Abstract
Fungal spoilage is one of the main reasons of economic losses in the food industry, especially in the wine sector. Consequently, the search for safer and new preservation techniques has gained importance in recent years. The objective of this study was to investigate the antifungal and anti-mycotoxigenic activity from 28 microorganisms (MO) isolated from red grape. The antifungal activity of a cell free supernatant of fermented medium by the isolated MO (CFS) was tested with the agar diffusion method and the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) assay. Additionally, different antifungal compounds from the CFS were identified and quantified (organic acids, phenolic compounds, and volatile organic compounds). Finally, the most active CFS were tested as red grape bio-preservative agents. Results evidenced that CFS fermented by the strain UTA 6 had the highest antifungal activity, above all isolates, and produced a wide pool of antifungal compounds. The use of UTA 6 CFS as bio-preservative agent showed a reduction of 0.4 and 0.6 log10 spores per gram of fruit in grapes contaminated by A. flavus and B. cinerea, respectively. Moreover, UTA 6 CFS treatment reduced the occurrence of aflatoxin B1 and fumonisin (B2, B3, and B4) production in grapes contaminated by 28–100%.
Key Contribution:
In vitro experiments showed that medium fermented by isolated microorganisms demonstrated significant antifungal activity against a broad spectrum of toxigenic fungi. In situ, the bio-preservation of grapes evidenced incomplete avoidance of the fungal growth, but reduced the fungal growth rates and the production of different toxic metabolites.
1. Introduction
Along the different stages of production, fruits and vegetables are susceptible to a wide variety of pathogens, such as nematodes, insects, and different microbial organisms [1]. Among those pathogenic agents, fungi represent a group that causes more damage to the agricultural sector [2]. Fungi alter food by decreasing the self-life and the appeal of the product and could produce a wide variety of toxigenic compounds such as mycotoxins [3,4].
Grape berries are one of those products primarily affected by fungal contamination, especially by the species of fungi belonging to the Aspergillus, Botrytis, Fusarium, and Penicillium genera [5]. The mycotoxins produced by them, including aflatoxins, ochratoxin A, fumonisins, zearalenone, trichothecens, penicillic acid, and patulin, are some of the most toxic secondary metabolites commonly found in food contaminated by fungi. Many of these mycotoxins are classified by the International Agency for Research on Cancer (IARC) as carcinogenic (aflatoxins), probably carcinogenic, and possibly carcinogenic (fumonisin B1, fumonisin B2, and ochratoxin A) to humans [6]. The use of synthetic compounds has been the regular measure against fungal contaminations. Nevertheless, the relation with different health and environmental problems and the rising concern of the consumer about those issues have increased the research interest to use and develop new and safer methods to manage fungal contamination [7].
New and safer techniques have been developed, such as the use of irradiation methods [8], thermal treatments [9], use of essential oils [10], biofilms, or the use of microbial antagonists [11]. Microbial antagonists, also known as bio-preservation agents, are a series of fungi, yeast, and bacteria which exhibit different antifungal activities [12]. The occurrence of antagonist microorganisms (MO) is a phenomenon which happens in nature as a result of the competition from the microbiome present in all foods [8]. Research and isolation of MO with antifungal properties has become a trend in the development of new methods to substitute the use of synthetic origin compounds [2]. In the bibliography, this antifungal activity has been reported with many MO, just as Brevibacillus laterosporus [13], Trichoderma asperellum [14], Pseudomonas sp. [15], and many acid lactic bacteria (LAB) like Lactobacillus plantarum [16,17], Lactobacillus coryniformis [18], or Leuconostoc mesenteroides [4].
The aim of this study was: (a) to evaluate the antifungal activity by quantitative and qualitative methods of a cell free Man Rogosa and Sharpe broth (MRS) fermented by MO isolated from red grape; (b) to identify and quantify the different components present in the fermented MRS; (c) to study the bio-preservative properties from the fermented MRS on red grape contaminated by fungi; and (d) to study the differential metabolomics profiling of fungal growth on contaminated grapes.
2. Results and Discussion
2.1. Isolation and Identification of LAB Strains
From the 33 MO isolated, a total of 28 were selected after the gram stain results. From the 28 isolates, the two MO with the highest activity against fungal contaminants of grape were selected and identified by identified by peptide mass fingerprinting MALDI-TOF MS. Identifications to the species level with a Log (score) superior to 2 were considered. Both isolates were classified to the species Leuconostoc fallax. The isolates were identified to the strain levels as L. fallax DSM 10614 (UTA 6) and L. fallax DSM 10615 (UTA 7). Moreover, the identity of the two strains was confirmed by the full sequence of the 16S rRNA obtained and compared to those already deposited in NBCI using BlastN tool.
Several studies report the presence of this bacterial species in different vegetal origin sources, like grapes [19], chickpeas, tapioca, and fermented derivates like wine [20] or sauerkraut [21], but only a few researches have reported the use of this species as bio-preservative agents. In Trias et al. [22] a cell free supernatant (CFS) made by the bacteria reduced the presence of Listeria monocytogenes in plants and vegetables. Nevertheless, this article is the first detailing the potential of this species as bio-protector agent of grapes.
2.2. Antifungal Activity Assays
The antifungal activity produced by the cell free supernatant from fermented MRS (CFS) obtained by the grape isolates was determined by the qualitative method agar diffusion test (Table 1). Thereby, 23 out of 28 the CFS manifested an inhibition halo against more than 80% of the fungi tested. Overall, the isolate UTA 6 manifested the greatest antifungal activity by showing inhibition halos against all 11 fungi. The highest inhibition halos were reported in the CFS from the strain UTA 2, which obtained inhibition halos more than 1 cm against P. expansum CECT 2278 and P. commune CECT 20767. The Fusarium spp., P. expansum CECT 2278, and P. commune CECT 20767 were the most sensitive fungi to the treatment. The strain P. commune CECT 20767 was inhibited by a 100% of the CFS used. Further, more than 85% of the CFS evidenced antifungal activity against the strains F. verticillioides ITEM 12043 and F. graminearum ITEM 126. On the contrary, Aspergillus genera evidenced the highest resistance against all CFS, especially the strain A. carbonarius ISPA 5010, which was only inhibited by five of the treatments tested.

Table 1.
Antifungal activity of isolated microorganisms against Penicillium, Aspergillus, Fusarium, Botrytis and Alternaria species by agar diffusion method. Inhibition halos were marked as “++” when the halo reached more than 1 cm of diameter, as “+” when the inhibition halo was inferior to 1 cm and “-” when no inhibition halo was observed. The concentration of CFS used was 400 g/L. MRS medium was used as control.
The antifungal activity of the CFS from the grape isolated MO was carried out by MIC MFC assay (Table 2). The MIC values reported for Penicillium spp., Aspergillus spp., Alternaria spp., Fusarium spp., and Botrytis cinerea were in ranges from 12.5 to >100.0, 25.0 to >100.0, 6.3–25.0, 3.1–50.0, and 25.0–50.0 g/L, respectively. The MFC values differ from 12.5 to >100.0 g/L against Penicillium spp., 50.0 to >100.0 g/L for Aspergillus spp., 25.0–50.0 g/L for Alternaria spp., 6.3–100.0 g/L for Fusarium spp., and 50.0 to >100.0 g/L for Botrytis cinerea. The CFS had low activity against Aspergillus spp. and, as in the diffusion agar test, the strain A. carbonarius ISPA 5010 evidenced the highest resistance reaching MFC values superior to 100 g/L. On the contrary, the Fusarium and Alternaria spp. were the most sensitive to the treatment. The strains Alternaria alternata ITEM 8121, Fusarium verticillioides ITEM 12043 and Fusarium proliferatum ITEM 12072 presented the average lower values of MIC and MFC. With respect to the CFS, the isolated UTA 6, UTA 7, and UTA 9 showed antifungal activity against a greater fungal range.

Table 2.
Results of the Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) for lyophilised CFS expressed in g/L. nd = not detected.
The CFS reported in this study exhibit similar results compared to other cell free supernatants made by different MO. The Aspergillus genera tends to be the most resistant to CFS fermented by MO. In opposition, Fusarium spp. and Alternaria spp. seem to be more susceptible to these treatments [11,23,24]. This is an interesting result because very few articles report the activity of a CFS against Botrytis cinerea [25].
Regarding the data obtained by the MIC MFC assay, alike values can also be seen in the literature using other CFS. Izzo et al. [26] reported on MFC values of CFS of sweet whey fermented by different strains of L. plantarum in a range of 31.0–250.0 g/L against Aspergillus spp. Or in Martí-Quijal et al. [27] where a fermented broth made out of by-products from the fish industry evidenced MFC of 16.0–31.0 g/L against a Fusarium spp.
2.3. Identification and Quantification of Compounds Present in the CFS
The accumulation of organic acids, lactic acid, and acetic acid in the CFS is presented in Table 3a. Concentrations of lactic acid and acetic acid ranged from 1.5 to 4.5 g/L and 0.3 to 2.2 g/L, respectively. Lactic acid was found in higher concentrations in the CFS produced by the MO UTA 7, UTA 8, and UTA 15. No significant statistical differences were detected in the content of acetic acid (p < 0.05) quantified by liquid chromatography. The analysis of the phenolic compounds revealed the presence from one of the five phenolic compounds studied, phenyllactic acid (PLA) (Table 3b). Among the CFS in which the phenyllactic acid was detected, the concentrations ranged from 0.6 to 1.4 mg/L. This compound was more abundant in the CFS produced by UTA 3, UTA 6, and UTA 9. Previous studies report the activity of these three compounds as antimicrobial agents against a wide spectrum of MO contaminants, from yeast to molds [28,29]. Nevertheless, there is not unanimous opinion concerning the concentrations of these compounds required to inhibit fungal growth. Gerez et al. [30] report MIC values of lactic acid from 2.5 to 300.0 mM, of acetic acid from 0.3 to 120.0 mM, and phenyllactic acid 0.02–6.0 mM against F. graminearum and A. niger. Izzo et al. [26] also link higher productions of PLA in medium fermented by acid lactic bacteria with rising antifungal activities against Aspergillus, Penicillium, and Fusarium spp.

Table 3.
Identification and quantification of the (a) organic acids (g/L) and (b) phenolic compounds (mg/L) produced by the microorganisms isolated from grape in CFS. The results are expressed as mean ± standard deviation. Statistically significant differences for each fermentation using one-way ANOVA Tukey HSD post hoc test are indicated with different letters (p < 0.05).
Between the volatile organic compounds (VOCs) studied a total of 36 compounds were detected and quantified in the CFS and the MRS control (Table S1). The VOCs were divided in four groups according to their chemical groups, they were classified as alcohols, aldehydes, pyrazines and others. Compared to the samples, the MRS control evidenced a tiny spectrum of different compounds and the more abundant chemicals were 2,4-Di-tert-butylphenol and the aldehydes. Fermentation of the MRS by the isolates showed an increase on the pool of compounds present in the CFS, such as ethanol, pyrazines, and acetic acid. In particular, the concentration of different pyrazines significantly increased between 10% and 40%. The antifungal activity of pyrazines is well known in the literature. Many articles report the effect of this volatile compound on the inhibition of different Penicillium and Fusarium [31,32].
2.4. Leuconostoc fallax DSM 10614 and DSM 10615 as a Grape Bio-Preservative
The bio-preservative effect of the CFS fermented by L. fallax DSM 10614 (UTA 6) and DSM 10615 (UTA 7) of red grapes inoculated with A. flavus, A. niger and B. cinerea during incubation can be seen in Figure 1. Fungal growth determination was performed as seen in Figure 2. After 12 days of incubation, grapes treated with CFS fermented by UTA 6 evidenced a significant reduction of 15% of the infected grapes inoculated with A. flavus (p < 0.05) compared to the MRS control. Moreover, in the control grapes contaminated with B. cinerea reached a contamination of 100% after 10 days, although when the CFS fermented by UTA 6 was applicated, contamination was reduced to 88%. No visible augment of the shelf life of the fruit was achieved in the grapes inoculated with A. niger. The results of the microbiological analysis from the grapes confirmed the results evidenced by the study of the self-life (Figure 3). After 12 days, the control grapes showed a population of 5.00 log10 spores/g, whereas the fungal population was reduced significantly to 4.60 and 4.50 log10 spores per gram of fruit (p < 0.05) in grapes treated with the CFS fermented by UTA 6 and UTA 7, respectively. In grapes inoculated with B. cinerea, the fungal population in control grapes reached 5.40 log10 spores/g, while in the grapes treated with CFS produced by UTA 6, a significant decrease of the fungal growth was achieved, 4.80 log10 spores/g (p < 0.05). However, the CFS fermented by UTA 7 showed a significant increase in the number of Botrytis cinera spores per g of grape compared to the control and the CFS by UTA 6 at the end of the storage period. The concentration of secondary metabolites derived from fermentation in the food assay was not sufficient to inhibit fungal growth, increasing the spore formation.

Figure 1.
Results of the bio-preservation test on red grape contaminated with B. cinerea (A), Aspergillus flavus (B), Aspergillus niger (C). Treatment used were MRS as control, and CFS fermented by the strains UTA 6 and UTA 7. Values were expressed as mean ± standard deviations of triplicates. Statistically significant differences between the mean of contaminated percentage grapes during storage time and the type of treatment are indicated with different letters, (p < 0.05).

Figure 2.
Fungal growth of A. flavus on grapes after 12 days at room temperature and in the dark conditions. A negative growth (A) and a positive growth (B).
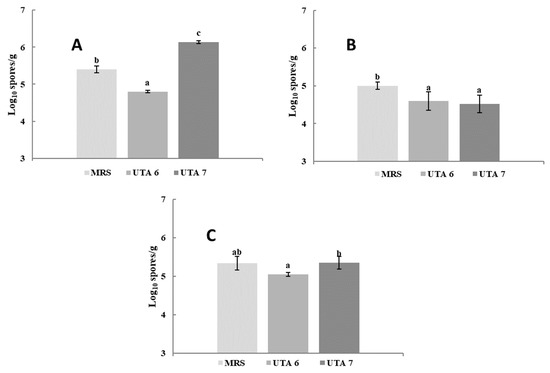
Figure 3.
Results of the determination of the fungal population in red grape contaminated with B. cinerea (A), Aspergillus flavus (B), Aspergillus niger (C). Treatment used were MRS as control, and CFS fermented by the strains UTA 6 and UTA 7. Values were expressed as mean ± standard deviations of triplicates. Statistically significant differences between the mean content of spores/g and the type of treatment are indicated with different letters, (p < 0.05).
There are no previous publications in the bibliography related to the use of CFS produced by LAB strains for the bio-preservation of red grapes. Nevertheless, different studies have proven the bio-preservation of red grape with other methods. For instance, Lappa et al. [33] evidenced the antifungal activity of a Lactobacillus plantarum strain against Aspergillus spp. The immersion of the grapes in a suspension of 108 CFU/mL of the LAB studied was able to delay the fungal growth two days. Moreover, other bacterial species have been used as bio-preservatives, such as in the work of Kasfi et al. [34], where strains of Bacillus spp. reduced the growth of A. flavus a 27% in grapes treated with a suspension (107 CFU/mL) of the antagonistic MO. Previous studies reported bio-preservative effects of CFS fermented by L. plantarum TR7 and TR71 against Aspergillus flavus ITEM 8111 and Penicillium expansum CECT 2278 on tomato [12].
2.5. Metabolite Profiling of Fungal Growth on Grape
The LC-MS-TOF-based metabolomics approach is an interesting tool to study changes in the fungal metabolomics profiling. In the present study, the differential metabolites profile expressed by A. flavus and A. niger on inoculated grapes and treated by CFS were evaluated. A total of 14 toxic metabolites were identified. A. flavus produced on inoculated grapes a differential production of aflatoxin B1, 3-O-methylsterigmatocystin, cyclopiazonic acid, aspinolide B, and asperloxin A. However, A. niger showed the production of other fungal metabolites like fumonisin B2, B3, B4, rubrofusarin, pyranonigrin A, funalenone, and aurasperone E, C, and B. In Figure 4 shows the heat map of the differential metabolite profiling produced by A. flavus and A. niger between the three grapes treatments. Differences were also shown in the abundance of fungal metabolites between the two bio-preservation treatments using CFS fermented by UTA 6 and UTA 7. Similar activities have been evidenced in the bibliography with other bio-preservative compounds. In the work of Císarová et al. [35], the use of different essential oils as bread preserving agents also evidenced an influence on the metabolite production of several Aspergillus species, reducing the occurrence of mycotoxins compared to the control.
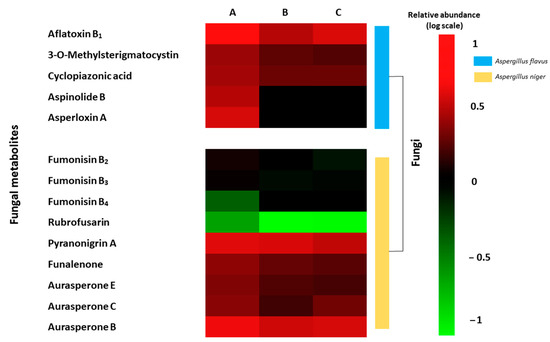
Figure 4.
Heat map of the fungal metabolites produced by Aspergillus flavus and Aspergillus niger on inoculated grapes treated ((A) Control; (B) UTA 6; (C) UTA 7) after 12 days of incubation at room temperature. Colors are based on relative abundance (logarithmic scale) of metabolites produced independently for each fungus, where red represents high abundance and green represents low abundance.
Frequently monitored mycotoxins in food like aflatoxin B1, fumonisin B2, fumonisin B3, fumonisin B4 produced by Aspergillus flavus and Aspergillus niger were quantified using calibration curves with commercial standards. In Figure 5, the content of these mycotoxins expressed in µg/Kg is reported. In the control grapes, the content of aflatoxin B1, fumonisin B2, fumonisin B3 and fumonisin B4 were 198 µg/Kg, 841 µg/Kg, 718 µg/Kg, and 264 µg/Kg. Bio-preservation of grapes using CFS evidenced a statistically significant reduction in the mean content of the four mycotoxins. Moreover, the fumonisin B2 and fumonisin B4 was not detected on treated grapes using CFS fermented by UTA 6. In general, the UTA 6 treatment showed significant reductions in the content of the 4 mycotoxins compared to the UTA 7 treatment. Other sources have report this anti-mycotoxigenic activity from other LAB strains. In the work of Nazareth et al. [36], the production of several metabolites in a CFS fermented by Lactobacillus plantarum CECT 749 has been linked to a reduction of aflatoxin B1 and fumonisin B1 in corn contaminated by A. flavus and F. verticillioides. Guimarães et al. [37] also reported anti-aflatoxigenic activity in vitro related with compounds such as lactic acid, PLA, hydroxyphenyllactic acid, and indol acetic acid present in CFS fermented by L. plantarum UM55 against Aspergillus parasiticus and A. flavus. They reported a percentage reduction in aflatoxins production in the range of 53–97%.
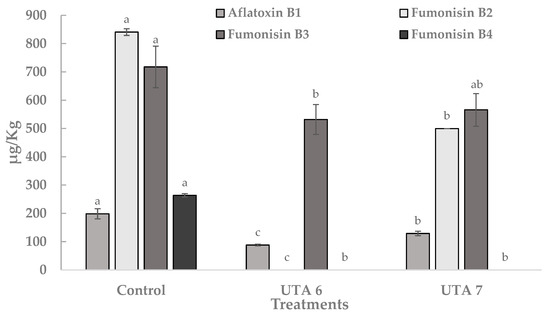
Figure 5.
Quantification of mycotoxin aflatoxin B1, fumonisin B2, fumonisin B3, fumonisin B4 produced by Aspergillus flavus and Aspergillus niger on inoculated grapes treated after 12 days of incubation at room temperature. Values were expressed as mean ± standard deviations of triplicates. Statistically significant differences between the mean content of each mycotoxin and the type of treatment are indicated with different letters, (p < 0.01).
3. Conclusions
The in vitro experiments demonstrated that CFS produced by UTA 6 has a significant antifungal activity against a wide variety of different fungal species. Furthermore, this antifungal activity may be related to the production of a biocomplex of antimicrobial compounds, including lactic acid, acetic acid, PLA, and pyrazines. The application of this CFS evidenced a reduction in the fungal growth in grapes contaminated with A. flavus and B. cinerea. The application of the UTA 6 CFS managed to significantly reduce the occurrence of aflatoxin B1, fumonisin B2, fumonisin B3, and fumonisin B4 in the grapes contaminated by A. flavus and A. niger. Therefore, applications of this method may present a promising increase in the self-life of grapes as a post-harvest treatment, reduce the use of traditional antifungal compounds, and have a significant impact on human health, reducing the presence of toxic metabolites produced by the species tested. Further investigations will focus on the pre-harvest application of CFS in the field to prevent fungal development and reduction of mycotoxin production in grapes.
4. Materials and Methods
4.1. Chemicals and Materials
The media cultures used for the study were Man Rogosa and Sharpe broth (MRS). Man Rogosa and Sharpe agar (MRS-A), potato dextrose broth (PDB), and potato dextrose agar (PDA) were all purchased from Liofilchem (Teramo, Italy). The deionized water (<18 MΩ/cm) was obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, USA).
Lactic acid was from Sigma-Aldrich (St. Louis, MO, USA) and acetic acid were obtained from Fisher Scientific (Waltham, MA, USA). Gallic acid, chlorogenic acid, caffeic acid, syringic acid, vanillic acid, p-coumaric, hydroxybenzoic acid, vanillin, hydroxycinnamic acid, sinapic acid, benzoic acid, phenyllactic acid, dihydrocaffeic acid, 3,4-dihydroxyhydrocinnamic acid, and DL-p-hydroxyphenyllactic acid were acquired from Sigma–Aldrich (Dublin, Ireland). Phenyllactic acid was obtained from BaChem (Weil am Rhein, Germany). Ferulic acid was purchased from MP Biomedicals, and protocatechuic acid came from HWI Pharma Services (Ruelzheim, Germany). All analytes had a purity of 95%.
Mycotoxins aflatoxin B1 and fumonisin B1 were provided from Sigma–Aldrich (St. Louis, MI, USA) with a purity ≥95%.
The filamentous fungi used in this study were Penicillium commune CECT 20767, Aspergillus niger CECT 2088, Botrytis cinerea CECT 20973, Penicillium expansum CECT 2278, and Penicillium digitatum CECT 2954 from the Colección Española de Cultivos Tipo (CECT) (Valencia, Spain) and Aspergillus carbonarius ITEM 5010, Fusarium verticillioides ITEM 12043, Fusarium proliferatum ITEM 12072, Fusarium graminearum ITEM 126, Aspergillus flavus ITEM 8111, Alternaria alternata ITEM 8121, purchased from the ITEM Collection from Istituto di Scienze delle Produzioni Alimentari (ISPA) (Bari, Italy).
4.2. Microorganisms Isolation
The MO investigated in this study were isolated from red grapes from different Tempranillo wine grapes vineyards located in Villar del Arzobispo (Valencia, Spain). Grapes were homogenized in distilled water with 0.1 % peptone water using a using a Stomacher (IUL, Barcelona, Spain). The homogenized mix was used to inoculate MRS. After 24 h at 37 °C, serial dilutions of the incubated MRS were sowed in MRS agar. After an incubation of 24 h at 37 °C different colonies were taken and cultured on new MRS agar plates by the streak plate technique [38]. This incubation was performed also under anaerobic conditions. After isolation, the MO were subjected to a Gram staining and a morphological analysis by microcopy. Gram positive bacteria and yeast were selected for the study. Selected MO were frozen with MRS broth, with 25 % of glycerol, at −80 °C. For the preparation of the treatments studied in this work, the MO were recovered in MRS broth medium 37 °C for 24 h.
4.3. Use of a MALDI-TOF MS System and 16S rRNA Gene Sequencing for Bacterial Identification
Identification of the strains with the highest antifungal activity was performed as described in [39]. The analysis was carried out from an isolated culture of the bacteria. The equipment used was a MALDI-TOF MS with a mass spectrophotometer Microflex L20 (Bruker Daltonics) and a N” laser. The acquisition of all spectrums was in positive linear ion mode. The voltage acceleration was 20 kV and the mass range was set from 2000–20,000 Da. Following the method MALDI Biotyper Realtime Classification (RTC), three spectrums were obtained from each sample. The identification corresponded to the highest log score. The results were compared with the database MBT 7854 y MBT 7311_RUO (Bruker Daltonics). Moreover, 16S rRNA gene sequencing of isolates was performed using the method described by Chenoll et al. [40].
4.4. Preparation of the CFS
The CFS was made following the next steps. After their defrost and recovery, the MO were cultivated in MRS at 37 °C until the exponential phase growth (12 h). Then, MRS was inoculated with MO in a 1/100 (v/v) proportion and incubated for 72 h at 37 °C. Afterwards, a CFS was obtained by centrifugation at 4000 RPM for 15 min at 4 °C and biomass was discarded. The CFS was frozen at −20 °C, then lyophilized (FreeZone 2.5 L, Labconco, Kansas City, MO, USA) to be used in subsequent assays.
4.5. Antifungal Activity Assays by Qualitative Method
Agar diffusion method was performed as described in Luz et al. [12]. A total of 11 fungal strains were cultured on PDA plates using sterile cotton swabs. Then wells were punched with sterile 1000 µL pipette tips. Finally, 100 µL from a 400 g/L suspension of each CFS was added to each well and the plates were incubated at 25 °C for 48 h under aerobic conditions. Each experiment was carried out in triplicate and the average of the three measured diameters was used. Antifungal activities were recorded as “++” when the inhibition halo reached more than 1 cm of diameter, as “+” when the inhibition halo was inferior to 1 cm, or “-” when no inhibition halo was observed. MRS suspended at 400 g/L was used as control.
4.6. Antifungal Activity Assays by Quantitative Method
The technique used for this analysis was the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) method as described by Nazareth et al. [36]. This test was performed using the CFS that evidenced the greatest results in the quantitative test. Trials were performed per quadruplicate. For each of them, a positive control, which consisted in 100 µL of sterile PDB inoculated with 100 µL of the spore suspension (105 spores/mL), and a negative control (200 µL sterile PDB) was performed. In a 96-well plate, 100 µL of a solution of PDB with 105 spores/mL was mixed at a 1:1 (v/v) ratio with different concentrations, from 200.0 to 0.1 g/L, of the CFS. Then, after 72 h of incubation at 25 °C, the MIC was identified as the smallest concentration in which there was an absence of fungal growth compared to the positive control.
Afterwards, 10 µL from the MIC well and higher concentrations were plated on PDA and incubated for 72 h at 25 °C to control the growth of the species tested. Then, the MFC was described as the lowest concentration in which no fungal growth was observed.
4.7. Determination of Organic Acids and Phenolic Compounds in the CFS
The organic acids (lactic acid and acetic acid) were analysed using a JASCO Analytica (28600 Mary's Ct, Easton, MD, USA) high-performance liquid chromatography (HPLC) system equipped with a quaternary pump, an MD 4015 PDA diode array detector, a 20 µL sample injection loop, and a Rezex ROA-Organic Acid (150 × 7.8 mm) reverse phase column (Phenomenex Inc. 411 Madrid Avenue, Torrance, CA, USA). Mobile phase consisted in an isocratic solution of water and formic acid at 0.1% (v/v) flowing at a rate of 0.8 mL/min for 20 min. Assay was performed with wavelength of 214 nm for quantification [41]. Calibration curves were performed using lactic acid and acetic acid in MRS Broth, diluted 1/20 (v/v), at a standard final concentration from 0.125 to 1 g/L. A total of 3 replicates were performed of each CFS (independent fermentations) and the results were given in g/L.
For the analysis of the phenolic compounds, fist a QuEChERS extraction of the CFS was performed [42]. A total of 10 mL of the samples was mixed by vortex with a solution of 4 g de MgSO4, 1 g NaCl, and 10 mL of ethyl acetate (with 1% of formic acid). After centrifugation, the supernatant was added to 150 mg C18 and 900 mg MgSO4 and vortexed for 1 min. Then samples were centrifuged again, and the supernatant was dried under nitrogen flow until the analysis.
Before the injection in the chromatograph samples were suspended in 1 mL with a solution of water with acetonitrile 10% (v/v). The chromatograph used was an Agilent 1200 (Palo Alto, CA, USA) equipped with a vacuum degasser, a binary pump and autosampler. A Gemini C18 column (50 9 2 mm, 100 Å and particle size 3 μm; Phenomenex) was used as stationary phase and 0.1% FA in water (solvent A) and 0.1% FA in ACN (solvent B) as the mobile phase. The elution rate used was: 0 min, 5% B; 30 min, 95% B; 35 min, 5% B. The volume used for each injection was 20 µL. The flow rate was set at 0.3 mL/min and a run time of 37 min.
The mass spectrophotometry (MS) study a was executed with a Q-TOF-MS (6540 Agilent Ultra High-Definition Accurate Mass), equipped with an Agilent Dual Jet Stream electrospray ionisation (Dual AJS ESI) operating in the negative ion mode. Parameters were set at; capillary voltage 3.5 kV, drying gas flow (N2) 8.0 L/min, nebuliser pressure 30 psig, temperature of 350 °C and fragmentor voltage of 175 V. Targeted MS/MS study was performed using collision energy of 10, 20 and 40 eV. Calibration curves with all phenolic compound standards described in the Section 4.1 were carried out using a concentration range of 0.01 to 1 mg/L. Software use for the data integration was MassHunter Qualitative Analysis Software B.08.00 [43]. A triplicate of analysis was performed for each sample.
4.8. Determination of Volatile Organic Compounds (VOC) in the CFS
The analysis of the VOC present in the CFS was performed using a headspace solid-phase microextraction (SPME) and a single quadrupole mass spectrometer detector (GC/MS) Agilent 6890N GC system (Agilent Technologies, Palo Alto, CA, USA). A quantity of 200 µg of the CFS was diluted in 2 mL of water and incubated at 55 °C for 45 min. Then, the VOC were extracted of the headspace using a SPME (Supelco, Bellafonte, PA, USA) with a fiber coated with a 50/30 μm layer of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS). Volatiles were desorbed from the DVB/CAR/PDMS fiber 250 °C for 5 min inside the injector from the GC/MS in splitless mode. Helium (99.999%) was used as carrier gas set at a flow rate of 1.0 mL/min. Column used in the assay was an HP-5MS (30 m × 0.25 mm, 0.25 μm 5% diphenyl/ 95% dimethylpolysiloxane) capillary column (J&W Scientific, Folsom, CA, USA), and was set at a temperature of 40 °C, for 2 min, increased to 160 °C at 6 °C per min, and to ramp to 260 °C at 10 °C per min (held for 4 min). The ion set temperature was set at 230 °C, a mass range of 40–450 Da and an electron ionization energy of 70 eV. Identification of the compounds was performed using NIST Atomic Spectra Database version 1.6 (Gaithersburg, MD, USA) setting the similarity spectra at 95% [44]. A total of 3 replicates were performed of each CFS (independent fermentations). Results were expressed as mean (n = 3) ± standard deviation of percentage of each area by the total area of the chromatogram peaks.
4.9. Protection of Red Grapes from Fungal Spoilage by CFS
Adapting the steps from Luz et al. the assay was performed as it follows [12]. Under sterile conditions in a Telstar MH 100 laminar flow hood (Terrassa, Spain) red grapes (from ESTABLECIMIENTOS MAS Y MAS S.L., Burjassot, Valencia, Spain), were decontaminated by submerging in a solution of water and sodium hypochlorite at 1%, then washed with sterile distilled water. A total of 81 grapes divided into 9 for each treatment and fungus were placed in groups of 3 in different petri dishes as replicates (n = 9). Each grape was punched with a sterile needle, then sprayed with 1 mL of a conidia solution (1.5 × 103 spores/g). Afterwards, grapes were sprayed with 2 mL a solution of 500 g/L of the CFS reaching a final concentration of 50 g of CFS per Kg of fruit. Between each steep the grapes dried for 30 min. Then, the fruits were stored in a closed sterile plastic box (dimensions 30 cm × 40 cm) for 10–12 days at room temperature and in a dark condition. Finally, six replicates were homogenized at random, in a ratio of 1/10 (w/v) with distilled water, 0.1% of peptone (w/v), and 0.1% of Tween 80 (v/v), and three replicates of a serial dilution were performed and cultured on PDA plates. After 48 h at 25 °C, the CFU were observed. Results were reported in percentage of contaminated grapes per day and spores per gram of fruit.
4.10. Metabolite Profiling of Fungal Growth on Grape
To investigate the effects of different bio-preservative treatments on inoculated grape on the metabolite profiling of A. flavus ITEM 8111 and A. niger CECT 2088, we collected contaminated grapes at the end of the incubation period, following the method described by Quiles et al. with some modifications [45]. The samples were frozen at −80 °C and lyophilized for 2 days. After, the grapes were crushed using a Cecotec electric coffee grinder (Valencia, Spain) to reduce particle size. Then, 5 g of sample were extracted with 25 mL of methanol for 2 min using a homogenizer T 50 Ultra-turrax (Staufen, Germany). The supernatant was dried under nitrogen flow with a Buchi R-215 Rotavapor system (Essen, Germany). The dry samples were dissolved with 2 mL of methanol, filtered with 0.22 µm and analysed using an UPLC (1290 Infinity LC, Agilent Technologies) coupled with a quadrupole time of flight mass spectrometer (Agilent 6546 LC/Q-TOF) operating in positive ionization mode. Chromatographic separation was performed with an Agilent Zorbax RRHD SB-C18, 2.1 × 50mm, 1.8 µm column. Mobile phase A was composed of Milli-Q water and acetonitrile was used for mobile phase B (both phases were acidified with 0.1% formic acid), with gradient elution, as follows: 0 min, 2% B; 22 min 95% B; 25 min, 5% B. The column was equilibrated for 3 min before every analysis. The flow rate was 0.4 mL/min, and 5 μL of sample was injected. Dual AJS ESI source conditions were as follows: gas temperature: 325 °C; gas flow: 10 L/min; nebulizer pressure: 40 psig; sheath gas temperature: 295 °C; sheath gas flow: 12 L/min; capillary voltage: 4000 V; nozzle voltage: 500 V; Fragmentor: 120 V; skimmer: 70 V; product ion scan range: 100–1500 Da; MS scan rate: 5 spectra/s; MS/MS scan rate: 3 spectra/s; maximum precursors per cycle: 2; and collision energy: 10, 20, 40 eV. The analysis of the metabolites was carried out in triplicate. Calibration curves of aflatoxin B1 and fumonisin B1 at a concentration of 0.01 to 5 mg/L were performed to quantify the most important toxic secondary metabolites produced by A. flavus and A. niger in treated grapes. Fumonisins (B2, B3 and B4) were quantified using the fumonisin B1 calibration curve as a reference. Untargeted LC/Q-TOF based metabolomics approach were used identify the differential metabolic profiling of fungi growing on grapes treated with CFS. Integration, data elaboration, and identification of metabolites were managed using MassHunter Qualitative Analysis software B.08.00 and library PCDL Manager B.08.00.
4.11. Statistical Analysis
The statistical analysis was performed using the software InfoStat 2019 (Universidad Nacional de Córdoba, Córdoba, Argentina). The differences between the groups were analysed with one-way ANOVA followed by the Tukey HSD post-hoc test for multiple comparisons. The differences have been considered statistically significant at p < 0.05 and p < 0.01.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13060412/s1, Table S1: Identification and quantification of the main VOC present in the CFS. Results as a percentage (%) of the VOC by dividing the area of each peak by the total area of the chromatogram peaks. Statistically significant differences for each fermentation are indicated with different letters (p < 0.05).
Author Contributions
Conceptualization, C.L., J.M.Q. and G.M.; methodology, V.D., C.L. and G.M.; investigation, V.D., C.L. and R.C.; writing—original draft, V.D., C.L. and J.C.; writing—review & editing, J.M.Q., J.C. and G.M.; resources, J.M.Q. and G.M.; supervision, J.M. and G.M.; project administration, J.M. and G.M.; funding acquisition, J.M. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was supported by the Ministry of Science and Innovation (PID2019-108070RB-100), by the project Prometeo/2018/126 supported by Generalitat Valenciana, by the project “Smart and innovative packaging, postharvest rot management and shipping of organic citrus fruit (BiorangePack)” Partnership for Research and Innovation in Mediterranean Area (PRISMA-S2-2019) (E69C20000130001) and by the pre PhD program of University of Valencia “Atracción de Talento (UV-INV-PREDOC17F1-534905-POP)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within article.
Acknowledgments
The authors would like to thank the scholarship support from the Ph.D. program of the University of Valencia (Atracció de Talent); the Spanish Ministry of Science, Innovation and Universities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-López, D.; Vázquez-Ovando, A. Antifungal Lactic Acid Bacteria Isolated from Fermented Beverages with Activity against Colletotrichum gloeosporioides. Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- Lan, W.T.; Chen, Y.S.; Wu, H.C.; Yanagida, F. Bio-Protective Potential of Lactic Acid Bacteria Isolated from Fermented Wax Gourd. Folia Microbiol. 2012, 57, 99–105. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Yang, Q.; Tchabo, W.; Castoria, R.; Zhang, X.; Zhang, H. Leuconostoc Mesenteroides subsp. Mesenteroides LB7 Isolated from Apple Surface Inhibits P. expansum in Vitro and Reduces Patulin in Fruit Juices. Int. J. Food Microbiol. 2021, 339, 109025. [Google Scholar]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, J. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological Control of Postharvest Diseases of Fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Calado, T.; Venâncio, A.; Abrunhosa, L. Irradiation for Mold and Mycotoxin Control: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef]
- Hou, L.; Kou, X.; Li, R.; Wang, S. Thermal inactivation of fungi in chestnuts by hot air assisted radio frequency treatments. Food Control 2018, 93, 297–304. [Google Scholar] [CrossRef]
- Basak, S.; Guha, P. A review on antifungal activity and mode of action of essential oils and their delivery as nano-sized oil droplets in food system. J. Food Sci. Technol. 2018, 55, 4701–4710. [Google Scholar] [CrossRef]
- Yi, Y.J.; Li, Y.S.; Xia, B.; Li, W.P.; Pang, L.; Tong, Z.D. Optimization of Medium Composition and Culture Conditions for Antifungal Activity of a Tomato Endophytic Bacterium. Biol. Control 2015, 82, 69–75. [Google Scholar] [CrossRef]
- Luz, C.; Dopazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of Tomatoes Using Fermented Media by Lactic Acid Bacteria. LWT Food Sci. Technol. 2020, 130, 109618. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Wang, J.; Lv, D.; Ma, Y.; Zhou, B.; Wang, B. Biocontrol Effects of Brevibacillus laterosporus AMCC100017 on Potato Common Scab and Its Impact on Rhizosphere Bacterial Communities. Biol. Control 2017, 106, 89–98. [Google Scholar] [CrossRef]
- Vinodkumar, S.; Indumathi, T.; Nakkeeran, S. Trichoderma Asperellum (NVTA2) as a Potential Antagonist for the Management of Stem Rot in Carnation under Protected Cultivation. Biol. Control 2017, 113, 58–64. [Google Scholar] [CrossRef]
- Dukare, A.; Paul, S. Biological Control of Fusarium Wilt and Growth Promotion in Pigeon Pea (Cajanus Cajan) by Antagonistic Rhizobacteria, Displaying Multiple Modes of Pathogen Inhibition. Rhizosphere 2021, 17, 100278. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The Lactic Acid Bacteria: A Literature Survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef]
- Li, K.; Zhang, W.; Kwok, L.Y.; Menghe, B. Screening of Lactobacillus plantarum with Broad-Spectrum Antifungal Activity and Its Application in Preservation of Golden-Red Apples. Czech J. Food Sci. 2020, 38, 315–322. [Google Scholar] [CrossRef]
- Salman, M.; Tariq, A.; Ijaz, A.; Naheed, S.; Hashem, A.; Abd-Allah, E.F.; Soliman, M.H.; Javed, M.R. In Vitro Antimicrobial and Antioxidant Activities of Lactobacillus coryniformis BCH-4 Bioactive Compounds and Determination of Their Bioprotective Effects on Nutritional Components of Maize (Zea Mays L.). Molecules 2020, 25, 4685. [Google Scholar] [CrossRef]
- Francesca, N.; Chiurazzi, M.; Romano, R.; Aponte, M.; Settanni, L.; Moschetti, G. Indigenous yeast communities in the environment of “Rovello bianco” grape variety and their use in commercial white wine fermentation. World J. Microbiol. Biotechnol. 2010, 26, 337–351. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, characterisation and exploitation of lactic acid bacteria capable of efficient conversion of sugars to mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef]
- Barrangou, R.; Yoon, S.S.; Breidt, F.; Fleming, H.P.; Klaenhammer, T.R. Identification and Characterization of Leuconostoc fallax Strains Isolated from an Industrial Sauerkraut. Ferment. Appl. Environ. Microbiol. 2002, 68, 2877–2884. [Google Scholar] [CrossRef]
- Trias, R.; Badosa, E.; Montesinos, E.; Bañeras, L. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and Quantification of Antifungal Compounds Produced by Lactic Acid Bacteria and Propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible Coatings Incorporating Pomegranate Peel Extract and Biocontrol Yeast to Reduce Penicillium digitatum Postharvest Decay of Oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Zadeh, M.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M. Biocontrol of Gray Mold Disease on Strawberry Fruit by Integration of Lactobacillus plantarum A7 with Ajwain and Cinnamon Essential Oils. J. Food Sci. 2013, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Luz, C.; Ritieni, A.; Quiles Beses, J.; Mañes, J.; Meca, G. Inhibitory Effect of Sweet Whey Fermented by Lactobacillus plantarum Strains against Fungal Growth: A Potential Application as an Antifungal Agent. J. Food Sci. 2020, 85, 3920–3926. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Príncep, A.; Tornos, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.J.; Barba, F.J.; Mañes, J. Isolation, Identification and Investigation of Fermentative Bacteria from Sea Bass (Dicentrarchus Labrax): Evaluation of Antifungal Activity of Fermented Fish Meat and by-Products Broths. Foods 2020, 9, 576. [Google Scholar] [CrossRef]
- Lipinska-Zubrycka, L.; Klewicki, R.; Sojka, M.; Bonikowski, R.; Milczarek, A.; Klewicka, E. Anticandidal Activity of Lactobacillus spp. in the Presence of Galactosyl Polyols. Microbiol. Res. 2020, 240, 126540. [Google Scholar] [CrossRef]
- Nischitha, R.; Shivanna, M.B. Antimicrobial Activity and Metabolite Profiling of Endophytic Fungi in Digitaria bicornis (Lam) Roem. and Schult. and Paspalidium flavidum (Retz.) A. Camus. 3 Biotech 2021, 11, 53. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Rollán, G.; Font de Valdez, G. Prevention of Bread Mould Spoilage by Using Lactic Acid Bacteria with Antifungal Properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal Activity of Volatile Organic Compounds Produced by Pseudomonas fluorescens ZX and Potential Biocontrol of Blue Mold Decay on Postharvest Citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Lappa, I.K.; Mparampouti, S.; Lanza, B.; Panagou, E.Z. Control of Aspergillus carbonarius in Grape Berries by Lactobacillus plantarum: A Phenotypic and Gene Transcription Study. Int. J. Food Microbiol. 2018, 275, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kasfi, K.; Taheri, P.; Jafarpour, B.; Tarighi, S. Characterization of Antagonistic Microorganisms against Aspergillus spp. from Grapevine Leaf and Berry Surfaces. J. Plant Pathol. 2018, 100, 179–190. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božik, M.; Klouček, P. The in vitro and in Situ Effect of Selected Essential Oils in Vapour Phase against Bread Spoilage Toxicogenic Aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Nazareth, T.M.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Antiaflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef]
- Erkmen, O. Practice 4—Pure culture techniques. In Laboratory Practices in Microbiology; Erkmen, O., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 41–50. [Google Scholar]
- Maier, T.; Klepel, S.; Renner, U.; Kostrzewa, M. Fast and Reliable MALDI-TOF MS-Based Microorganism Identification. Nat. Methods 2006, 3, 4. [Google Scholar] [CrossRef]
- Chenoll, E.; Moreno, I.; Sánchez, M.; Garcia-Grau, I.; Silvia, A.; González-Monfort, M.; Genovés, S.; Vilella, F.; Seco-Durban, C.; Simón, C.; et al. Selection of new probiotics for endometrial health. Front. Cell. Infect. Microbiol. 2019, 9, 114. [Google Scholar] [CrossRef]
- Khosravi, F.; Rastakhiz, N.; Iranmanesh, B.; Olia, S. Determination of organic acids in fruit juices by UPLC. Int. J. Biol. Sci. 2015, 9, 41–44. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS Approach in a Novel Application for the Identification of Antifungal Compounds Produced by Lactic Acid Bacteria Cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Rodriguez, L.; Romano, R.; Mañes, J.; Meca, G. A Natural Strategy to Improve the Shelf Life of the Loaf Bread against Toxigenic Fungi: The Employment of Fermented Whey Powder. Int. J. Dairy Technol. 2020, 73, 88–97. [Google Scholar] [CrossRef]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in Autochthonous Microbial Diversity and Volatile Metabolites during the Fermentation of Chili Pepper (Capsicum Frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; De Melo Nazareth, T.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an Antifungal and Antimycotoxigenic Device Containing Allyl Isothiocyanate for Silo Fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).