In Vitro and In Vivo Inhibitory Activities of Selected Traditional Medicinal Plants against Toxin-Induced Cyto- and Entero- Toxicities in Cholera
Abstract
1. Introduction
2. Results
2.1. Estimation of Polyphenolic Content
2.2. In Vitro Studies
2.2.1. Cytotoxicity Assay
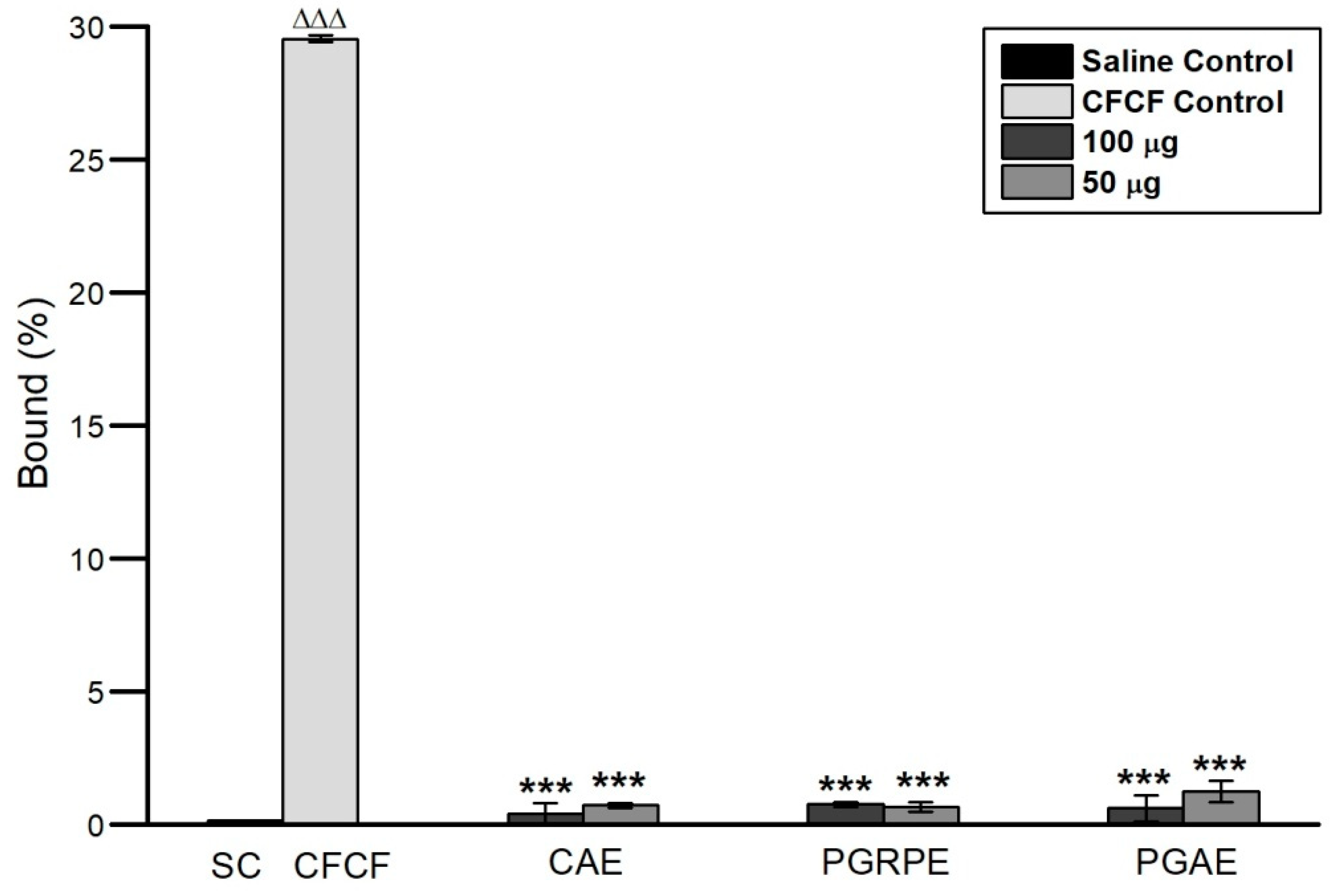
2.2.2. Binding Inhibition of CT to GM1
2.2.3. Cyclic AMP Assay
2.2.4. Protective Activity against Morphological Alterations
2.3. In Vivo Studies
2.3.1. Mice Ligated-Ileal Loop Assay
2.3.2. Estimation of cAMP Levels in Ligated-Ileal Loops
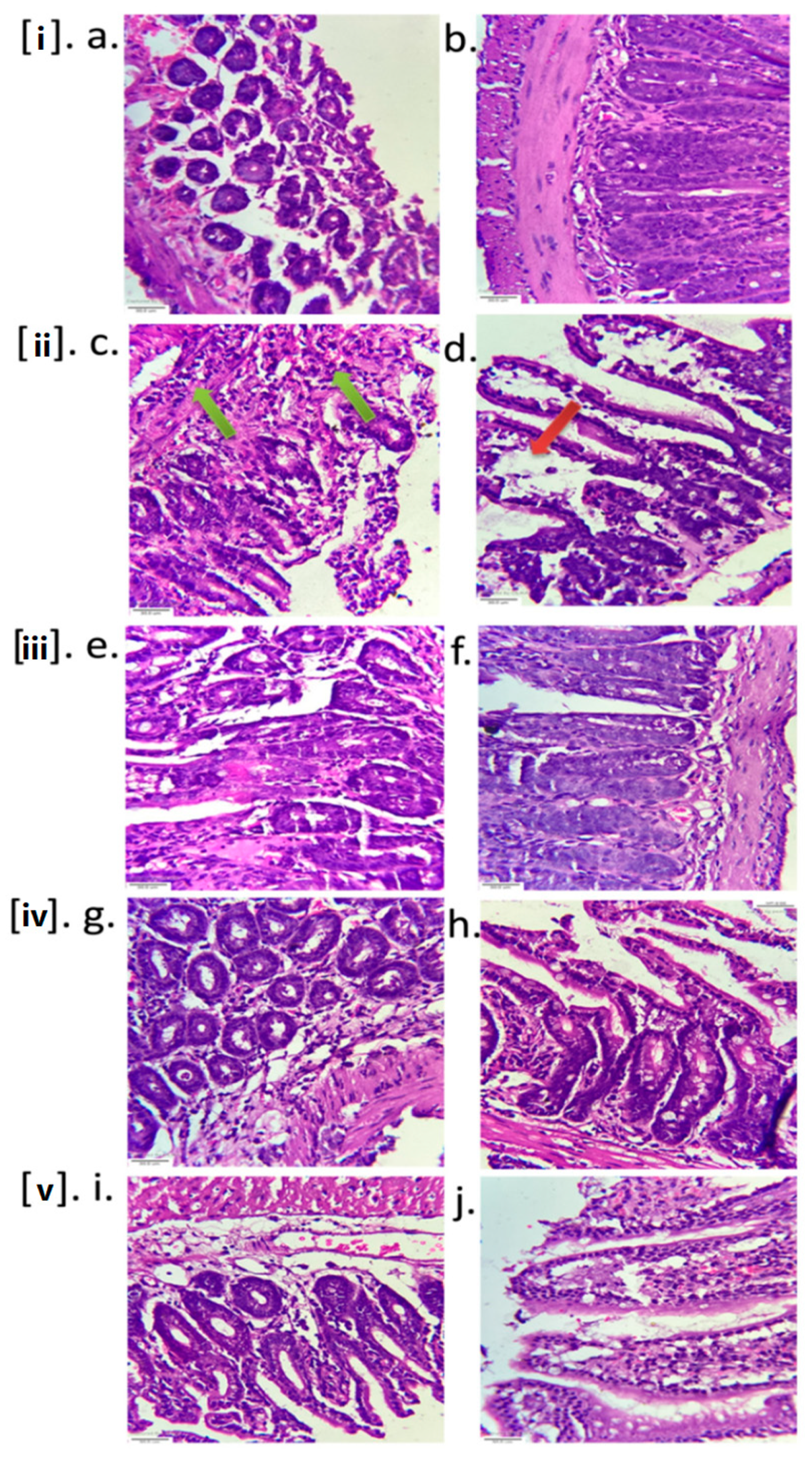
2.3.3. Histopathology
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Plant Collection, Authentication and Preparation of Extract
5.3. Estimation of Polyphenolic Content
5.3.1. Phenolic Content in Total (TPC)
5.3.2. Flavonoid Total Content (TFC)
5.4. In Vitro Studies
5.4.1. CFCF Preparation
5.4.2. CT Concentration Estimation in CFCF
5.4.3. Cytotoxicity Assay
5.4.4. Binding Inhibition of CT to Ganglioside GM1
5.4.5. Cyclic AMP Assay
5.4.6. Protective Activity against Morphological Changes
5.5. In Vivo Studies
5.5.1. Mice Ligated-Ileal Loop Assay
5.5.2. Estimation of cAMP Levels in Ligated-Ileal Loops
5.5.3. Histopathology
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical branch at Galveston: Galveston, TX, USA, 1996; Volume 273. [Google Scholar]
- Holmgren, J.; Lönnroth, I. Cholera toxin and the adenylate cyclase-activating signal. J. Infect. Dis. 1976, 133, S64–S74. [Google Scholar] [CrossRef]
- Tsai, S.C.; Noda, M.; Adamik, R.; Moss, J.; Vaughan, M. Enhancement of choleragen ADP-ribosyltransferase activities by guanyl nucleotides and a 19-kDa membrane protein. Proc. Natl. Acad. Sci. USA 1987, 84, 5139–5142. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Nandy, R.K.; Mukhopadhyay, A.K.; Dutta, S.; Mutreja, A.; Okamoto, K.; Miyoshi, S.I.; Nair, G.B.; Ghosh, A. Virulence regulation and innate host response in the pathogenicity of Vibrio cholerae. Front. Cell. Infect. Microbiol. 2020, 10, 572096. [Google Scholar] [CrossRef]
- Basu, I.; Mitra, R.; Saha, P.K.; Ghosh, A.N.; Bhattacharya, J.; Chakrabarti, M.K.; Takeda, Y.; Nair, G.B. Morphological and cytoskeletal changes caused by non-membrane damaging cytotoxin of Vibrio cholerae on Int 407 and HeLa cells. FEMS Microbiol. Lett. 1999, 179, 255–263. [Google Scholar] [CrossRef][Green Version]
- Saha, P.K.; Nair, G.B. Production of monoclonal antibodies to the non-membrane-damaging cytotoxin (NMDCY) purified from Vibrio cholerae O26 and distribution of NMDCY among strains of Vibrio cholerae and other enteric bacteria determined by monoclonal-polyclonal sandwich enzyme-linked immunosorbent assay. Infect. Immun. 1997, 65, 801–805. [Google Scholar] [PubMed]
- Sharma, C.; Thungapathra, M.; Ghosh, A.; Mukhopadhyay, A.K.; Basu, A.; Mitra, R.; Basu, I.; Bhattacharya, S.K.; Shimada, T.; Ramamurthy, T.; et al. Molecular analysis of non-O1, non-O139 Vibrio cholerae associated with an unusual upsurge in the incidence of cholera-like disease in Calcutta, India. J. Clin. Microbiol. 1998, 36, 756–763. [Google Scholar] [CrossRef]
- Brijesh, S.; Daswani, P.; Tetali, P.; Antia, N.; Birdi, T. Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: Validating its traditional usage. BMC Complement. Altern. Med. 2009, 9, 47. [Google Scholar] [CrossRef]
- Birdi, T.; Daswani, P.; Brijesh, S.; Tetali, P.; Natu, A.; Antia, N. Newer insights into the mechanism of action of Psidium guajava L. leaves in infectious diarrhoea. BMC Complement. Altern. Med. 2010, 10, 33. [Google Scholar] [CrossRef]
- Sharma, D.K.; Gupta, V.K.; Kumar, S.; Joshi, V.; Mandal, R.S.K.; Prakash, A.B.; Singh, M. Evaluation of antidiarrheal activity of ethanolic extract of Holarrhena antidysenterica seeds in rats. Vet. World. 2015, 8, 1392. [Google Scholar] [CrossRef]
- Zhao, S.S.; Ma, D.X.; Zhu, Y.; Zhao, J.H.; Zhang, Y.; Chen, J.Q.; Sheng, Z.L. Antidiarrheal effect of bioactivity-guided fractions and bioactive components of pomegranate (Punica granatum L.) peels. J. Neurogastroenterol. Motil. 2018, 30, 13364. [Google Scholar] [CrossRef]
- Souli, A.; Sebai, H.; Rtibi, K.; Chehimi, L.; Sakly, M.; Amri, M.; El-Benna, J.; Marzouki, L. Inhibitory effects of two varieties of Tunisian Pomegranate (Punica granatum L.) extracts on gastrointestinal transit in rat. J. Med. Food. 2015, 18, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Khan, O.F.; Saha, S.; Alimuzzaman, M. Antidiarrhoeal activity of the bark extract of Careya arborea Roxb. Fitoterapia 2003, 74, 116–118. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Shah, A.J.; Gilani, A.H. Insight into the possible mechanism of antidiarrheal and antispasmodic activities of piperine. Pharm. Biol. 2009, 47, 660–664. [Google Scholar] [CrossRef]
- Dey, S.; Parande, M.V.; Parande, A.M.; Lakkannavar, S.L.; Rathore, P.K.; Mantur, B.G.; Kholkute, S.D.; Roy, S. Twin outbreak of cholera in rural North Karnataka, India. Indian J. Med. Res. 2014, 140, 420–426. [Google Scholar]
- Roy, S.; Parande, M.V.; Mantur, B.G.; Bhat, S.; Shinde, R.; Parande, A.M.; Meti, R.S.; Chandrasekhar, M.R.; Kholkute, S.D.; Saini, A.; et al. Multidrug-resistant Vibrio cholerae O1 in Belgaum, south India. J. Med. Microbiol. 2012, 61, 1574–1579. [Google Scholar] [CrossRef][Green Version]
- Baek, Y.; Lee, D.; Lee, J.; Yoon, Y.; Nair, G.B.; Kim, D.W.; Kim, E.J. Cholera toxin production in Vibrio cholerae O1 El tor biotype strains in single-phase culture. Front. Microbiol. 2020, 11, 825. [Google Scholar] [CrossRef]
- Saha, P.K.; Koley, H.; Nair, G.B. Purification and characterization of an extracellular secretogenic non-membrane-damaging cytotoxin produced by clinical strains of Vibrio cholerae non-O1. Infect. Immun. 1996, 64, 3101–3108. [Google Scholar] [CrossRef]
- Aoun, J.; Hayashi, M.; Sheikh, I.A.; Sarkar, P.; Saha, T.; Ghosh, P.; Bhowmick, R.; Ghosh, D.; Chatterjee, T.; Chakrabarti, P.; et al. Anoctamin 6 contributes to Cl− secretion in accessory cholera enterotoxin (Ace)-stimulated diarrhea: An essential role for phosphatidylinositol 4, 5-bisphosphate (PIP2) signaling in cholera. J. Biol. Chem. 2016, 291, 26816–26836. [Google Scholar] [CrossRef]
- Uzzau, S.; Lu, R.; Wang, W.; Fiore, C.; Fasano, A. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol. Lett. 2001, 194, 1–5. [Google Scholar] [CrossRef]
- Cordero, C.L.; Kudryashov, D.S.; Reisler, E.; Satchell, K.J.F. The actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 2006, 281, 32366–32374. [Google Scholar] [CrossRef]
- Yamamoto, K.; Al-Omani, M.; Honda, T.; Takeda, Y.; Miwatani, T. Non-O1 Vibrio cholerae hemolysin: Purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect. Immun. 1984, 45, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Yamamoto, K.; Higa, N.; Ichinose, Y.; Nakasone, N.; Tanabe, M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 1986, 30, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, S.; Citorik, R.; LaRocque, R.C.; Ramoni, M.F.; Calderwood, S.B. A systems biology approach to modeling Vibrio cholerae gene expression under virulence-inducing conditions. J. Bacteriol. 2010, 192, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Cassel, D.; Pfeuffer, T. Mechanism of cholera toxin action: Covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc. Natl. Acad. Sci. USA 1978, 75, 2669–2673. [Google Scholar] [CrossRef]
- Saha, P.; Das, B.; Chaudhuri, K. Role of 6-gingerol in reduction of cholera toxin activity in vitro and in vivo. Antimicrob. Agents Chemother. 2013, 57, 4373–4380. [Google Scholar] [CrossRef]
- Jobling, M.G.; Yang, Z.; Kam, W.R.; Lencer, W.I.; Holmes, R.K. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. MBio 2012, 3, 00401-12. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Verkman, A.S. CFTR pharmacology and its role in intestinal fluid secretion. Curr. Opin. Pharmacol. 2003, 3, 594–599. [Google Scholar] [CrossRef]
- Saito, T.; Miyake, M.; Toba, M.; Okamatsu, H.; Shimizu, S.; Noda, M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol. Immunol. 2002, 46, 249–255. [Google Scholar] [CrossRef]
- Sawasvirojwong, S.; Srimanote, P.; Chatsudthipong, V.; Muanprasat, C. An adult mouse model of Vibrio cholerae-induced diarrhea for studying pathogenesis and potential therapy of cholera. PLoS Negl. Trop. Dis. 2013, 7, 2293. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ikigai, H.; Suzuki, T.; Suzuki, Y.; Hara, Y.; Shimamura, T. The protective activity of tea catechins against experimental infection by Vibrio cholerae O1. Microbiol. Immunol. 1992, 36, 999–1001. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food. Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.; Craft, J.W., Jr.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of cholera toxin and other AB toxins by polyphenolic compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food. Chem. 1999, 64, 555–559. [Google Scholar]
- Svennerholm, A.M.; Wiklund, G. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J. Clin. Microbiol. 1983, 17, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.M. Characterization of the binding of cholera toxin to ganglioside GM1 immobilized onto microtitre plates. J. Appl. Toxicol. 2005, 25, 30–38. [Google Scholar] [CrossRef]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar]







| Plant Name | IC50 (µg/mL) | NC1 (µg/mL) | Viability (%) | NC2 (µg/mL) | Viability (%) |
|---|---|---|---|---|---|
| Careya arborea bark (CAE) | 349.3 ± 19.3 | 85 | 90.1 | 42.5 | 95.1 |
| Punica granatum peel (PGRPE) | 446 ± 5.1 | 110 | 93.6 | 55 | 98.6 |
| Punica granatum juice (PGRJ) | ND* | 500 | 98.8 | 250 | 98.4 |
| Psidium guajava leaf (PGAE) | 486.4 ± 30.8 | 120 | 90.7 | 60 | 96.2 |
| Holarrhena antidysenterica (HAE) | 321.4 ± 22.1 | 80 | 89.9 | 40 | 91.2 |
| Aegle marmelos fruit (AME) | 646.3 ± 33.7 | 160 | 97.6 | 80 | 98.8 |
| Piper longum fruit (PLE) | 128.9 ± 14.2 | 35 | 91.3 | 17.5 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charla, R.; Patil, P.P.; Bhatkande, A.A.; Khode, N.R.; Balaganur, V.; Hegde, H.V.; Harish, D.R.; Roy, S. In Vitro and In Vivo Inhibitory Activities of Selected Traditional Medicinal Plants against Toxin-Induced Cyto- and Entero- Toxicities in Cholera. Toxins 2022, 14, 649. https://doi.org/10.3390/toxins14100649
Charla R, Patil PP, Bhatkande AA, Khode NR, Balaganur V, Hegde HV, Harish DR, Roy S. In Vitro and In Vivo Inhibitory Activities of Selected Traditional Medicinal Plants against Toxin-Induced Cyto- and Entero- Toxicities in Cholera. Toxins. 2022; 14(10):649. https://doi.org/10.3390/toxins14100649
Chicago/Turabian StyleCharla, Rajitha, Priyanka P. Patil, Arati A. Bhatkande, Nisha R. Khode, Venkanna Balaganur, Harsha V. Hegde, Darasaguppe R. Harish, and Subarna Roy. 2022. "In Vitro and In Vivo Inhibitory Activities of Selected Traditional Medicinal Plants against Toxin-Induced Cyto- and Entero- Toxicities in Cholera" Toxins 14, no. 10: 649. https://doi.org/10.3390/toxins14100649
APA StyleCharla, R., Patil, P. P., Bhatkande, A. A., Khode, N. R., Balaganur, V., Hegde, H. V., Harish, D. R., & Roy, S. (2022). In Vitro and In Vivo Inhibitory Activities of Selected Traditional Medicinal Plants against Toxin-Induced Cyto- and Entero- Toxicities in Cholera. Toxins, 14(10), 649. https://doi.org/10.3390/toxins14100649






