Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control
Abstract
:1. Introduction
2. The Use of Sensorics for Determination of DON and ZON
2.1. Optical Immunosensors
2.1.1. Label-Free Optical Immunosensors
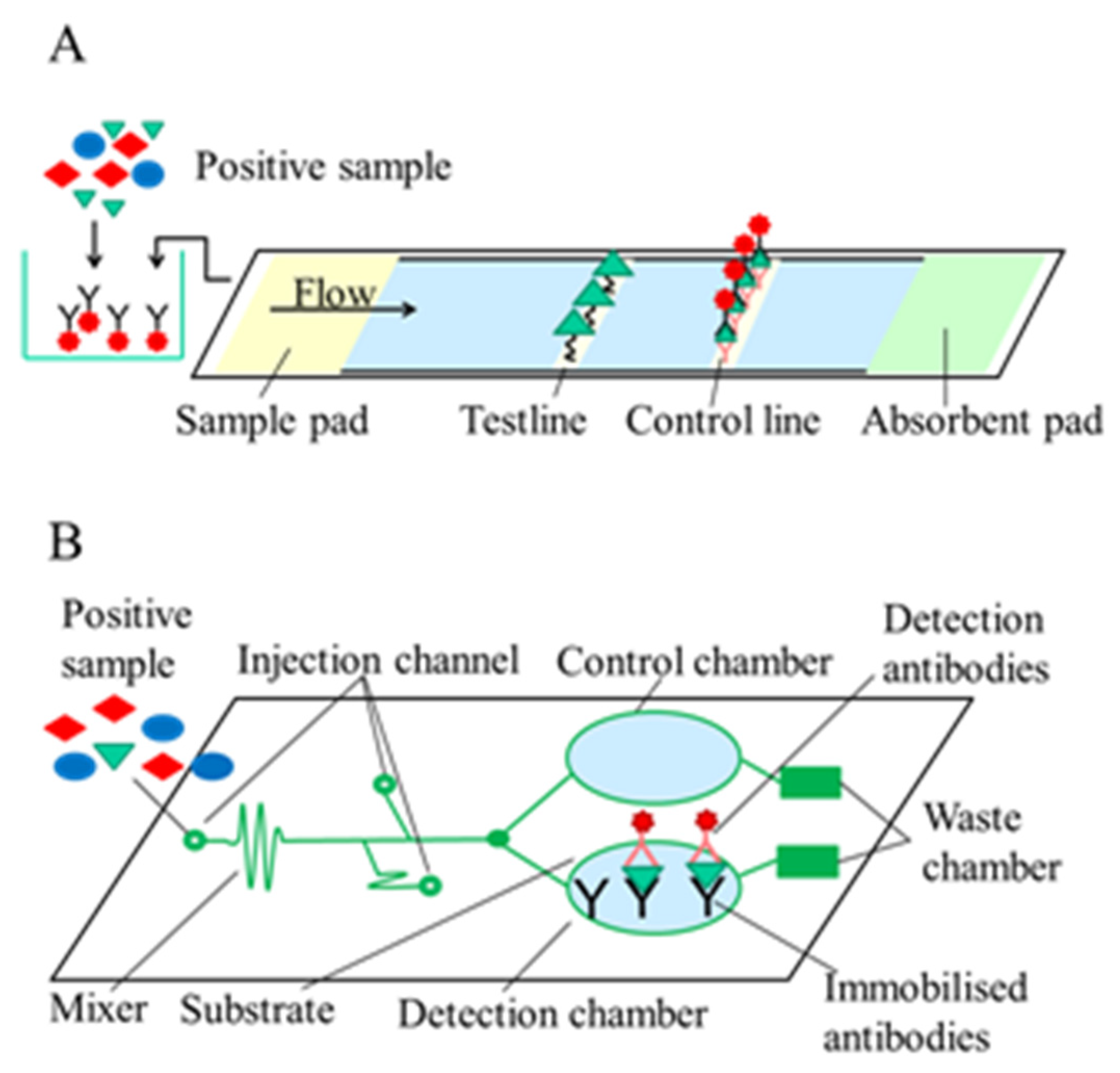
2.1.2. Label-Based Optical Immunosensors
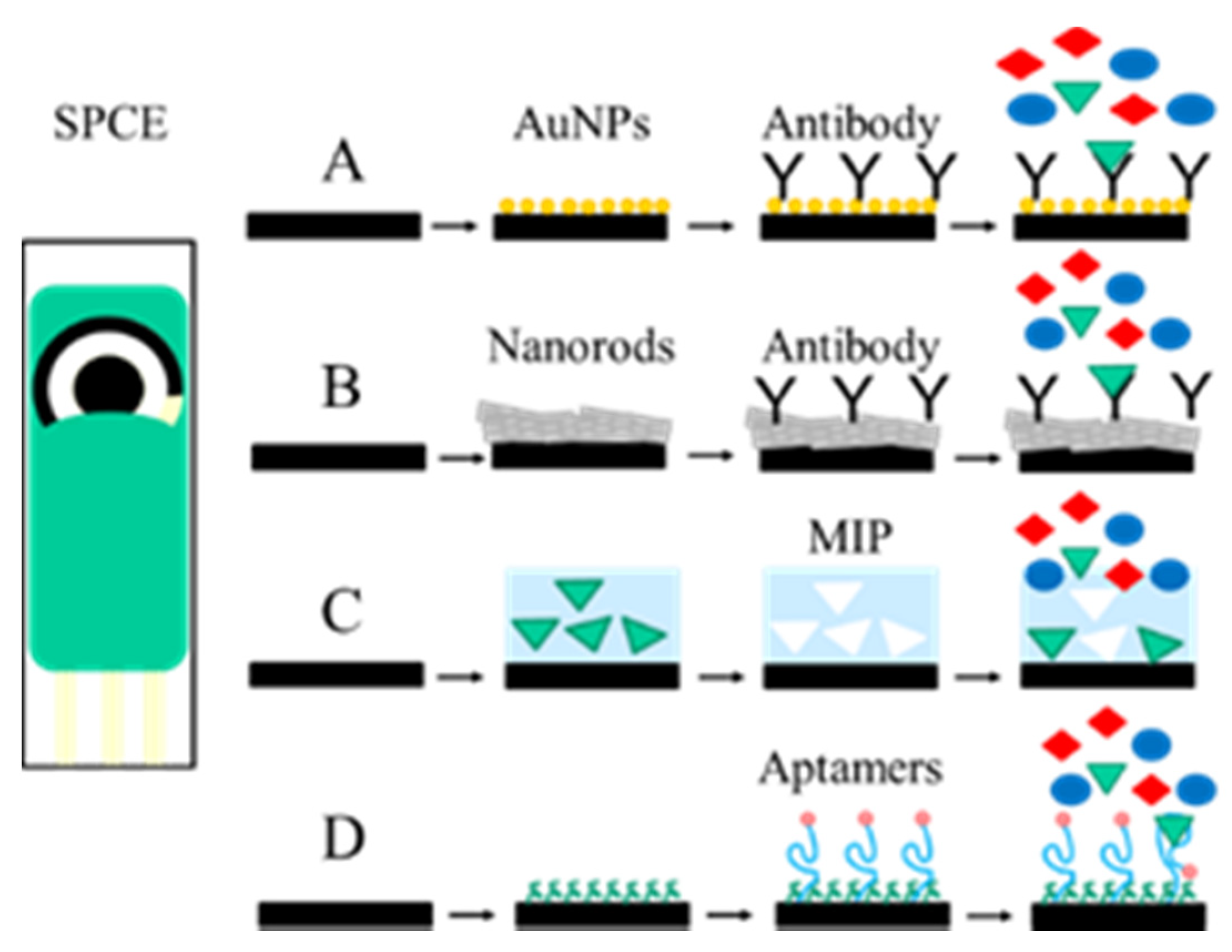
2.2. Electrochemical Immunosensors
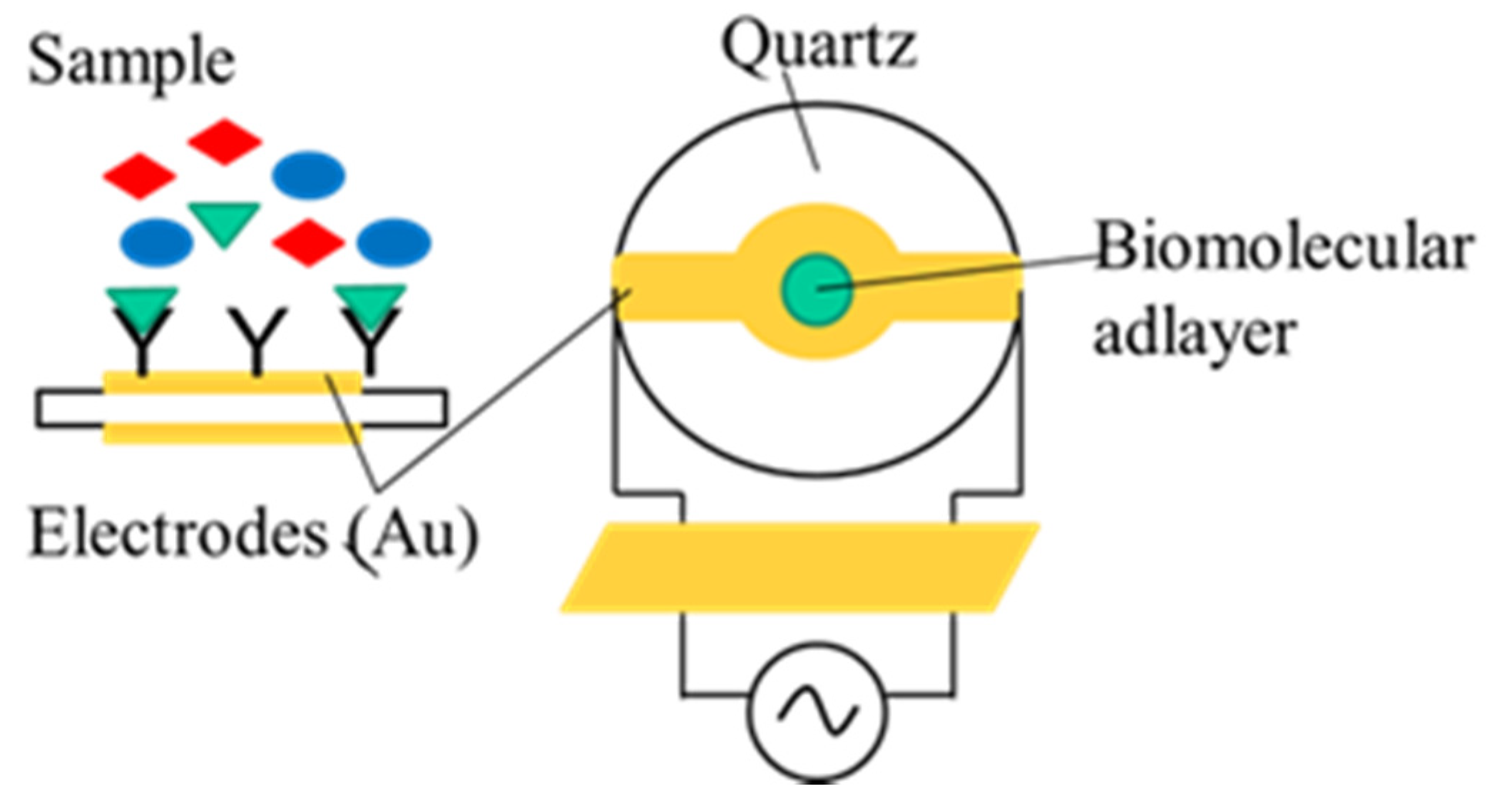
2.3. Piezoelectric Immunosensors
3. Sensors Based on Artificial Recognition Elements
3.1. Aptasensors
3.2. Molecularly Imprinted Polymer Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ricciardi, C.; Castagna, R.; Ferrante, I.; Frascella, F.; Marasso, S.L.; Ricci, A.; Canavese, G.; Lorè, A.; Prelle, A.; Gullino, M.L.; et al. Development of a microcantilever-based immunosensing method for mycotoxin detection. Biosens. Bioelectron. 2013, 40, 233–239. [Google Scholar] [CrossRef]
- CAST. Mycotoxins: Risk in plant, animal and human systems. In Task Force Report-139; Council for agricultural Science and Technology: Ames, IA, USA, 2003; ISBN -1887383220. [Google Scholar]
- RASSF–Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/food/rasff-food-and-feed-safety-alerts_en (accessed on 23 June 2021).
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.-U. Thermal Degradation of the Fusarium Mycotoxin Deoxynivalenol. J. Agric. Food Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Gbashi, S.; Njobeh, P.B. Occurrences of Deoxynivalenol, Zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. NFS J. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Becker-Algeri, T.A.; Castagnaro, D.; De Bortoli, K.; De Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [Green Version]
- Cressey, P.; Pearson, A.; Baoumgren, A. Mycotoxins. In Contaminants in Animal Feed. New Zealand Food Safety Technical Paper No: 2020/21; Ministry for Primary Industries: Wellington, New Zealand, 2020; pp. 26–33. [Google Scholar]
- Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. OJ 2006, L229, 7–9.
- Valenta, H. Chromatographic methods for the determination of ochratoxin A in animal and human tissues and fluids. J. Chromatogr. A 1998, 815, 75–92. [Google Scholar] [CrossRef]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, X.-W.; Zhang, C.; Logrieco, A.F.; Yang, M.-H. A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, M.; De Girolamo, A.; Lippolis, V.; Stroka, J.; Mol, H.G.J.; Lattanzio, V.M.T. Performance Evaluation of LC-MS Methods for Multimycotoxin Determination. J. AOAC Int. 2019, 102, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.Y.; Ryu, S.Y.; Tian, F.; Lee, S.Y.; Park, S.B.; Chun, H.S. Simultaneous Determination of Twenty Mycotoxins in the Korean Soybean Paste Doenjang by LC-MS/MS with Immunoaffinity Cleanup. Toxins 2019, 11, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Hong, S.-Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous Determination of Multi-Mycotoxins in Cereal Grains Collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oueslati, S.; Berrada, H.; Juan-Garcia, A.; Mañes, J.; Juan, C. Multiple Mycotoxin Determination on Tunisian Cereals-Based Food and Evaluation of the Population Exposure. Food Anal. Methods 2020, 13, 1271–1281. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Development of microextraction techniques in combination with GC-MS/MS for the determination of mycotoxins and metabolites in human urine. J. Sep. Sci. 2017, 40, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Shotwell, O.L.; Goulden, M.L.; Bennett, G.A. Determination of Zearalenone in Corn: Collaborative Study. J. Assoc. Off. Anal. Chem. 1976, 59, 666–670. [Google Scholar] [CrossRef] [Green Version]
- Eppley, R.M.; Trucksess, M.W.; Nesheim, S.; Thorpe, C.W.; Pohland, A.E.; Applegate, S.L.; Bean, G.A.; Chang, H.; Chatel, R.; Van Deteghem, C.; et al. Thin Layer Chromatographic Method for Determination of Deoxynivalenol in Wheat: Collaborative Study. J. Assoc. Off. Anal. Chem. 1986, 69, 37–40. [Google Scholar] [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.-Y.; Kajikawa, K.; Mihara, H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef] [Green Version]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef] [Green Version]
- Piliarik, M.; Vaisocherová, H.; Homola, J. Surface Plasmon Resonance Biosensing. Methods Mol. Biol. 2009, 503, 65–88. [Google Scholar] [CrossRef]
- Englebienne, P.; Van Hoonacker, A.; Verhas, M. Surface plasmon resonance: Principles, methods and applications in biomedical sciences. J. Spectrosc. 2003, 17, 372913. [Google Scholar] [CrossRef]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Recent Advances in Mycotoxin Determination for Food Monitoring via Microchip. Toxins 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karczmarczyk, A.; Dubiak-Szepietowska, M.; Vorobii, M.; Rodriguez-Emmenegger, C.; Dostalek, J.; Feller, K.-H. Sensitive and rapid detection of aflatoxin M1 in milk utilizing enhanced SPR and p(HEMA) brushes. Biosens. Bioelectron. 2016, 81, 159–165. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Reiner-Rozman, C.; Hageneder, S.; Dubiak-Szepietowska, M.; Dostalek, J.; Feller, K.-H. Fast and sensitive detection of ochratoxin A in red wine by nanoparticle-enhanced SPR. Anal. Chim. Acta 2016, 937, 143–150. [Google Scholar] [CrossRef]
- Sun, L.; Wu, L.; Zhao, Q. Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim. Acta 2017, 184, 2605–2610. [Google Scholar] [CrossRef]
- Rehmat, Z.; Mohammed, W.S.; Sadiq, M.B.; Somarapalli, M.; Anal, A.K. Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloids Surf. B Biointerfaces 2019, 174, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; Van Beek, T.A.; Nielen, M.W.F. Multiplex surface plasmon resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef]
- Hossain, Z.; Maragos, C.M. Gold nanoparticle-enhanced multiplexed imaging surface plasmon resonance (iSPR) detection of Fusarium mycotoxins in wheat. Biosens. Bioelectron. 2018, 101, 245–252. [Google Scholar] [CrossRef]
- Hossain, Z.; McCormick, S.P.; Maragos, C.M. An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat. Toxins 2018, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Chen, H.; Zhang, H.; He, G.; Li, X.; Zhang, X.; Liu, Y.; Li, C.M. Sensitive detection of multiple mycotoxins by SPRi with gold nanoparticles as signal amplification tags. J. Colloid Interface Sci. 2014, 431, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Adányi, N.; Majer-Baranyi, K.; Székács, A. Evanescent field effect-based nanobiosensors for agro-environmental and food safety. In Nanobiosensors; Grumezescu, A.M., Ed.; Elsevier: Cambridge, MA, USA, 2017; pp. 429–474. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Székács, A.; Szendrő, I.; Kiss, A.; Adányi, N. Optical waveguide lightmode spectroscopy technique–based immunosensor development for deoxynivalenol determination in wheat samples. Eur. Food Res. Technol. 2011, 233, 1041–1047. [Google Scholar] [CrossRef]
- Székács, I.; Adányi, N.; Szendrő, I.; Székács, A. Direct and Competitive Optical Grating Immunosensors for Determination of Fusarium Mycotoxin Zearalenone. Toxins 2021, 13, 43. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Gémes, B.; Takács, E.; Székács, A. An Optical Planar Waveguide-Based Immunosensors for Determination of Fusarium Mycotoxin Zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Τsialla, Z.; Petrou, P.; Misiakos, K.; Goustouridis, D.; Moreno, A.U.; Fernandez-Alba, A.R.; Raptis, I.; Kakabakos, S.E. Fast simultaneous detection of three pesticides by a White Light Reflectance Spectroscopy sensing platform. Sens. Actuators B Chem. 2017, 238, 1214–1223. [Google Scholar] [CrossRef]
- Anastasiadis, V.; Raptis, I.; Economou, A.; Kakabakos, S.E.; Petrou, P.S. Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor. Biosensors 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, J.; Yao, K.; Yin, Y.; Gong, M.M.; Yang, C.; Lin, F. Paper-Based Microfluidic Device (DON-Chip) for Rapid and Low-Cost Deoxynivalenol Quantification in Food, Feed, and Feed Ingredients. ACS Sens. 2019, 4, 3072–3079. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Q.; Luo, S.; He, L.; Fan, R.; Zhang, S.; Yang, C.; Chen, Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021, 336, 127718. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2018, 99, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- AlHamoud, Y.; Yang, D.; Kenston, S.S.F.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Molecularly Imprinted Impedimetric Sensor for Determination of Mycotoxin Zearalenone. Electroanalysis 2020, 32, 1788–1794. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical Immuno- and Aptasensors for Mycotoxin Determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.E.; Gobi, K.V.; Kim, K.-H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef] [PubMed]
- Shoala, T. Carbon nanostructures: Detection, controlling plant diseases and mycotoxins. In Micro and Nano Technologies; Abd-Elsalam, K.A., Ed.; Elsevier: Cambridge, MA, USA, 2020; pp. 261–277. [Google Scholar] [CrossRef]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.L. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Kailasa, S.K.; Park, T.J.; Singhal, R.K.; Basu, H. Nanoparticle-integrated electrochemical devices for identification of mycotoxins. In Handbook of Nanomaterials in Analytical Chemistry; Hussain, C.M., Ed.; Elsevier: Cambridge, MA, USA, 2020; pp. 275–296. [Google Scholar] [CrossRef]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arévalo, F.; Fernández, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuators B Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kumar, V.S.; Hayat, A.; Gobi, K.V.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Regiart, M.; Fernández, O.; Vicario, A.; Villarroel-Rocha, J.; Sapag, K.; Messina, G.A.; Raba, J.; Bertolino, F.A. Mesoporous immunosensor applied to zearalenone determination in Amaranthus cruentus seeds. Microchem. J. 2018, 141, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Gunasekaran, S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 2019, 194, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Tuantranont, A.; Wisitsora-At, A.; Sritongkham, P.; Jaruwongrungsee, K. A review of monolithic multichannel quartz crystal microbalance: A review. Anal. Chim. Acta 2011, 687, 114–128. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Solanki, P.R.; Basu, T.; O’Kennedy, R.; Malhotra, B.D. Electrochemical piezoelectric reusable immunosensor for aflatoxin B1 detection. Biochem. Eng. J. 2015, 103, 103–113. [Google Scholar] [CrossRef]
- Chauhan, R.; Solanki, P.R.; Singh, J.; Mukherjee, I.; Basu, T.; Malhotra, B. A novel electrochemical piezoelectric label free immunosensor for aflatoxin B1 detection in groundnut. Food Control 2015, 52, 60–70. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Solanki, P.R.; Manaka, T.; Iwamoto, M.; Basu, T.; Malhotra, B. Label-free piezoelectric immunosensor decorated with gold nanoparticles: Kinetic analysis and biosensing application. Sens. Actuators B 2016, 222, 804–814. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Haupt, K.; Feller, K.-H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef]
- Spinella, K.; Mosiello, L.; Palleschi, G.; Vitali, F. Development of a QCM (Quartz Crystal Microbalance) Biosensor to Detection of Mycotoxins. In Sensors and Microsystems. Lecture Notes in Electrical Engineering; Di Natale, C., Ferrari, V., Ponzoni, A., Sberveglieri, G., Ferrari, M., Eds.; Springer: Cham, Switzerland, 2014; Volume 268, pp. 195–198. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Pan, Q.; Dai, Z.; Pan, M.; Wang, S. A Portable, Label-Free, Reproducible Quartz Crystal Microbalance Immunochip for the Detection of Zearalenone in Food Samples. Biosensors 2021, 11, 53. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of Mass Sensitive Micro-Array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2021, 222, 121521. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Z.O.; Uygun, H.D.E.; Ermis, N.; Canbay, E. Molecularly Imprinted Sensors—New Sensing Technologies. In Biosensors: Micro and Nanoscale Applications; Rinken, T., Ed.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Naseri, M.; Mohammadniaei, M.; Sun, Y.; Ashley, J. The Use of Aptamers and Molecularly Imprinted Polymers in Biosensors for Environmental Monitoring: A Tale of Two Receptors. Chemosensors 2020, 8, 32. [Google Scholar] [CrossRef]
- Hianik, T. Aptamer-Based Biosensors. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Cambridge, MA, USA, 2018; Volume 7.1, pp. 11–19. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Jin, Z.; Xu, E. A fluorometric method for aptamer-based simultaneous determination of two kinds of the fusarium mycotoxins zearalenone and fumonisin B1 making use of gold nanorods and upconversion nanoparticles. Microchim. Acta 2020, 187, 254. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, E.; Chughtai, M.F.; Jin, Z.; Irudayaraj, J. Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem. 2017, 230, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Zhang, D.; Tan, W.; Shi, J.; Li, Z.; Liu, H.; Yu, Y.; Yang, L.; Wang, X.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. LWT 2021, 147, 111541. [Google Scholar] [CrossRef]
- Azri, F.A.; Eissa, S.; Zourob, M.; Chinnappan, R.; Sukor, R.; Yusof, N.A.; Raston, N.H.A.; Alhoshani, A.; Jinap, S. Electrochemical determination of zearalenone using a label-free competitive aptasensor. Microchim. Acta 2020, 187, 266. [Google Scholar] [CrossRef]
- He, B.; Yan, X. An amperometric zearalenone aptasensor based on signal amplification by using a composite prepared from porous platinum nanotubes, gold nanoparticles and thionine-labelled graphene oxide. Microchim. Acta 2019, 186, 383. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Ong, C.C.; Sangu, S.S.; Illias, N.M.; Gopinath, S.C.B.; Saheed, M.S.M. Iron nanoflorets on 3D-graphene-nickel: A ‘Dandelion’ nanostructure for selective deoxynivalenol detection. Biosens. Bioelectron. 2020, 154, 112088. [Google Scholar] [CrossRef]
- Ji, X.; Yu, C.; Wen, Y.; Chen, J.; Yu, Y.; Zhang, C.; Gao, R.; Mu, X.; He, J. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Jiang, K.; Huang, Q.; Meng, J.; Nie, D.; Zhao, Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and Au NPs (rMoS2-Au) for multiplex detection of mycotoxins. Biosens. Bioelectron. 2020, 150, 111894. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Chang, H.-J.; Lee, N.; Kim, J.-H.; Chun, H.S. Detection of Mycoestrogen Zearalenone by a Molecularly Imprinted Polypyrrole-Based Surface Plasmon Resonance (SPR) Sensor. J. Agric. Food Chem. 2009, 57, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Chang, H.-J.; Lee, N.; Chun, H.S. A Surface Plasmon Resonance Sensor for the Detection of Deoxynivalenol Using a Molecularly Imprinted Polymer. Sensors 2011, 11, 8654–8664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeyeva, T.; Yarynka, D.; Dubey, L.; Dubey, I.; Piletska, E.; Linnik, R.; Antonyuk, M.; Ternovska, T.; Brovko, O.; Piletsky, S.; et al. Sensor Based on Molecularly Imprinted Polymer Membranes and Smartphone for Detection of Fusarium Contamination in Cereals. Sensors 2020, 20, 4304. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxin | Method | Detection Range | LOD | Matrix | Selectivity/cross Reactivity | Reference |
|---|---|---|---|---|---|---|
| AFB1 OTA ZON DON | SPR | 0.99–21.92 ng/mL 1.98–28.22 ng/mL 10.37–103.31 ng/mL 5.31–99.37 ng/mL | 0.59 ng/mL, 1.27 ng/mL, 7.07 ng/mL 3.26 ng/mL | Spiked corn and wheat | AFB2 19.1% OTB 6.2% α-ZEL 15,3% 15-AcDON 16.2% | [34] |
| DON ZON T-2 | iSPR | 48–2827 µg/kg 54–790 µg/kg 42–1836 µg/kg | 15 µg/kg 24 µg/kg 12 µg/kg | wheat | 15-AcDON 150% α-ZEL 104% HT-2 n.s. | [36] |
| T-2 T2-G | iSPR | 1.2 ng/mL 0.9 ng/mL | spiked wheat | 15-AcDON < 1% HT-2Glc < 1% HT-2 < 1% | [37] | |
| AFB1 OTA ZON | iSPR | 8 pg/mL 30 pg/mL 15 pg/mL | spiked peanut | n.d. | [38] | |
| DON | OWLS | 0.01–100 ng/mL | 0.005 ng/mL | spiked wheat flour | n.d. | [40] |
| ZON | OWLS | 0.01–1 pg/mL | 0.002 pg/mL | spiked maize | α -ZEL 25.2% Zeranol 12.8% | [41] |
| ZON | PW | 0.01–1000 ng/mL | 0.01 ng/mL | ZON standard | AFB1 n.s. OTA n.s. | [42] |
| DON | WLRS | 62.5 μg/kg–12.5 mg/kg | 62.5 μg/kg | spiked maize wheat | 3-AcDON 929% 3DON-Glc 23% | [44] |
| DON | DON-Chip | 0.01–20 μg/g | 4.7 ng/g | food, feed | n.d. | [45] |
| ZON DON | NIR-based LFIA | 0.012–0.33 ng/mL 0.082–6.7 ng/mL | 0.55 μg/kg 3.8 μg/kg | maize | AFB1 <1% FB1 <1% OTA <1% T-2 <1% | [46] |
| DON OTA AFB1 | Microfluidic immunoassay | 10 ng/mL 40 ng/mL 0.1 ng/mL | spiked corn feed | OTA, AFB1 n.s. DON, AFB1 n.s. OTA, DON n.s. | [47] | |
| FB1 ZEN T-2 DON AFB1 | LFIA | 0.5–10 μg/kg 0.25–5 μg/kg 0.3–1 μg/kg 1–20 μg/kg 0.25–0.5 μg/kg | 10 μg/kg 2.5 μg/kg 1.0 μg/kg 10 μg/kg 0.5 μg/kg | maize | α-ZEL 70.6% Zeranol 32% HT-2 37% 3-AcDON 347% 15-AcDON 34% AFM1 45% | [48] |
| Mycotoxin | Method | Detection Range | LOD | Matrix | Selectivity | Reference |
|---|---|---|---|---|---|---|
| ZON | Amperometry | 0.1 to 100 pg/mL | 0.15 pg/mL | spiked maize | n.d. | [60] |
| ZON | DPV | 0.25–256 ng/mL | 0.25 ng/mL | spiked beer, wine | AFB1 AFM1 85–90% OTA OTB | [61] |
| ZON | Amperometry | 1.88–45 ng/mL | 0.57 ng/mL | Amaranthus cruentus seeds | n.d. | [62] |
| FB1 DON | DPV | 0.3–140 ng/mL 0.2–60 ng/mL | 97 pg/mL 35 pg/mL | spiked corn sample | n.d. | [63] |
| Mycotoxin | Method | Detection Range | LOD | Matrix | Selectivity | Reference |
|---|---|---|---|---|---|---|
| ZON FB1 | Fluorometric method | 0.05–100 μg/L 0.01–100 ng/L | 0.01 μg/L 0.003 ng/L | spiked corn sample | AFB1, OTA, PAT, OTB n.s. | [75] |
| ZON | Upconversion fluorescence | 0.005–100 ng/mL | 0.0018 ng/mL | maize | AFB1, AFB2, OTA, DON, FB1 ≈Low n.d. | [77] |
| ZON | Fluorescense | 0.05–100 μg/L | 0.126 μg/kg | spiked corn | AFB1, AFB2, OTA, FB1, FB2, a-ZEL, β-ZEL <13% | [76] |
| ZON | Square wave voltammetry | 0.01–1000 ng/mL | 0.017 ng/mL | spiked maize | α-ZEL, β-ZEL, ZON-14-Glc, DON, FB1 ≈high n.d. | [78] |
| ZON | Voltammetry | 0.5 pg/mL–0.5 μg/mL | 0.17 pg/mL | spiked maize | DON, AFB1, PAT ≈Low n.d. | [79] |
| ZON | DPV | 10.0 fg/mL– 10.0 ng/mL | 1.37 fg/mL | spiked maize | DON, OTA, AFB1, PAT, FB1 n.s. | [80] |
| DON | Voltammerty | 1 pg/mL–1 ng/mL | 2.11 pg/mL | spiked rice | OTA, ZON <14% | [81] |
| ZON | Cronoamperometry | 1 fg/mL to 100 ng/mL | 0.45 fg/mL | spiked beer | T-2, OTA, FB1, AFB1 n.d. | [82] |
| ZON FB1 | DPV | 0.001–10 ng/mL 0.001–100 ng/mL | 0.0005 ng/mL | maize | α-ZEL, FB2, AFB1, DON, T-2, OTA n.d. | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majer-Baranyi, K.; Adányi, N.; Székács, A. Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control. Toxins 2021, 13, 499. https://doi.org/10.3390/toxins13070499
Majer-Baranyi K, Adányi N, Székács A. Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control. Toxins. 2021; 13(7):499. https://doi.org/10.3390/toxins13070499
Chicago/Turabian StyleMajer-Baranyi, Krisztina, Nóra Adányi, and András Székács. 2021. "Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control" Toxins 13, no. 7: 499. https://doi.org/10.3390/toxins13070499
APA StyleMajer-Baranyi, K., Adányi, N., & Székács, A. (2021). Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control. Toxins, 13(7), 499. https://doi.org/10.3390/toxins13070499






