Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK)
Abstract
1. Introduction
2. Results
2.1. mRNA Extraction, cDNA Synthesis, Target Gene Amplification and Sequencing Analysis
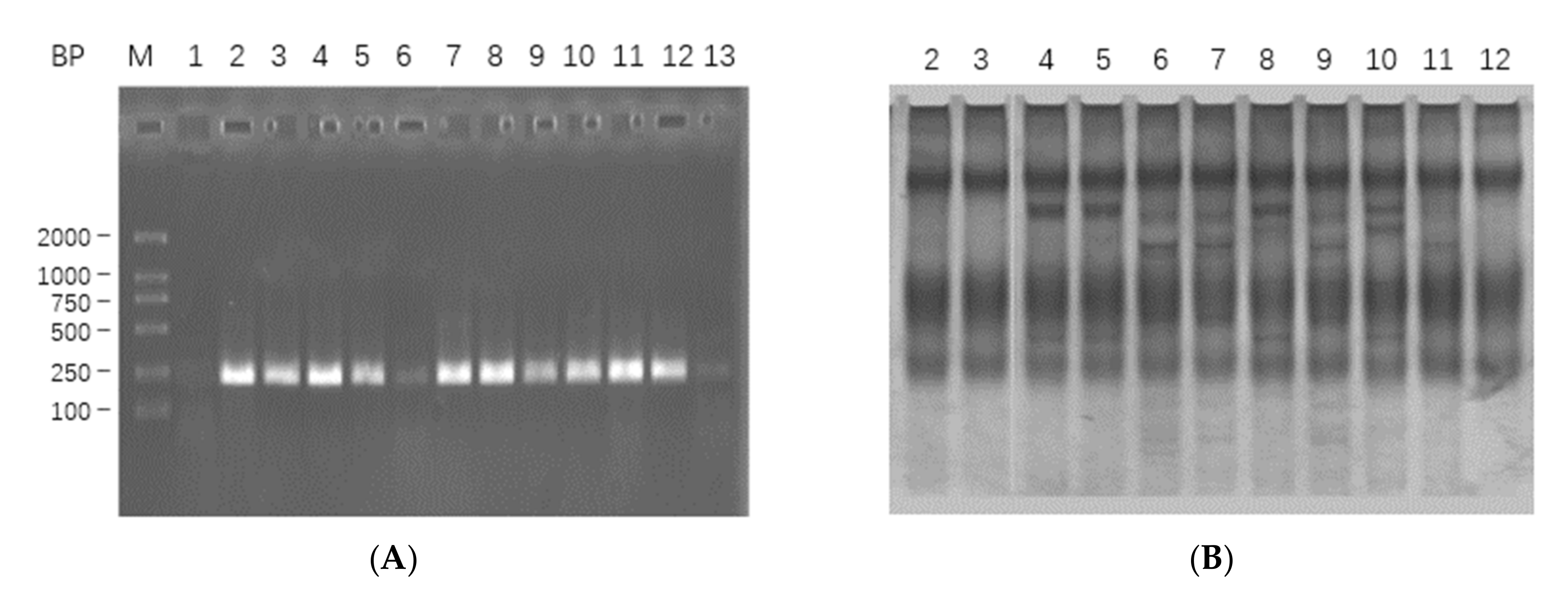
2.1.1. mRNA Extraction, cDNA Synthesis and Target Gene Amplification
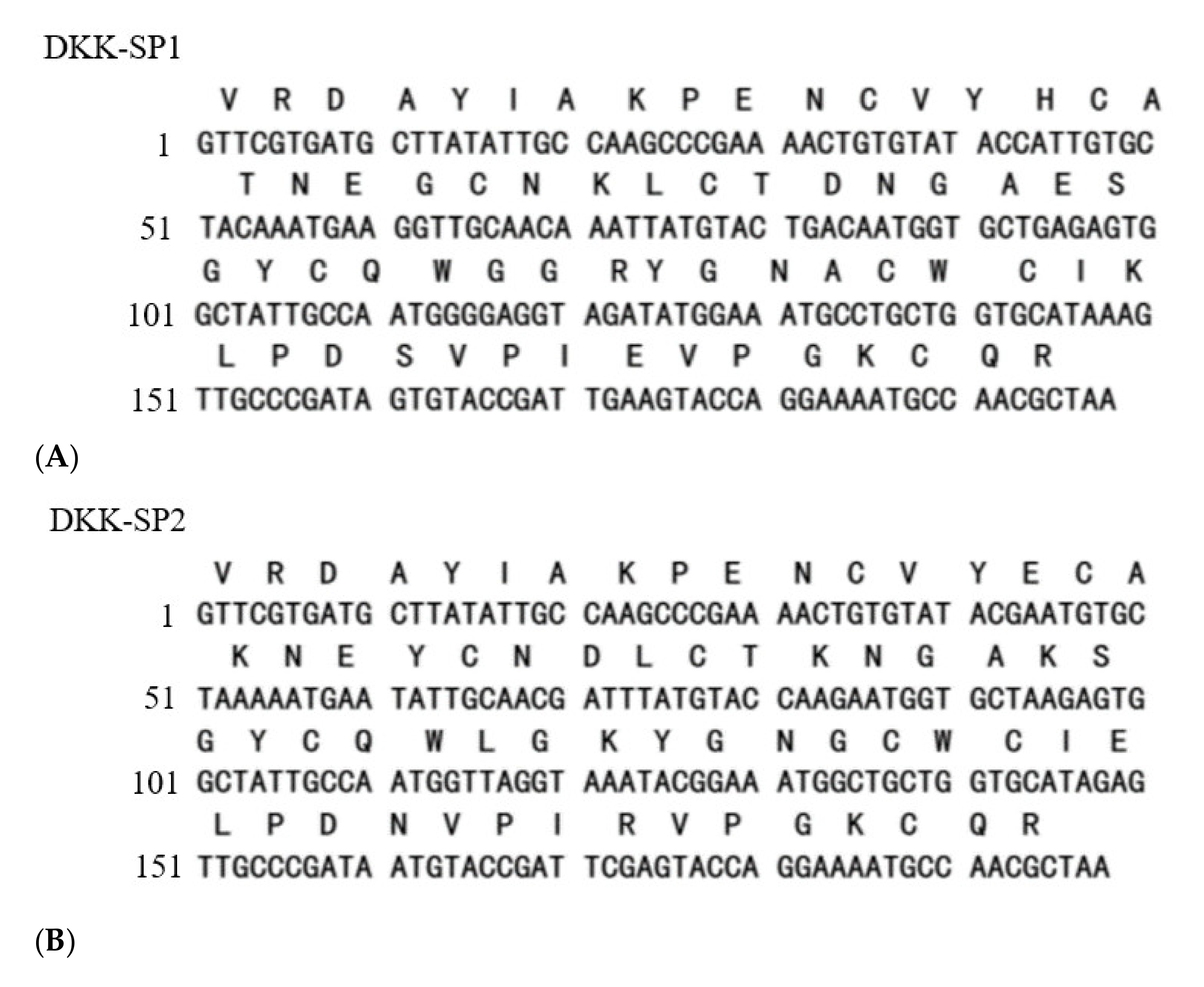
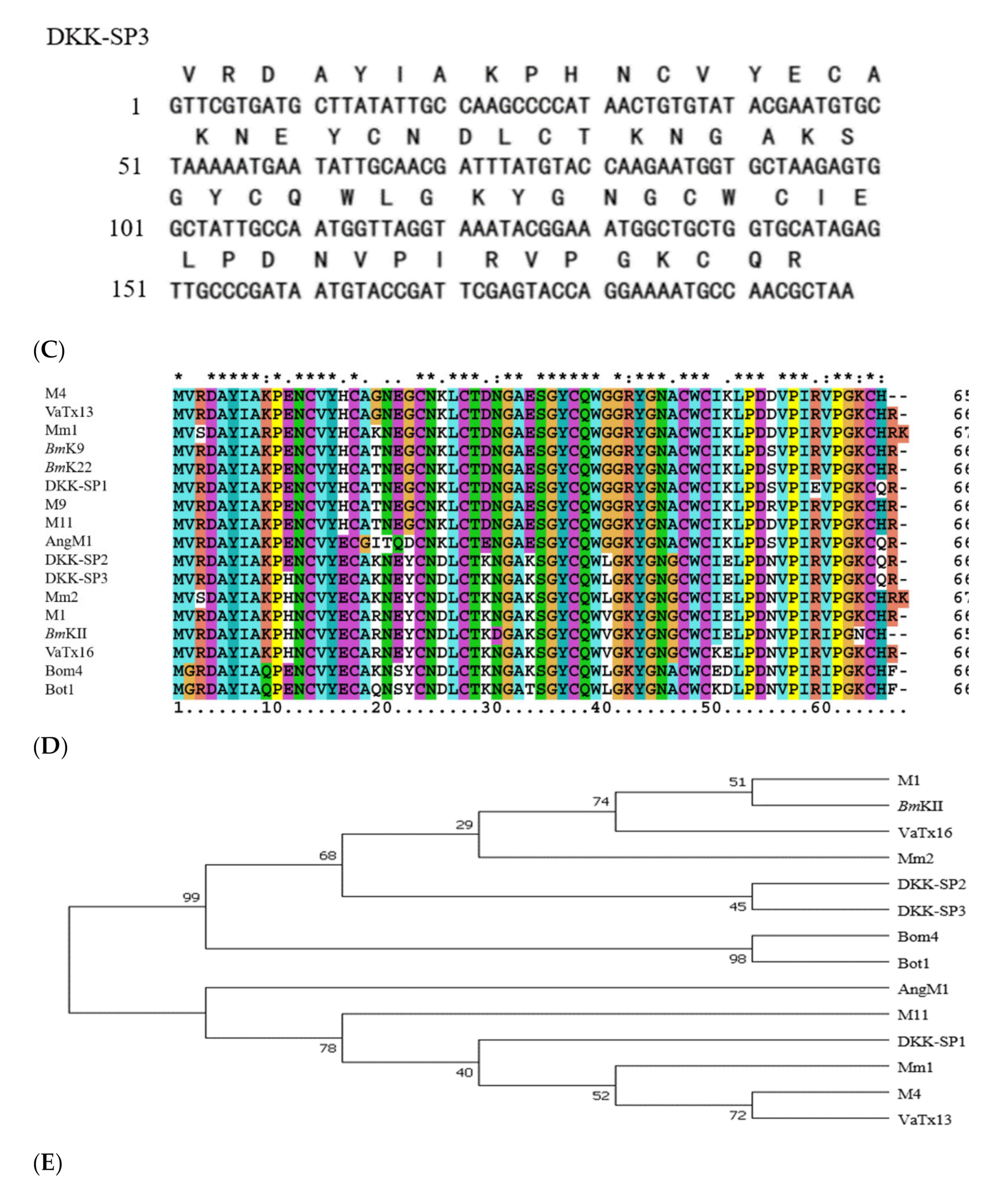
2.1.2. Analysis of Sequencing Results
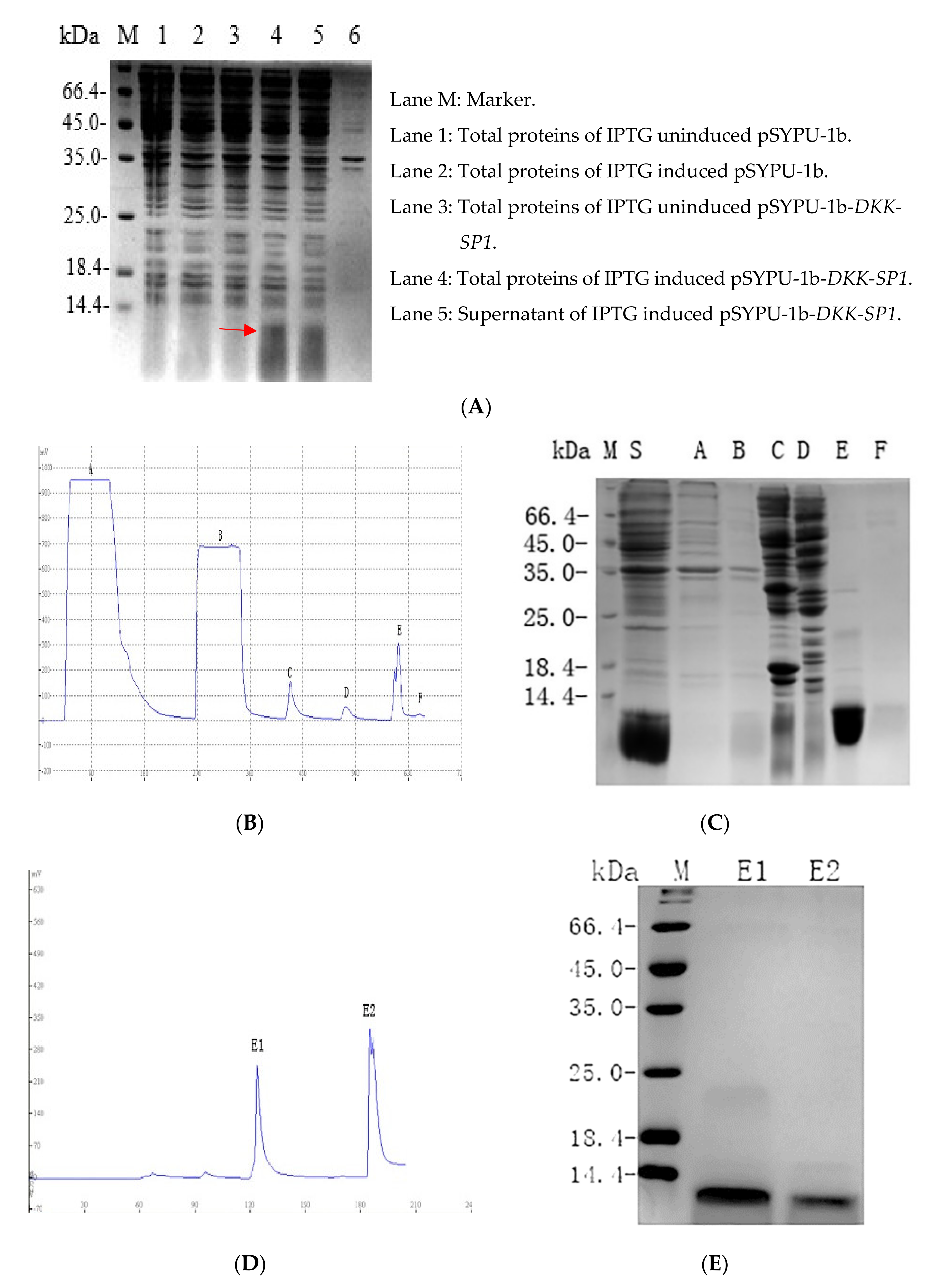
2.2. Purification and Solubility Analysis of pSYPU-1b-DKK-SP1/SP2/SP3
2.3. Acute Toxicity on DKK-SP1/SP2/SP3
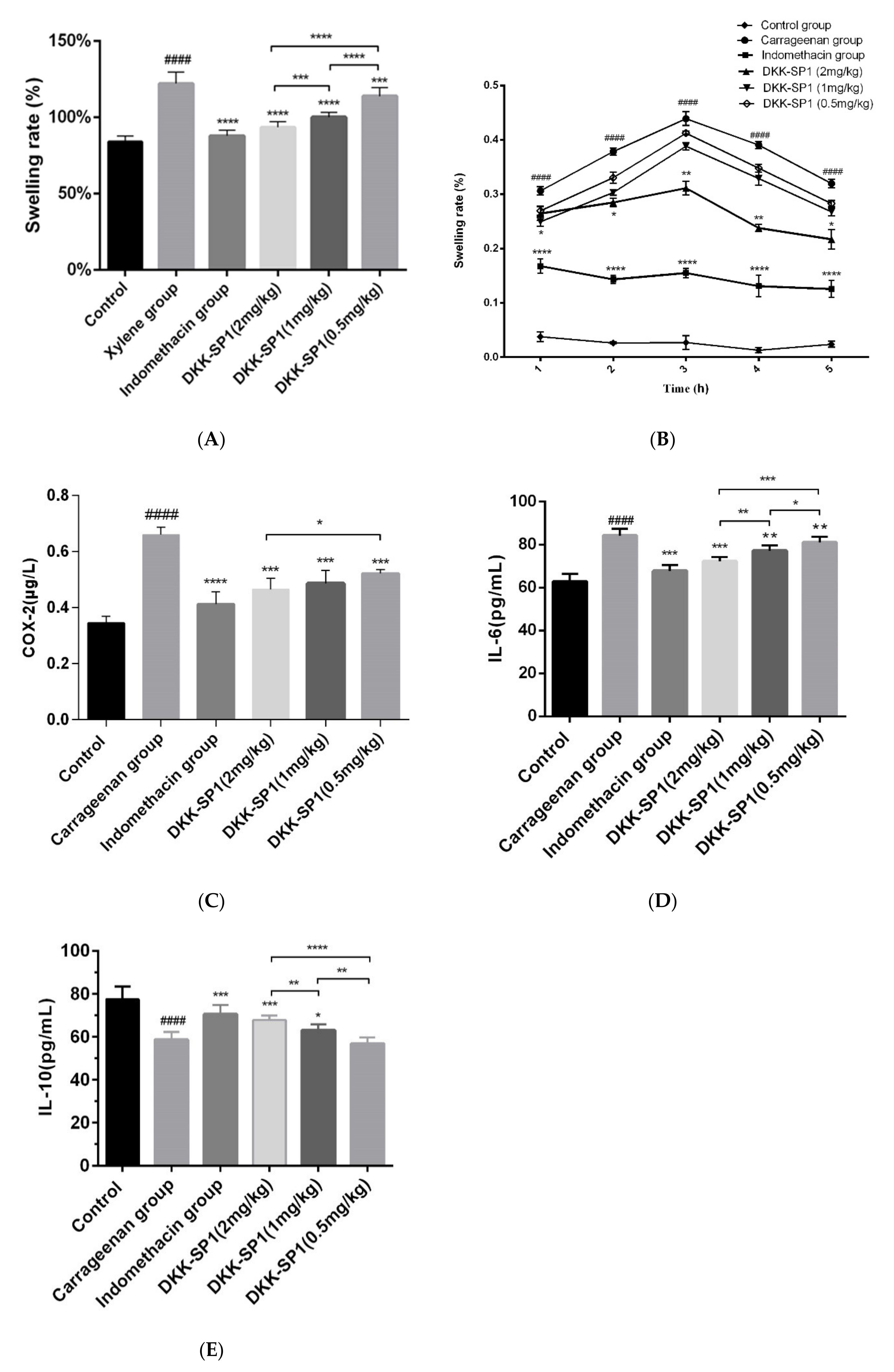
2.4. Research on Anti-Inflammatory Activity of DKK-SP1
2.4.1. Anti-Inflammatory Activity of DKK-SP1
2.4.2. Effects of DKK-SP1 on Cytokine Secretion
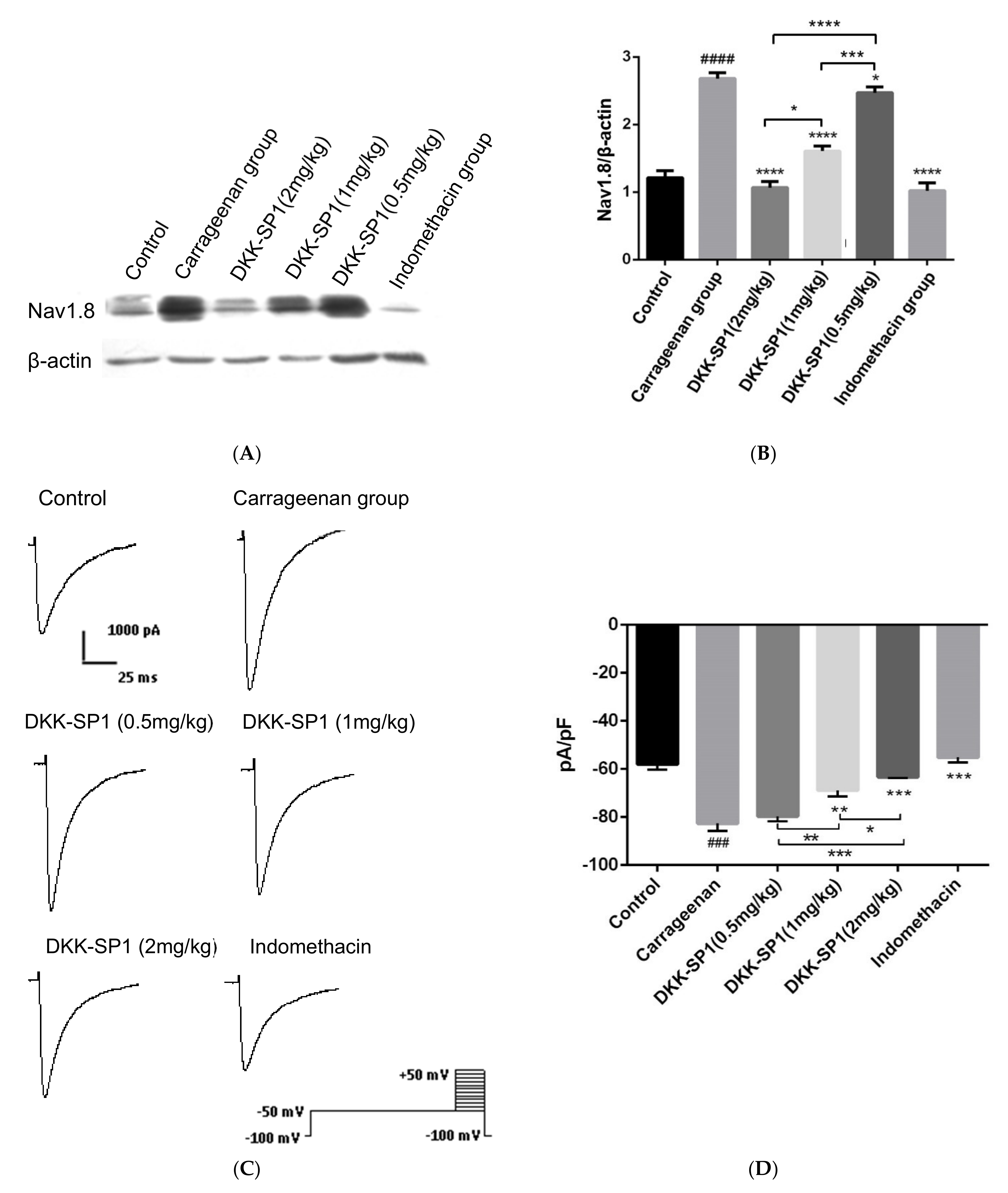
2.4.3. Effects of DKK-SP1 on the Nav1.8 Expression and Current Density of Nav1.8
2.5. Research on Analgesic Activity of DKK-SP2
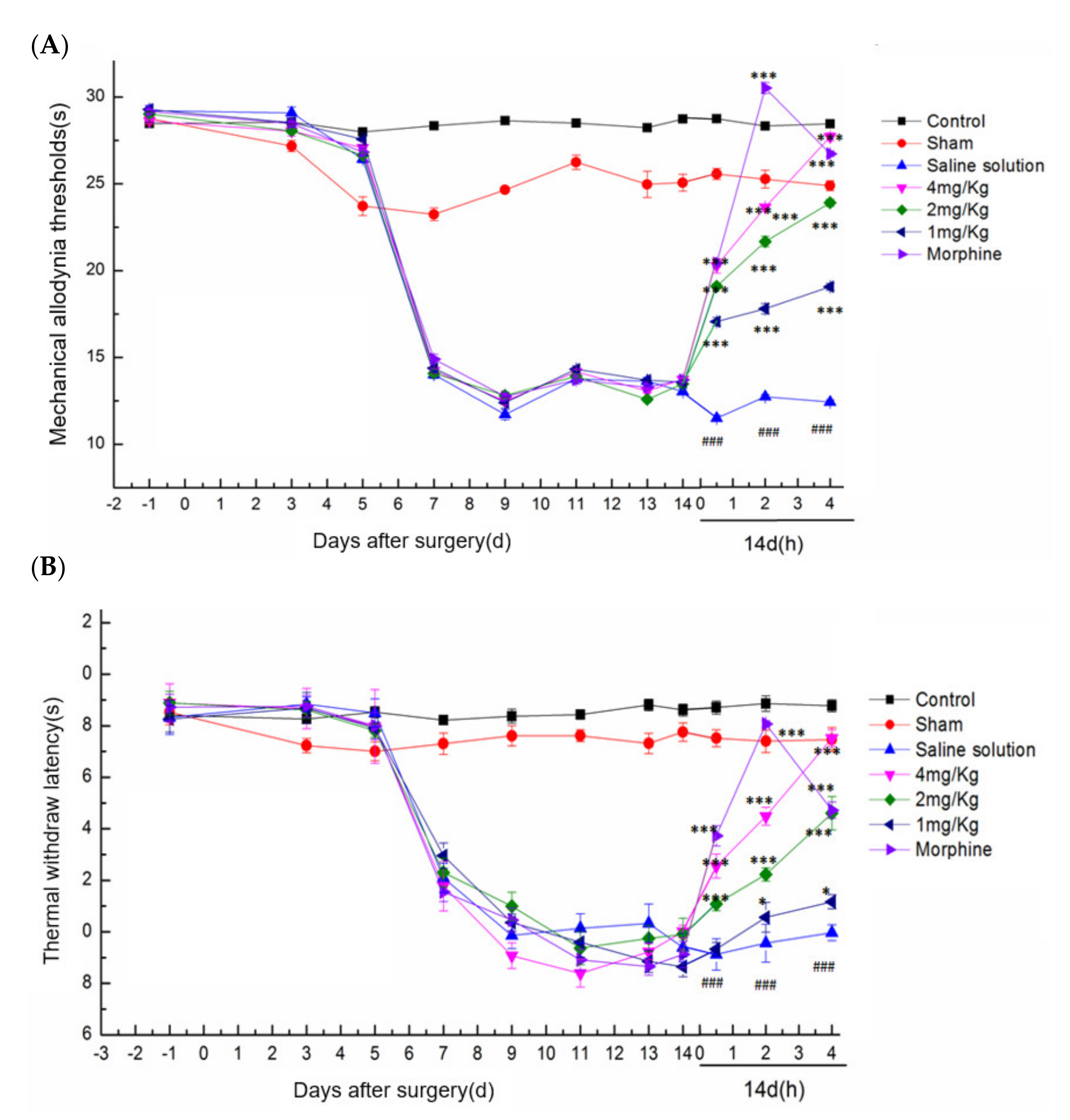
2.5.1. Analgesic Activities of DKK-SP1/SP2
2.5.2. Effect of DKK-SP2 on Mechanical Pain Threshold and Thermal Pain Threshold
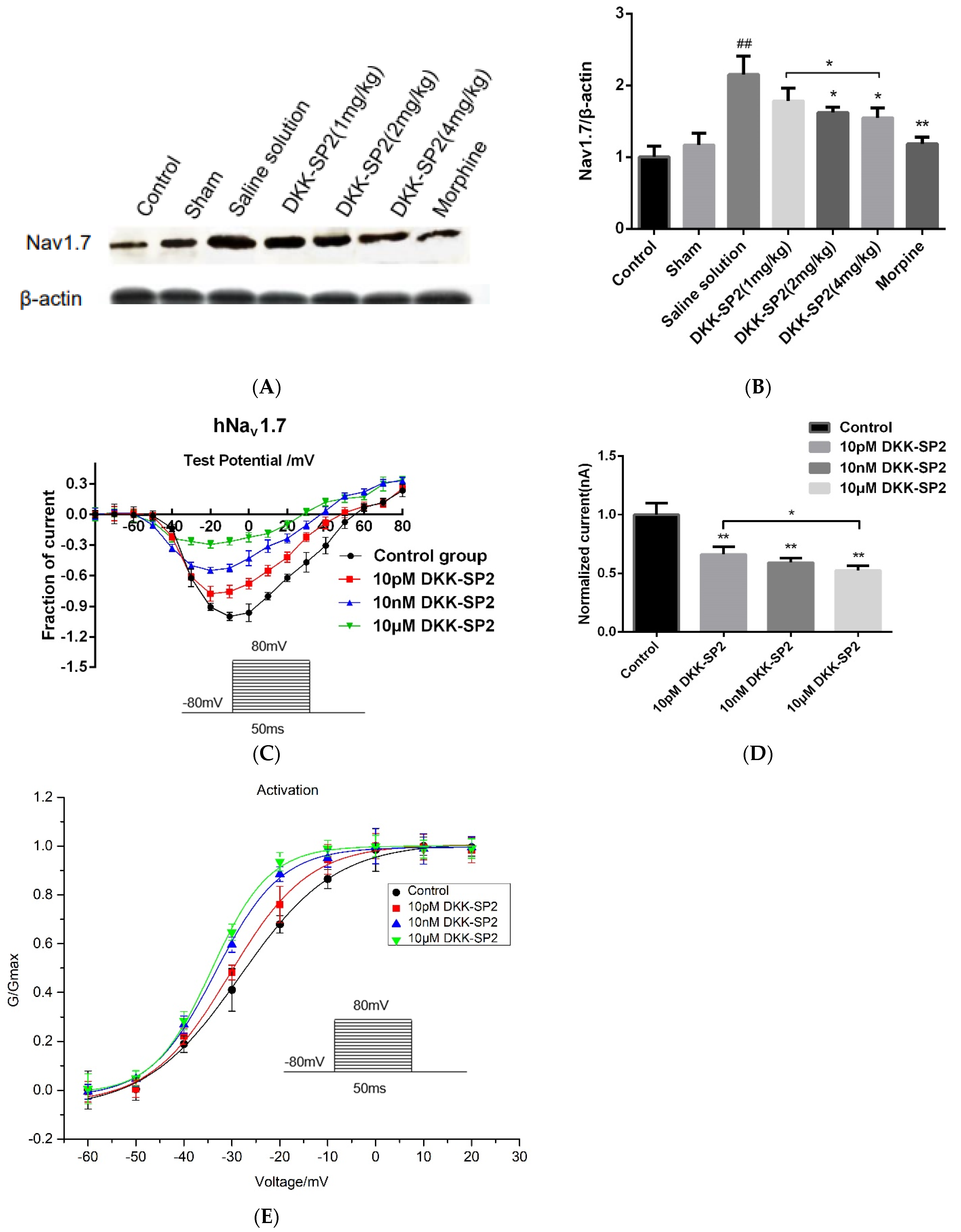
2.5.3. Effects of DKK-SP2 on Nav1.7 Expression and Currents
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal and Cell Cultures
5.2. Materials
5.3. Gene Cloning and Sequencing
5.3.1. cDNA Synthesis and Target Gene Amplification
- Oligo(dT)17: 5′-CGGAATTCGGATCCGATATC (T)17(G/C/A)-3′
- DKKSP F0: 5′-CATGCCATGGTTCGTGATGCTTATATTGCCAAG-3′
- DKKSP R0: 5′-CGGGATCCTTAGCGTTGGCATTTTCCTGGTACT-3′
5.3.2. Construction and Verification of the Expression pSYPU-1b-DKK-SP1/SP2/SP3
5.3.3. Sequencing Analysis Softwares
5.4. Purification and Solubility of DKK-SP1 and DKK-SP2
- DKKSP R0: 5′-CGGGATCCTTAGCGTTGGCATTTTCCTGGTACT-3′
- DKKSP F1: 5′-CATGCCATGGGACATCATCATCATCATCACGTTCGTGATGCTTATATTGCCAAG-3′
5.5. Acute Toxicity Test
5.6. Research on Anti-Inflammatory Activity of DKK-SP1
5.6.1. Xylene-Induced Ear Edema Model
5.6.2. Carrageenan-Induced Paw Edema Model
5.6.3. Enzyme-Linked Immunosorbent Assays
5.7. Research on Analgesic Activity of DKK-SP2
5.7.1. Acetic Acid-Writhing Test
5.7.2. The Trigeminal Neuralgia Model (ION-CCI)
5.8. Western Blotting
5.9. Electrophysiology
6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowell, G.; Diaz-Duenas, P.; Bustos-Saldana, R.; Mireles, A.A.; Fet, V. Epidemiological and clinical characteristics of scorpionism in Colima, Mexico (2000–2001). Toxicon 2006, 47, 753–758. [Google Scholar] [CrossRef]
- Kang, A.M.; Brooks, D.E. Geographic Distribution of Scorpion Exposures in the United States, 2010–2015. Am. J. Public Health 2017, 107, 1958–1963. [Google Scholar] [CrossRef]
- Lavon, O.; Bentur, Y. Poison exposures in young Israeli military personnel: A National Poison Center Data analysis. Clin. Toxicol. 2017, 55, 322–325. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Shao, Z.; Gao, B.; Li, J.; Ma, J.; Li, J.; Che, H.; Zhang, W. Eukaryotic expression and purification of anti-epilepsy peptide of iKarsch and its protein interactions. Mol. Cell. Biochem. 2009, 330, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sarfo-Poku, C.; Eshun, O.; Lee, K.H. Medical application of scorpion venom to breast cancer: A mini-review. Toxicon 2016, 122, 109–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Wang, Z.; Zhang, X.; Liang, X.; Civelli, O. BmK-YA, an Enkephalin-Like Peptide in Scorpion Venom. PLoS ONE 2012, 7, e40417. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Tytgat, J. Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon 2007, 49, 142–158. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Caliskan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Housley, D.M.; Housley, G.D.; Liddell, M.J.; Jennings, E.A. Scorpion toxin peptide action at the ion channel subunit level. Neuropharmacology 2017, 127, 46–78. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Selisko, B.; Gurrola, G.B. Structure and function of scorpion toxins affecting K+-channels. Perspect. Drug Discov. Design 1999, 15, 15–40. [Google Scholar] [CrossRef]
- He, Y.; Zou, X.; Li, X.; Chen, J.; Jin, L.; Zhang, F.; Yu, B.; Cao, Z. Activation of sodium channels by alpha-scorpion toxin, BmK NT1, produced neurotoxicity in cerebellar granule cells: An association with intracellular Ca2+ overloading. Arch. Toxicol. 2017, 91, 935–948. [Google Scholar] [CrossRef]
- Catterall, W.A.; Cestele, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Yarov-Yarovoy, V.; Scheuer, T.; Karbat, I.; Cohen, L.; Gordon, D.; Gurevitz, M.; Catterall, W.A. Mapping the Interaction Site for a beta-Scorpion Toxin in the Pore Module of Domain III of Voltage-gated Na+ Channels. J. Biol. Chem. 2012, 287, 30719–30728. [Google Scholar] [CrossRef]
- Argoff, C.E.; Alford, D.P.; Fudin, J.; Adler, J.A.; Bair, M.J.; Dart, R.C.; Gandolfi, R.; McCarberg, B.H.; Stanos, S.P.; Gudin, J.A.; et al. Rational Urine Drug Monitoring in Patients Receiving Opioids for Chronic Pain: Consensus Recommendations. Pain Med. 2018, 19, 97–117. [Google Scholar] [CrossRef]
- Derry, S.; Karlin, S.M.; Moore, R.A. Single dose oral ibuprofen plus codeine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2015, 2. [Google Scholar] [CrossRef]
- Jo, W.S.; Yang, K.M.; Choi, Y.J.; Jeong, C.H.; Ahn, K.J.; Nam, B.H.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. In Vitro and in vivo anti-inflammatory effects of pegmatite. Mol. Cell. Toxicol. 2010, 6, 195–202. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Azevedo Neto Mahon, C.P.; Marson Ascencio, P.G.; Ascencio, S.D.; Balogun, S.O.; de Oliveira Martins, D.T. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J. Ethnopharmacol. 2014, 155, 387–395. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent Developments in Anti-Inflammatory Natural Products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Bai, Z.T.; Chai, Z.F.; Zhang, J.W.; Liu, Y.; Ji, Y.H. Suppressive effects of BmK IT2 on nociceptive behavior and c-Fos expression in spinal cord induced by formalin. J. Neurosci. Res. 2003, 74, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-M.; Tao, J.; Tan, M.; Yang, H.-T.; Ji, Y.-H. U-shaped dose-dependent effects of BmK AS, a unique scorpion polypeptide toxin, on voltage-gated sodium channels (vol 158, 1895, 2009). Br. J. Pharmacol. 2010, 159, 1557. [Google Scholar]
- Ruan, J.-P.; Mao, Q.-H.; Lu, W.-G.; Cai, X.-T.; Chen, J.; Li, Q.; Fu, Q.; Yan, H.-J.; Cao, J.-L.; Cao, P. Inhibition of spinal MAPKs by scorpion venom peptide BmK AGAP produces a sensory-specific analgesic effect. Mol. Pain 2018, 14. [Google Scholar] [CrossRef]
- Chai, Z.-F.; Zhu, M.-M.; Bai, Z.-T.; Liu, T.; Tan, M.; Pang, X.-Y.; Ji, Y.-H. Chinese-scorpion (Buthus martensi Karsch) toxin BmK alpha IV, a novel modulator of sodium channels: From genomic organization to functional analysis. Biochem. J. 2006, 399, 445–453. [Google Scholar] [CrossRef]
- Li, F.; Lu, S.N.; Pan, L.S. The experimental evaluation of scorpion toxins physical dependence. Chin. J. Pharmacol. Toxin 1997, 11, 154–158. [Google Scholar]
- Zeng, X.-C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a multifunctional peptide from the Chinese scorpion Mesobuthus martensii Karsch: Gaining insight into a new mechanism for the functional diversification of scorpion venom peptides. Peptides 2012, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Wang, S.X.; Zhu, Y.; Zhu, S.Y.; Li, W.X. Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii Karsch. Peptides 2004, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, M.N.; Al-Hejin, A.M.; Bora, R.S.; Ahmed, M.M.M.; Ramadan, H.A.I.; Saini, K.S.; Baeshen, N.A.; Redwan, E.M. Production of Biopharmaceuticals in E. coli: Current Scenario and Future Perspectives. J. Microbiol. Biotechnol. 2015, 25, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.S.; Ahmed, M.M.; Liao, Y.C.; Waheed, M.T.; Sameeullah, M.; Darkhshan; Hussain, S.; Saud, S.; et al. Recent developments in therapeutic protein expression technologies in plants. Biotechnol. Lett. 2015, 37, 265–279. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Contreras-Gomez, A.; Sanchez-Miron, A.; Garcia-Camacho, F.; Molina-Grima, E.; Chisti, Y. Protein Production Using the Baculovirus-Insect Cell Expression System. Biotechnol. Prog. 2014, 30, 1–18. [Google Scholar] [CrossRef]
- Han, T.; Ming, H.; Deng, L.; Zhu, H.; Liu, Z.; Zhang, J.; Song, Y. A novel expression vector for the improved solubility of recombinant scorpion venom in Escherichia coli. Biochem. Biophys. Res. Commun. 2017, 482, 120–125. [Google Scholar] [CrossRef]
- Chugunov, A.O.; Koromyslova, A.D.; Berkut, A.A.; Peigneur, S.; Tytgat, J.; Polyansky, A.A.; Pentkovsky, V.M.; Vassilevski, A.A.; Grishin, E.V.; Efremov, R.G. Modular Organization of alpha-Toxins from Scorpion Venom Mirrors Domain Structure of Their Targets, Sodium Channels. J. Biol. Chem. 2013, 288, 19014–19027. [Google Scholar] [CrossRef]
- Luo, M.J.; Xiong, Y.M.; Wang, M.; Wang, D.C.; Chi, C.W. Purification and sequence determination of a new neutral mammalian neurotoxin from the scorpion Buthus martensii Karsch. Toxicon Off. J. Int. Soc. Toxinol. 1997, 35, 723–731. [Google Scholar] [CrossRef]
- Ye, X.; Bosmans, F.; Li, C.; Zhang, Y.; Wang, D.C.; Tytgat, J. Structural basis for the voltage-gated Na+ channel selectivity of the scorpion alpha-like toxin BmK M1. J. Mol. Biol. 2005, 353, 788–803. [Google Scholar] [CrossRef]
- Karimi, Z.; Falsafi-Zadeh, S.; Galehdari, H.; Jalali, A. Homology modeling and molecular dynamics simulation of odonthubuthus doriae (Od1) scorpion toxin in comparison to the BmK M1. Bioinformation 2012, 8, 474–478. [Google Scholar] [CrossRef][Green Version]
- Kharrat, R.; Darbon, H.; Rochat, H.; Granier, C. Structure/activity relationships of scorpion alpha-toxins. Multiple residues contribute to the interaction with receptors. Eur. J. Biochem. 1989, 181, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Wang, D.C.; Zeng, Z.H.; Jin, L.; Hu, R.Q. Crystal structure of an acidic neurotoxin from scorpion Buthus martensii Karsch at 1.85 A resolution. J. Mol. Biol. 1996, 261, 415–431. [Google Scholar] [CrossRef]
- Hongmin, L.I.; Jin, L.; Zeng, Z.; Wang, M.; Zhang, Y.; Wang, D. Crystal structure determination of an acidic neurotoxin (BmK M8) from scorpion Buthus martensii Karsch at 0.25 nm resolution. Sci. China 1996, 39, 373–384. [Google Scholar]
- Gold, M.S.; Reichling, D.B.; Shuster, M.J.; Levine, J.D. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc. Natl. Acad. Sci. USA 1996, 93, 1108–1112. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Vasko, M.R.; Nicol, G.D. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J. Physiol. Lond. 2002, 544, 385–402. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Fehrenbacher, J.C.; Vasko, M.R.; Nicol, G.D. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J. Neurophysiol. 2006, 96, 1042–1052. [Google Scholar] [CrossRef][Green Version]
- Su, Y.-Y.; Ye, M.; Li, L.; Liu, C.; Pan, J.; Liu, W.-W.; Jiang, Y.; Jiang, X.-Y.; Zhang, X.; Shu, Y.; et al. KIF5B Promotes the Forward Transport and Axonal Function of the Voltage-Gated Sodium Channel Na(v)1.8. J. Neurosci. 2013, 33, 17884–17896. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium Channels in Normal and Pathological Pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef]
- Ji, Y.H.; Mansuelle, P.; Terakawa, S.; Kopeyan, C.; Yanaihara, N.; Hsu, K.; Rochat, H. Two neurotoxins (BmK I and BmK II) from the venom of the scorpion Buthus martensi Karsch: Purification, amino acid sequences and assessment of specific activity. Toxicon Off. J. Soc. Toxinol. 1996, 34, 987–1001. [Google Scholar]
- Vogel, H.G.; Vogel, W.H. Drug Discovery and Evaluation: Pharmacological Assays; Springer Berlin Heidelberg: Heidelberg, Germany, 1997. [Google Scholar]
- Goudet, C.; Chi, C.W.; Tytgat, J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 2002, 40, 1239–1258. [Google Scholar] [CrossRef]
- Koster, R.M.; Anderson, M.; Beer, E.J.D.J.F.P. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412. [Google Scholar]
- Wang, Y.; Hao, Z.; Shao, J.; Song, Y.; Li, C.; Li, C.; Zhao, Y.; Liu, Y.; Wei, T.; Wu, C.; et al. The role of Ser54 in the antinociceptive activity of BmK9, a neurotoxin from the scorpion Buthus martensii Karsch. Toxicon 2011, 58, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Meng, D. Chemical constituents from Stauntonia brachyanthera Hand-Mazz. Biochem. Syst. Ecol. 2013, 48, 182–185. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, T.; Liu, W.; Wang, H.; Jiang, J.; Wei, Y.; Tian, H.; Zou, N.; Zhu, Y.; Shi, H.; et al. In Vivo evaluation of the anti-inflammatory and analgesic activities of compound Muniziqi granule in experimental animal models. BMC Complement. Altern. Medicine 2016, 16, 20. [Google Scholar] [CrossRef]
- Bouassida, K.Z.; Makni, S.; Tounsi, A.; Jlaiel, L.; Trigui, M.; Tounsi, S. Effects of Juniperus phoenicea Hydroalcoholic Extract on Inflammatory Mediators and Oxidative Stress Markers in Carrageenan-Induced Paw Oedema in Mice. Biomed. Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Amir, R.; Argoff, C.E.; Bennett, G.J.; Cummins, T.R. The role of sodium channels in chronic inflammatory and neuropathic pain. J. Pain 2006, 7, S1–S29. [Google Scholar] [CrossRef]
- Wu, C.; Xie, N.; Lian, Y.; Xu, H.; Chen, C.; Zheng, Y.; Chen, Y.; Zhang, H. Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ban, M.; Bai, F.; Chen, J.; Jin, X.; Song, Y. Anti-Nociceptive and Anti-Inflammation Effect Mechanisms of Mutants of Syb-prII, a Recombinant Neurotoxic Polypeptide. Toxins 2019, 11, 699. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Hou, X.; Sun, J.; Kong, X.; Sun, Y.; Liu, Z.; Ma, Y.; Niu, Y.; Song, Y.; et al. A mutant of the Buthus martensii Karsch antitumor-analgesic peptide exhibits reduced inhibition to hNa(v)1.4 and hNa(v)1.5 channels while retaining analgesic activity. J. Biol. Chem. 2017, 292, 18270–18280. [Google Scholar] [CrossRef] [PubMed]
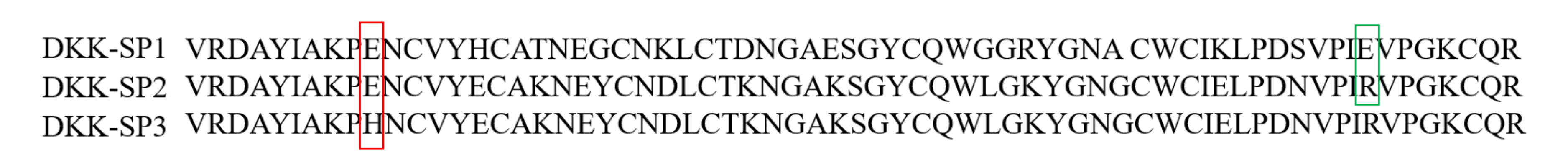
| Groups | Dose (mg/kg) | Number of Writhes (Mean±SEM) | Inhibition Efficiency (%) 1 |
|---|---|---|---|
| NS | - | 36.5 ± 3.7 | - |
| Morphine | 1.0 | 8.1 ± 2.4 *** | 77.81 |
| DKK-SP2 (2 mg/kg) | 2.0 | 8.5 ± 3.1 *** | 76.71 |
| DKK-SP2 (1 mg/kg) | 1.0 | 13.6 ± 3.4 ** | 62.85 |
| DKK-SP2 (0.5 mg/kg) | 0.5 | 30.4 ± 4.8 | 16.71 |
| DKK-SP1 | 1.0 | 31.7 ± 2.5 | 13.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Zhu, Y.; Zhang, L.; Ji, J.; Gui, M.; Li, C.; Song, Y. Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK). Toxins 2021, 13, 498. https://doi.org/10.3390/toxins13070498
Liu Y, Li Y, Zhu Y, Zhang L, Ji J, Gui M, Li C, Song Y. Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK). Toxins. 2021; 13(7):498. https://doi.org/10.3390/toxins13070498
Chicago/Turabian StyleLiu, Yunxia, Yan Li, Yuchen Zhu, Liping Zhang, Junyu Ji, Mingze Gui, Chunli Li, and Yongbo Song. 2021. "Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK)" Toxins 13, no. 7: 498. https://doi.org/10.3390/toxins13070498
APA StyleLiu, Y., Li, Y., Zhu, Y., Zhang, L., Ji, J., Gui, M., Li, C., & Song, Y. (2021). Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK). Toxins, 13(7), 498. https://doi.org/10.3390/toxins13070498




