.; Dall’Asta, C.; Bennett, J.; Park, Y. Mycotoxin contamination in maize is controlled by oxylipin signals. Phytopathology 2018, 108, S1.240–S1.319. [Google Scholar] [CrossRef][Green Version]
- Park, Y.S.; Kunze, S.; Ni, X.; Feussner, I.; Kolomiets, M.V. Comparative molecular and biochemical characterization of segmentally duplicated 9-lipoxygenase genes ZmLOX4 and ZmLOX5 of maize. Planta 2010, 231, 1425–1437. [Google Scholar] [CrossRef]
- He, Y.; Borrego, E.J.; Gorman, Z.; Huang, P.C.; Kolomiets, M.V. Relative contribution of LOX10, green leaf volatiles and JA to wound-induced local and systemic oxylipin and hormone signature in Zea mays (maize). Phytochemistry 2020, 174, 112334. [Google Scholar] [CrossRef]
- Payne, G.A. Aflatoxin in maize. Crit. Rev. Plant Sci. 1992, 10, 423–440. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]

| Plant Signaling | Structural Components | Defense Compounds | Other Metabolites | ||||
|---|---|---|---|---|---|---|---|
| PW-ID | Pathway Name | PW-ID | Pathway Name | PW-ID | Pathway Name | PW-ID | Pathway Name |
| R-ZMA-1119618.1 | 13-LOX and 13-HPL PW | Phenylpropanoids | PWY-5409 | divinyl ether biosyn. II (13-LOX) | Coenzyme A | ||
| R-ZMA-5608118.1 | auxin signalling | PWY1F-467 | phenylpropanoid biosyn., initial rxns | PWY-5410 | traumatin and (Z)-3-hexen-1-yl acetate biosyn. | COA-PWY | coenzyme A biosyn. I |
| R-ZMA-1119456.1 | brassinosteroid biosyn. II | PWY1F-FLAVSYN | flavonoid biosyn. | PWY-5751 | phenylethanol biosyn. | PWY-4221-1 | superPW of pantothenate and coenzyme A biosyn. II |
| PWY-2981 | diterpene phytoalexins precursors biosyn. | PWY-3181 | L-tryptophan degrad. VI | PWY-6040 | chlorogenic acid biosyn. II | COA-PWY-1 | coenzyme A biosyn. |
| PWY-5409 | divinyl ether biosyn. II (13-LOX) | PWY-361 | phenylpropanoid biosyn. | PWY-6330 | acetaldehyde biosyn. II | LIPAS-PWY | triacylglycerol degrad. |
| R-ZMA-1119566.1 | divinyl ether biosyn. II (13-LOX) | PWY-5059 | pinobanksin | PWY-6333 | acetaldehyde biosyn. I | PWY-5046 | 2-oxoisovalerate decarbox. to isobutanoyl-CoA |
| PWY-5032 | ent-kaurene biosyn. I | PWY-5160 | rose anthocyanin biosyn. I | PWY-6673 | caffeoylglucarate biosyn. | Vitamin biosynthesis | |
| PWY-5120 | geranylgeranyldiphosphate biosyn. | PWY-5313 | superPW of anthocyanin biosyn. | PWY-6922 | L-Nδ-acetylornithine biosyn. | ARO-PWY | chorismate biosyn. I |
| PWY-5035 | gibberellin biosyn. III | PWY-5868 | simplecoumarins biosyn. | PWY-861 | dhurrin biosyn. | CAROTENOID-PWY | superPW of carotenoid biosyn. |
| PWY-102 | gibberellin inactivation I (2β-hydroxylation) | PWY-641 | proanthocyanidins biosyn. from flavanols | R-ZMA-1119261.1 | salicylate biosyn. | FOLSYN-PWY-1 | superPW of tetrahydrofolate biosyn. |
| R-ZMA-5679411.1 | gibberellin signaling | PWY-6435 | 4-hydroxybenzoate biosyn. V | R-ZMA-1119344.1 | hydroxycinnamic acid serotonin amides biosyn. | PWY-1422 | vitamin E biosyn. (tocopherols) |
| R-ZMA-1119486.1 | indole-3-acetate biosyn. I | PWY-6457 | trans-cinnamoyl-CoA biosyn. | R-ZMA-1119444.1 | canavanine biosyn. | PWY-3841 | folate transformations II |
| PWY-581 | indole-3-acetate biosyn. II | PWY-6673 | caffeoylglucarate biosyn. | R-ZMA-1119495.1 | citrulline biosyn. | PWY-4221-1 | superPW of pantothenate and CoA biosyn. II |
| PWY-6219 | indole-3-acetate inactivation VIII | PWY-6787 | flavonoid biosyn. | R-ZMA-1119566.1 | divinyl ether biosyn. II (13-LOX) | PWY-5944 | zeaxanthin biosyn. |
| PWY-735 | jasmonic acid biosyn. | R-ZMA-1119316.1 | Phenylpropanoid biosyn. | R-ZMA-1119618.1 | 13-LOX and 13-HPL PW | PWY-5947 | lutein biosyn. |
| PWY-6220 | jasmonoyl-amino acid conjugates biosyn. I | R-ZMA-1119582.1 | Phenylpropanoid biosyn., initial rxns | PWY-882 | ascorbate biosyn. I | ||
| PWY-6233 | jasmonoyl-amino acid conjugates biosyn. II | Other Cell Wall Components | PWYBWI-7081 | carotenoid biosyn. (from lycopene) | |||
| NONMEVIPP-PWY | methylerythritol phosphate PW | PWY-1001 | cellulose biosyn. | R-ZMA-1119309.1 | aminopropanol biosyn. | ||
| R-ZMA-1119615.1 | Mevalonate PW | PWY-1121 | suberin monomers biosyn. | PWY-5188 | tetrapyrrole biosyn. I | ||
| PWY-5805-ARA | nonaprenyl diphosphate biosyn. III | PWY-3181 | L-tryptophan degrad. VI | ||||
| R-ZMA-1119261.1 | salicylate biosyn. | PWY-4821 | UDP-α-D-xylose biosyn. | ||||
| R-ZMA-1119438.1 | Secologanin and strictosidine biosyn. | PWY-5659 | GDP-mannose biosyn. | ||||
| PWY-5203 | soybean saponin I biosyn. | PWY-7120 | esterified suberin biosyn. | ||||
| PWY-5121 | superPW of geranylgeranyldiphosphate biosyn. II (MEP) | PWY-7343 | UDP-α-D-glucose biosyn. I | ||||
| PWY-5053 | superPW of gibberellin GA12 biosyn. | R-ZMA-1119316.1 | Phenylpropanoid biosyn. | ||||
| PWY-5410 | traumatin and (Z)-3-hexen-1-yl acetate biosyn. | R-ZMA-1119582.1 | Phenylpropanoid biosyn., initial rxns. | ||||
| PWY-6275 | β-caryophyllene biosyn. | R-ZMA-5655101.1 | xyloglucan biosyn. | ||||
| PWY-3461 | tyrosine biosyn. II | ||||||
| PWY-3481 | superPW of phenylalanine and tyrosine biosyn. | ||||||
| ALANINE-DEG3-PWY | L-alanine degrad. III | ||||||
| ALANINE-SYN2-PWY | L-alanine biosyn. II | ||||||
| ARO-PWY | chorismate biosyn. I | ||||||
| PWY-6629 | superPW of tryptophan biosyn. | ||||||
| Cell Membrane Components | |||||||
| PWY-3561 | choline biosyn. III | ||||||
| PWY4FS-5 | superPW of phosphatidylcholine biosyn. | ||||||
| PWY-5129 | sphingolipid biosyn. | ||||||
| R-ZMA-1119276.1 | choline biosyn. III | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warburton, M.L.; Jeffers, D.; Smith, J.S.; Scapim, C.; Uhdre, R.; Thrash, A.; Williams, W.P. Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. flavus Infection and Aflatoxin Accumulation in Maize. Toxins 2022, 14, 738. https://doi.org/10.3390/toxins14110738
Warburton ML, Jeffers D, Smith JS, Scapim C, Uhdre R, Thrash A, Williams WP. Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. flavus Infection and Aflatoxin Accumulation in Maize. Toxins. 2022; 14(11):738. https://doi.org/10.3390/toxins14110738
Chicago/Turabian StyleWarburton, Marilyn L., Dan Jeffers, Jessie Spencer Smith, Carlos Scapim, Renan Uhdre, Adam Thrash, and William Paul Williams. 2022. "Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. flavus Infection and Aflatoxin Accumulation in Maize" Toxins 14, no. 11: 738. https://doi.org/10.3390/toxins14110738
APA StyleWarburton, M. L., Jeffers, D., Smith, J. S., Scapim, C., Uhdre, R., Thrash, A., & Williams, W. P. (2022). Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. flavus Infection and Aflatoxin Accumulation in Maize. Toxins, 14(11), 738. https://doi.org/10.3390/toxins14110738





