Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver cancer in adults, the fifth most common malignancy worldwide and the third leading cause of cancer related death. An alternative to the surgical treatments and drugs, such as sorafenib, commonly used in medicine is necessary to overcome this public health problem. In this study, we determine the anticancer effect on HCC of Moroccan cobra Naja haje venom and its fraction obtained by gel filtration chromatography against Huh7.5 cancer cell line. Cells were grown together with WI38 human fibroblast cells, LX2 human hepatic stellate cell line, and human endothelial cells (HUVEC) in MCTS (multi-cellular tumor spheroids) models. The hepatotoxicity of venom and its fractions were also evaluated using the normal hepatocytes cell line (Fa2N-4 cells). Our results showed that an anti HCC activity of Moroccan cobra Naja haje venom and, more specifically, the F7 fraction of gel filtration chromatography exhibited the greatest anti-hepatocellular carcinoma effect by decreasing the size of MCTS. This effect is associated with a low toxicity against normal hepatocytes. These results strongly suggest that the F7 fraction of Moroccan cobra Naja haje venom obtained by gel filtration chromatography possesses the ability to inhibit cancer cells proliferation. More research is needed to identify the specific molecule(s) responsible for the anticancer effect and investigate their mechanism of action.
Keywords:
anticancer molecules; Hepatocellular carcinoma; multicellular tumor spheroids; Naja haje; venom Key Contribution:
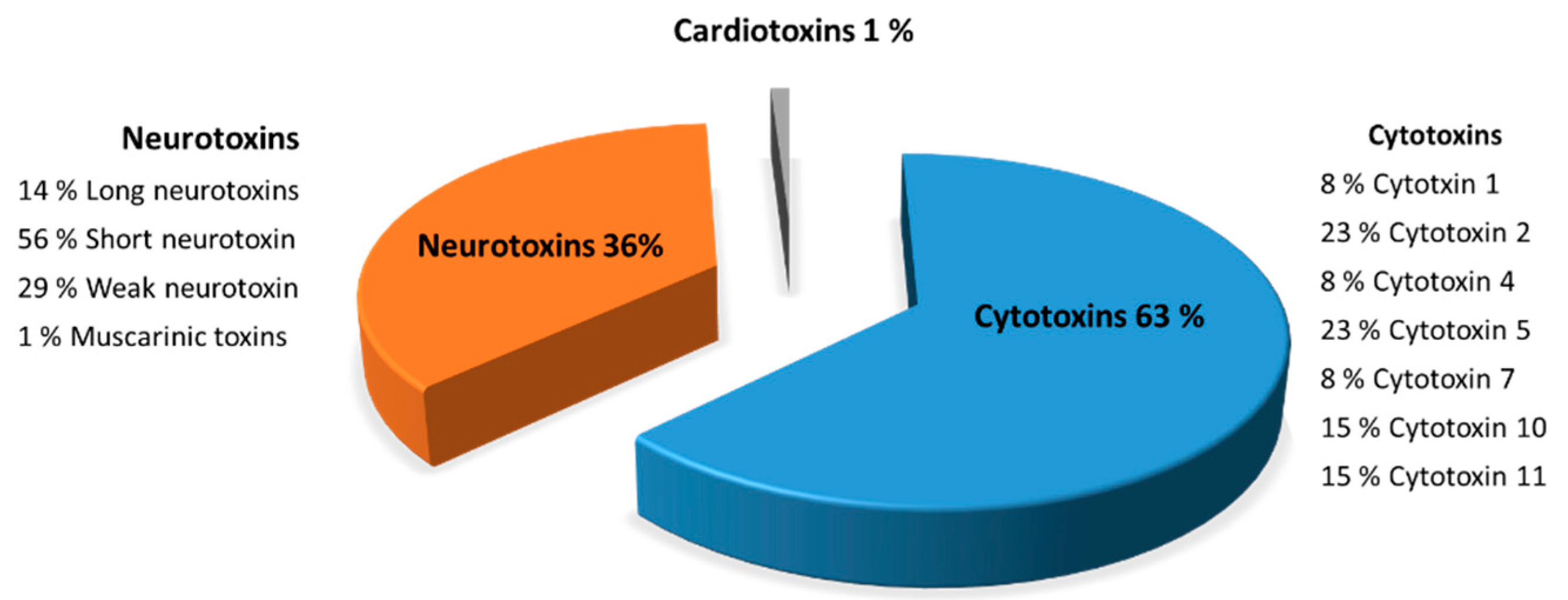
The purification by gel filtration chromatography of the Naja haje venoms showed the presence of several fractions which showed different toxicity. Naja haje venom has an in vitro anti-cancer activity against hepatocellular carcinoma and the F7 fraction from gel filtration was the most active. Naja haje venom—more specifically, the F7 fraction—did not show a cytotoxic effect against normal hepatocytes. F7 fraction has a comparable effect to sorafineb, which is used for the systematic treatment of hepatocellular carcinoma. Three families of toxins from F7 fraction—cytotoxins, neurotoxins, cardiotoxins—were identified, showing 63, 36, and 1%, respectively.
1. Introduction
More than 745,000 deaths worldwide were directly caused by hepatocellular carcinoma (HCC) in 2012 [1]. It is the most common primary malignancy, currently the sixth most common cancer worldwide, the second deadliest cancer for men and sixth for women [2]. HCC represents the major form of liver cancer; several factors are involved in the development of this cancer, notably chronic infection with hepatitis B (HBV) or hepatitis C (HCV). Numerous other factors such as autoimmune hepatitis, high alcohol consumption and other metabolic diseases such as diabetes and obesity are also risk factors [3,4]. Tumor progression, metastasis and a high frequency of tumor recurrence are still the main cause of death [5]. Cancer cells differ from normal cells as they have the capacity to bypass the cell cycle checkpoints, which are responsible for maintaining intracellular balance [6]. Hallmarks of cancer include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. In addition, there are two emerging characteristics, notably deregulating cellular energetics and avoiding immune destruction [7]. The development of a therapy of choice with high potency and effectiveness has led to an increased use of anticancer drugs produced by natural resources [8].
Therefore, there is an urgent need to develop new molecules to overcome this problem. To the best of our knowledge, sorafenib is currently the only systemic drug adopted for the treatment of advanced cases of HCC, in addition to other medical and surgical treatment options [9,10]. Liver resection and liver transplantation are not efficient treatments for patients with HCC due to the advanced stage of the disease at the time of diagnosis [11]. There has been a high level of interest in the establishment of natural products for therapeutic purposes. The search for new molecules could be used as antitumor agents with less severe side effects than the usual chemotherapy, and could serve as molecular models for the development of anticancer drugs [12,13].
Over the years, animal venoms and toxins from several species such as snakes, scorpions, cone snails, bees and wasps have been widely studied for their potential as a major source of bioactive molecules [14]. Animal venom has long been considered as a natural therapeutic source. Over time, scorpions have developed a variety of proteins within their venom gland for predator defense as well as to aid in the digestion of prey. This venom is a mixture of salts, nucleotides, lipids, neurotoxins, peptides and proteins. Toxins are the most studied proteins for their neurotoxic and pharmacological activities on ion channels [15]. They are disulfide-bridged peptides (DBPs), which are classified according to their specific target channels into four major groups—the toxin interacting/targeting the Na+, K+, Ca++ and Cl− channels [16]. They are commonly known for their deleterious effects on cells and some of them have important activities for the development of antimicrobial, antimalarial, immunosuppressive and anticancer drugs [17]. Many toxins isolated from snake venom have been used as medical tools to understand different pathophysiological effects, since these proteins have diverse biological effects, such as antiparasitic, antimicrobial and antitumor activities [18,19].
Snake venom has been applied for thousands of years in different communities such as China and Africa. Substantial in vivo and in vitro evidence has shed light on the ability of scorpion venom to decrease cancer growth, inhibit cancer progression and metastasis. The anticancer activity of snake venom is based on the immunosuppressive, cytotoxic and antiproliferative power of their proteins, which induce the apoptosis of cancer cells [20]. Recently, venoms have been used in medicine for treating a variety of disease [21]. Several studies have shown a potential anticancer effect of peptides extracted from animal venom. Therefore, the use of peptides from snake and scorpion venoms is one of the therapeutic methods providing very relevant results in the field of fight against cancer [22,23]. Recently, the 3D cell culture model has been widely used in order to create an in vitro microenvironment of cancer similar to that in vivo. This culture technique allows us to test the efficacy as well as the specificity of the compounds against the HCC.
In our study, we studied the potential anti-cancer effect of Moroccan cobra Naja haje venom and its fractions purified by gel filtration chromatography on MCTS-based phenomic screening system. The multicellular tumor spheroid model was used by combining HCC cell line Huh7.5 together with WI38 human fibroblast cell, LX2 human hepatic stellate cell line, and human endothelial cell (HUVECs). Moreover, we have studied the effect of venom and its fraction on the normal hepatocytes cell line (Fa2N-4 cell) in order to determine their toxicity and their specificity against the hepatocellular carcinoma in 3D cell culture. The fraction of venom that has an anti-cancerous effect against HCC has been characterized by liquid chromatography coupled with tandem mass spectrometry to determine the molecules responsible for the anticancer effect.
2. Results
2.1. Fractionation of Venom by Gel Filtration Chromatography
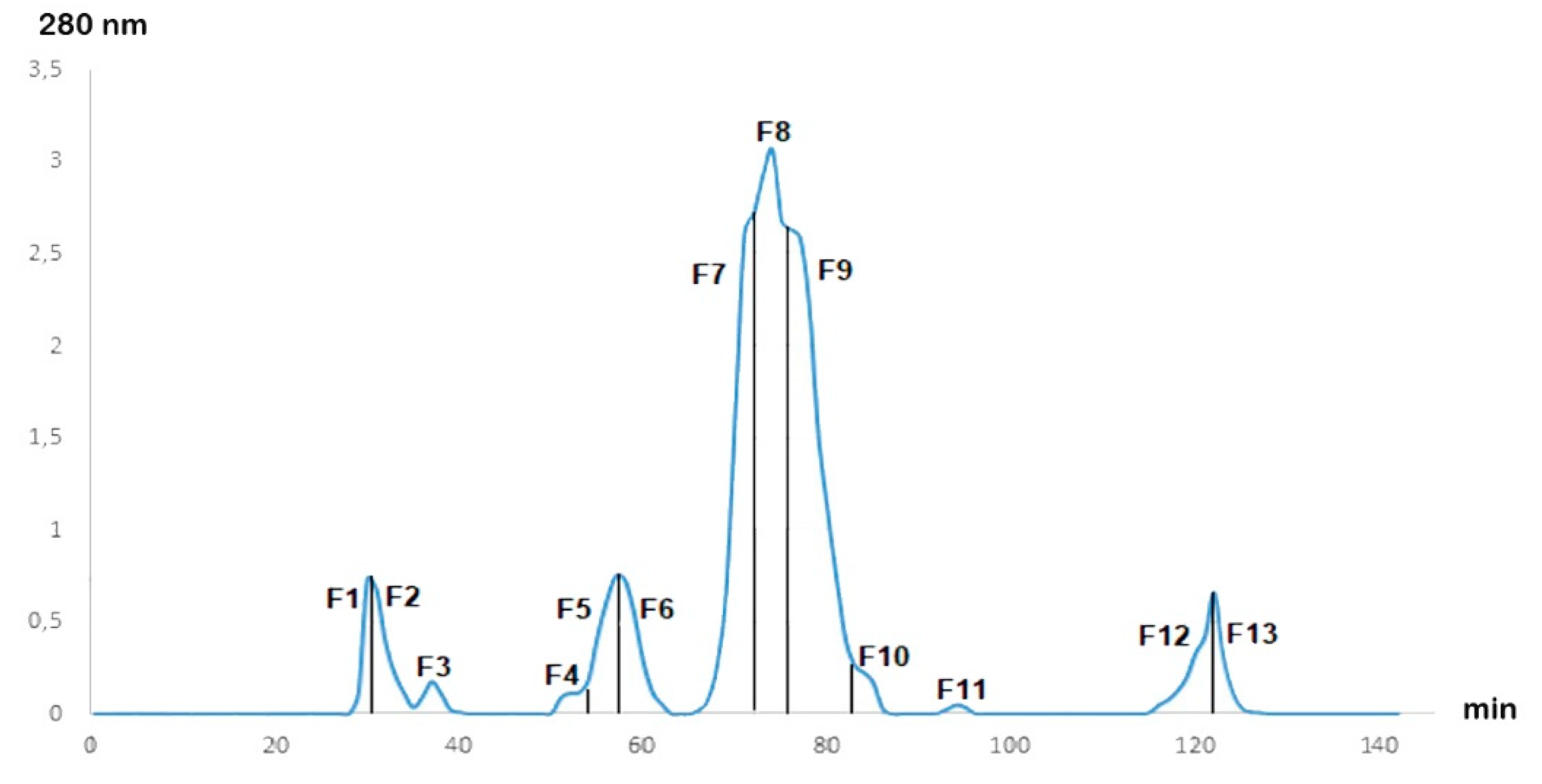
After fractionation of the Cobra Naja haje venom by gel filtration chromatography, the chromatogram (Figure 1) showed the presence of 13 fractions at different concentrations. The 13 fractions were separated by their molecular weight. F7, F8 and F9 were the major fractions present in Naja haje venom.
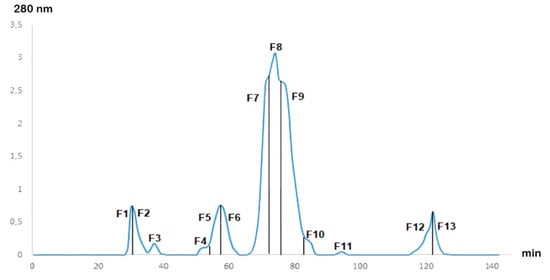
Figure 1.
Gel filtration chromatogram of Naja haje venom.
2.2. Cytotoxicity Test of Venoms and Its Fractions to Normal Hepatocytes
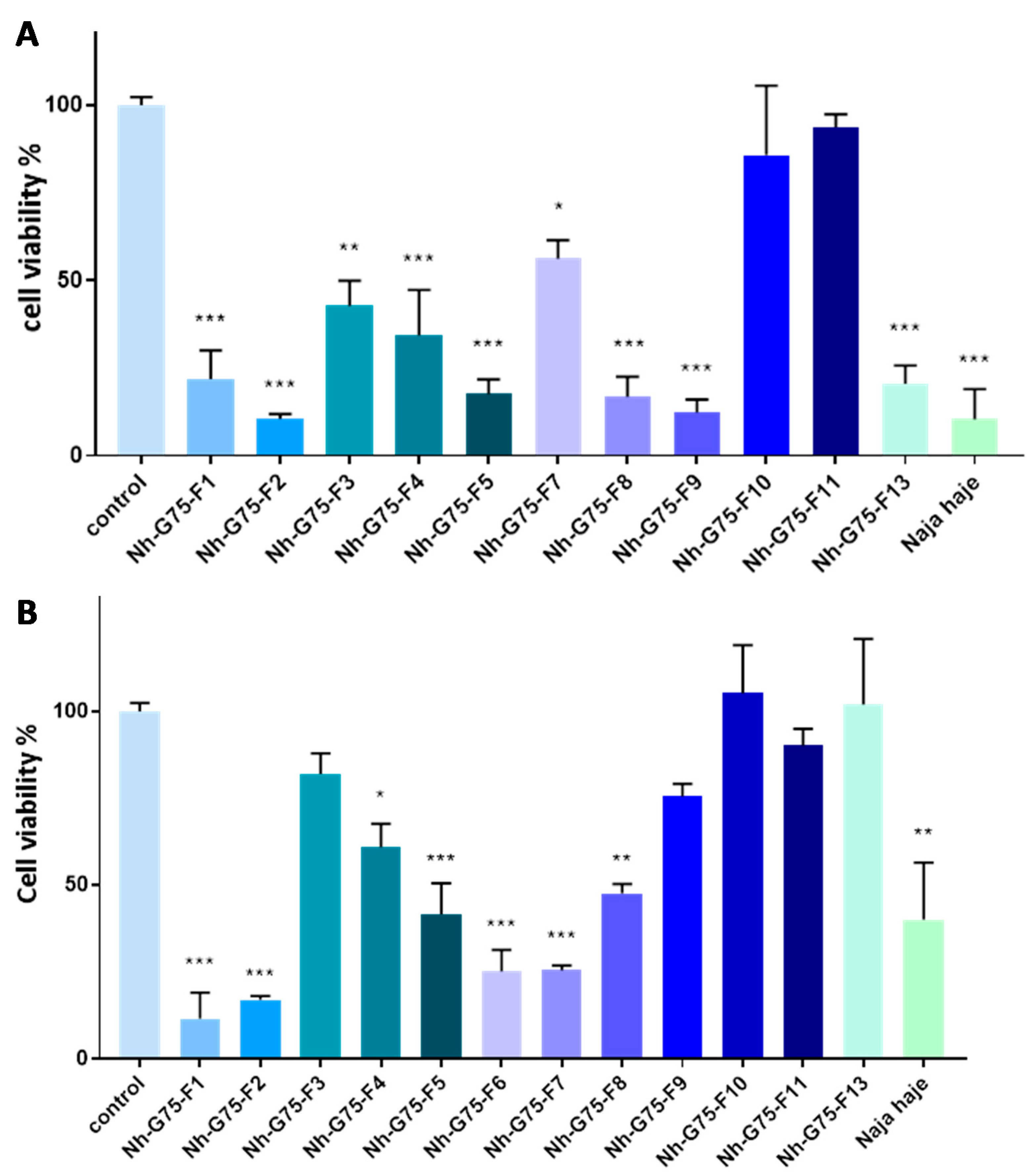
Figure 2A showed cytotoxicity of the venom of Naja haje and its fraction at 10 µg/mL on normal hepatocytes (Fa2N-4 cell). F3, F9, F10, F11 and F13 demonstrated a cytotoxic effect in comparison with the negative control (PBS). F4, F8 and the crude venom showed a significant cytotoxic moderated effect; F1, F2, F5, F6 and F7 demonstrated a significant high toxic effect on Fa2N-4 cell.
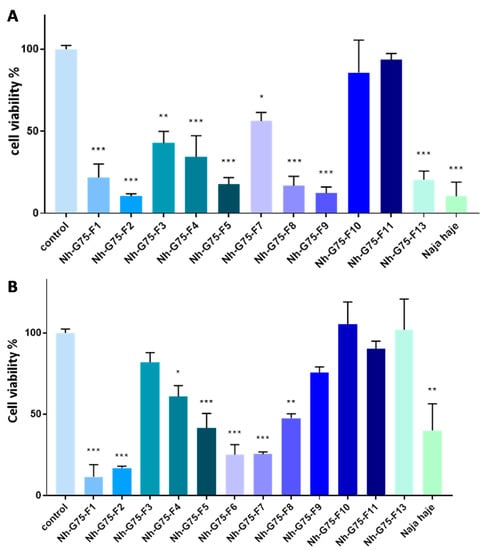
Figure 2.
Cytotoxic effect of Naja haje venom and its fractions (F1, F2, F3, F4, F5, F6, F7, F8, F9, F10, F11 and F13) and control (PBS) on normal hepatocytes (Fa2N-4). (A) Concentration of 10 µg/mL. (B) Concentration of 50 µg/mL. Each value is expressed as mean ± standard deviation (n = 2). * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
The cytotoxic effect of the Naja haje venom samples were tested on normal hepatocytes (Fa2N-4) at 50 µg/mL (Figure 2B). F10 and F11 showed a non-significant cytotoxic effect in comparison with the negative control. F3 and F7 showed a moderated cytotoxicity effect. However, it is important to note that F1, F2, F4, F5, F8, F9, F13 and the crude venom demonstrated a high toxic effect on the Fa2N-4 cell line at 50 µg/mL.
2.3. Anticancer Activity of Venoms and Its Fractions against MCTS
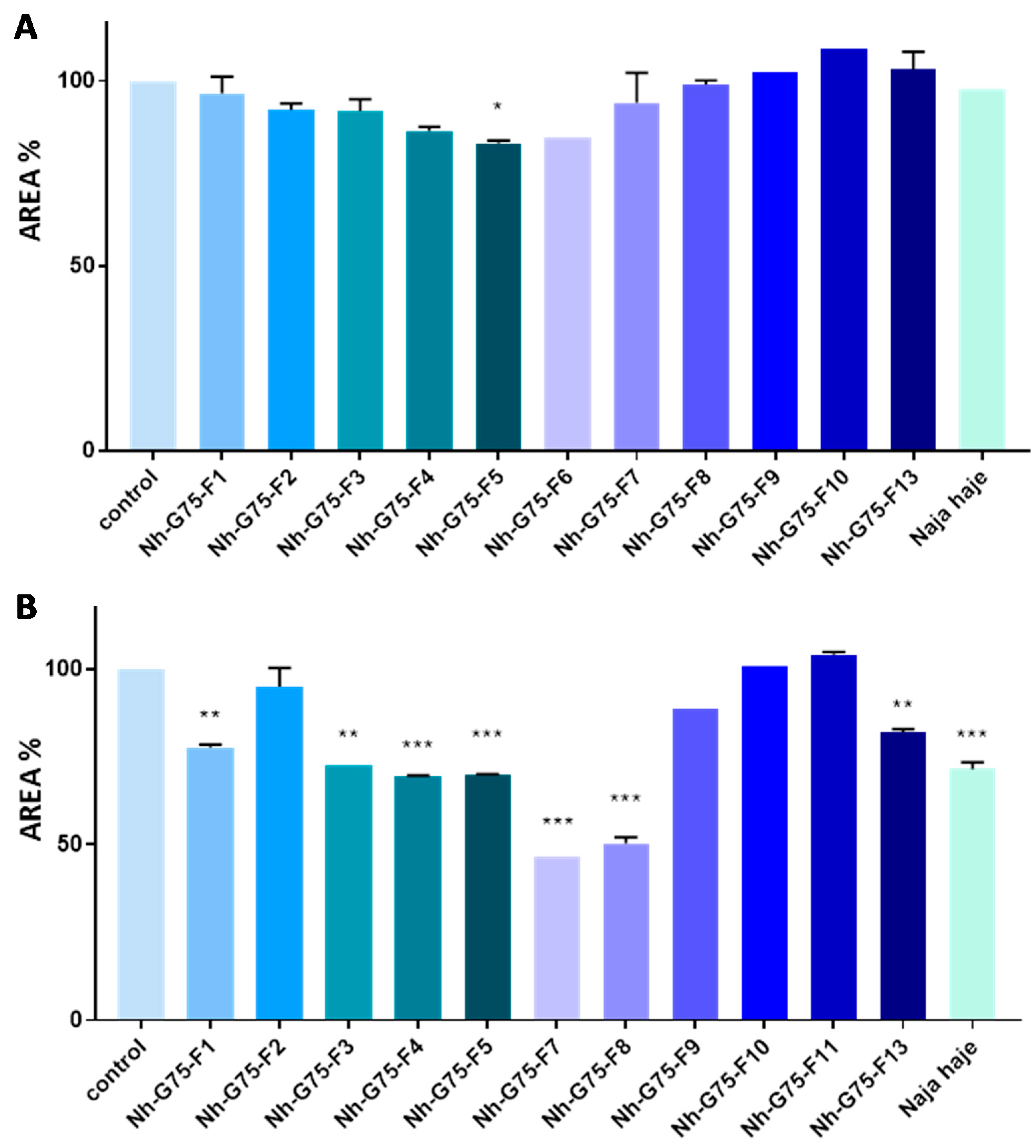
Naja haje venom and its fraction were evaluated against MCTSs at 10 µg/mL. All fractions did not show any reduction in the MCTSs area. However, only the F5 fraction showed a low reduction in the MCTSs area in comparison with the negative control (MCTSs treated with PBS) (Figure 3A).
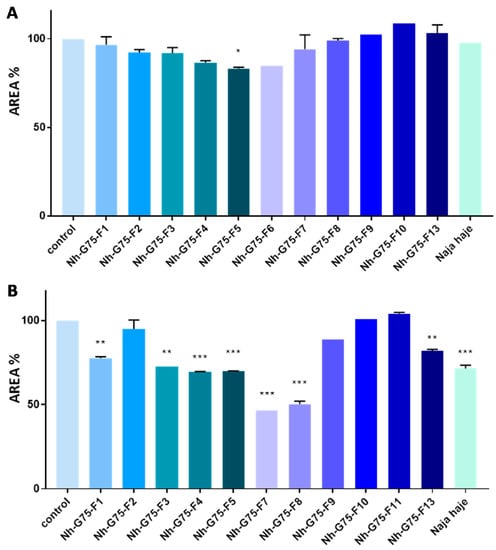
Figure 3.
Anticancer effect of Naja haje venom and its fractions (F1, F2, F3, F4, F5, F6, F7, F8, F9, F10, F11 and F13) and control (PBS) on the size of the MCTSs. (A) Concentration of 10 µg/mL. (B) Concentration of 50 µg/mL. Each value is expressed as mean ± standard deviation (n = 2). * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
Naja haje venom and its fraction were evaluated against MCTSs at 50 µg/mL. F2, F9, F10 and F11 did not show any effect on the MCTSs area. F1, F3 and F13, however, showed a moderated decrease of the MCTSs area in comparison with the negative control. F4, F5, F7, F8 and the crude venom showed a significant inhibition of MCTSs cells proliferation then a diminution of spheroids size (Figure 3B).
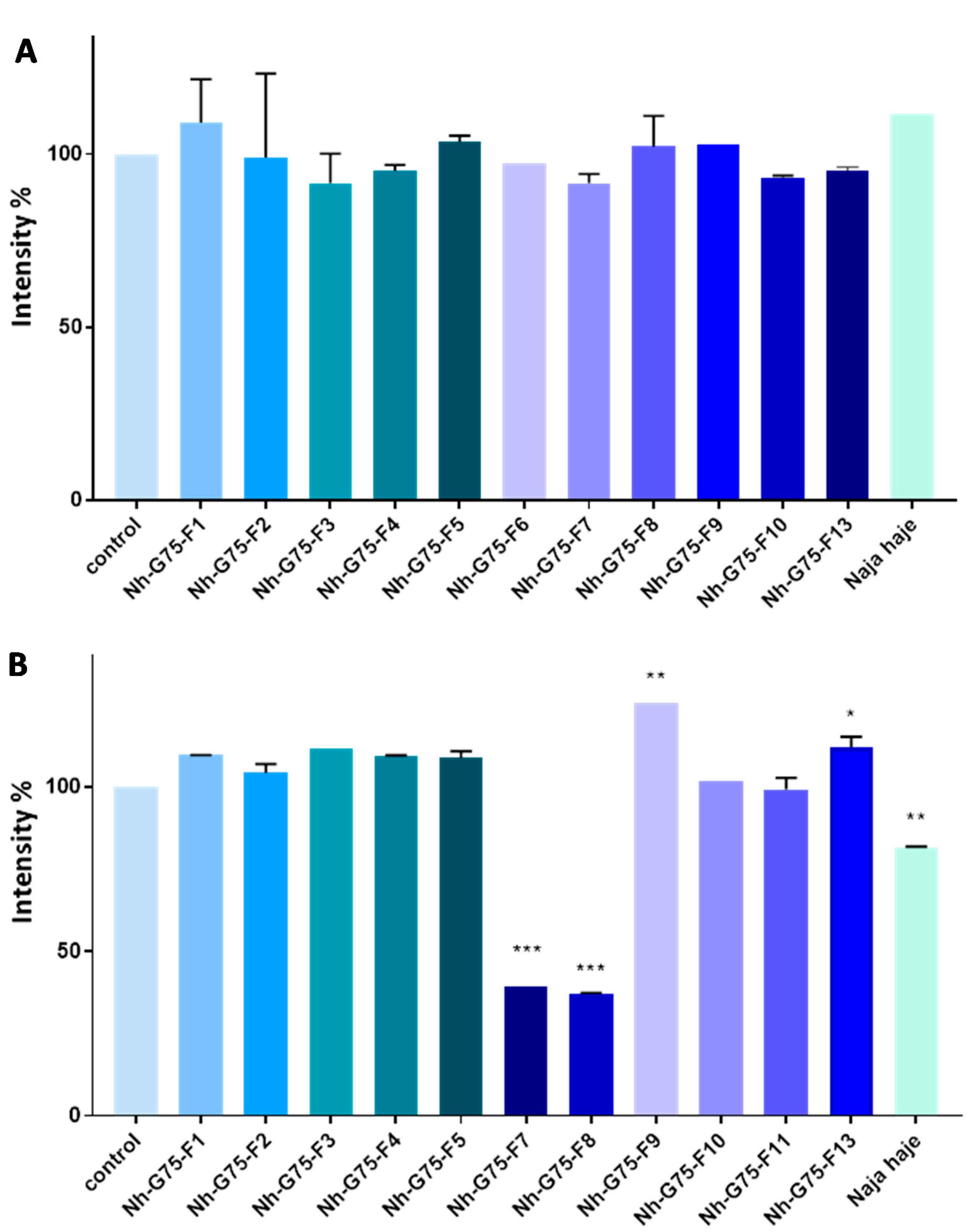
RFP imaging showed that after treatment with 10 µg/mL of Moroccan cobra Naja haje venom and its fractions, no significant reduction in the intensity was observed (Figure 4A).
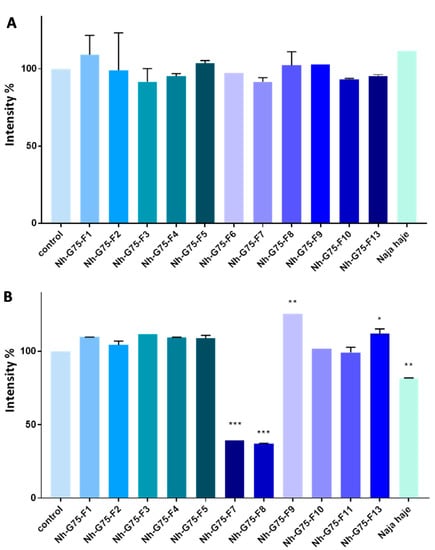
Figure 4.
Anti-cancer effect of snake Naja haje venom and its fractions (F1, F2, F3, F4, F5, F6, F7, F8, F9, F10, F11 and F13) and control (PBS) on the intensity of RFP of the MCTSs. (A) Concentration of 10 µg/mL. (B) Concentration of 50 µg/mL. Each value is expressed as mean ± standard deviation (n = 2). * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
Figure 4A shows MCTSs-RFP intensity of cobra Naja haje venom and its fractions treatment at 50 µg/mL. F1, F2, F3, F4, F5, F10 and F11 did not enhance any effect on MCTS intensity, while F9 and F13 raised it. Crude cobra venom and both F7 and F8 showed low intensity in comparison with the negative control (PBS).
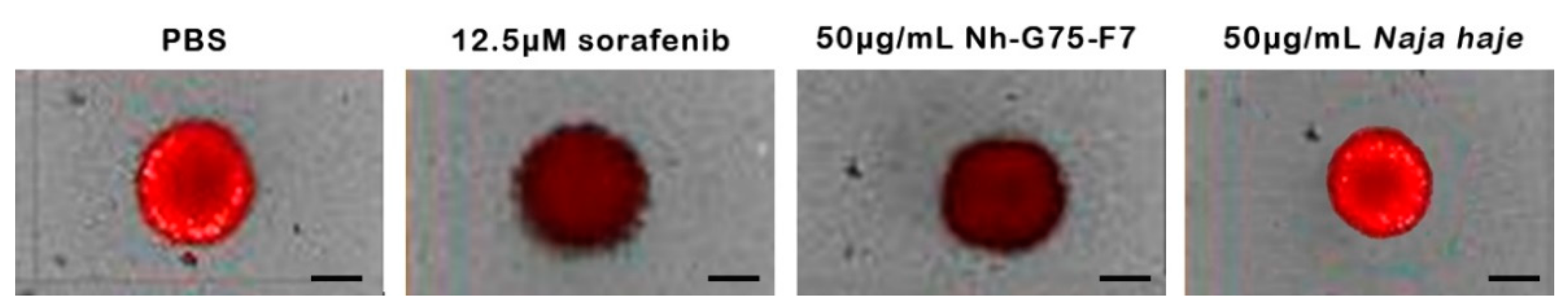
Figure 5 showed the appearance of the spheroids to show the ability of the F7 fraction which is similar to sorafenib. The positive and negative controls were 12.5 µM sorafenib and PBS, respectively. Spheroids treated by sorafenib and Nh-G75-F7 showed a decrease in the intensity of RFP, while Naja haje crude extract showed similar intensity to the negative control.
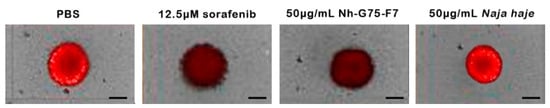
Figure 5.
MCTSs towards sorafenib and fraction Nh-G75-F7 (PBS, 12.5 µM sorafenib, 50 μg/mL Nh-G75-F7, 50 μg/mL Naja haje crude venom). Scale bar = 200 μm.
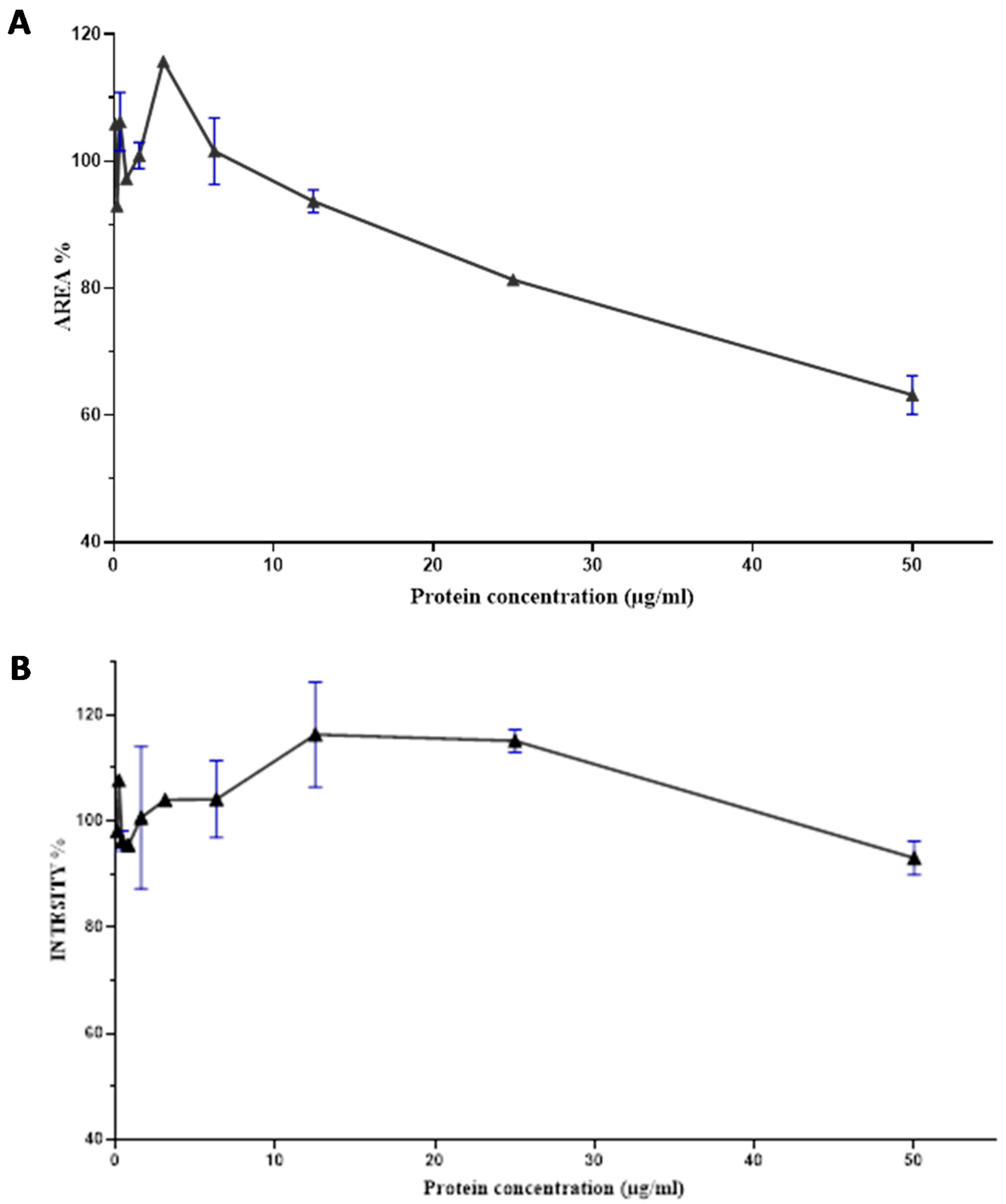
2.4. Dose Response Curve of the Nh-G75-F7 Fraction
The F7 fraction of cobra Naja haje venom showed that the higher the concentration of proteins, the more the size of spheroids decreases. Thus, an increase in the dose causes a decrease in area spheroids and its toxic effect. This is the dose–effect relationship (Figure 6A).
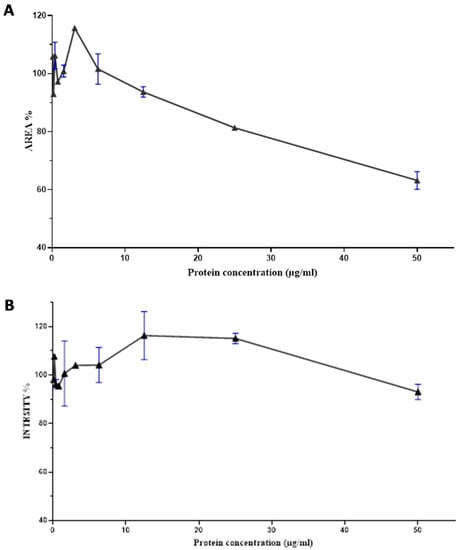
Figure 6.
Dose–response curve of the effect of the Nh-G75-F7 fraction. (A) AREA of MCTSs. (B) RFP intensity of MCTSs.
According to Figure 6B, there is a correlation between the dose and the effect generated by the F7 fraction of cobra Naja haje venom; that being said, an increase in the dose leads to an increase in RFP intensity up to 15ug/mL and then a decrease reaching up to 50 µg/mL.
2.5. Molecules Identification in the F7 Fraction of the Naja haje Venom by Mass Spectrometry
Characterization of the toxins from F7 fraction (Figure 7) is described in detail to illustrate the peptides composition from Naja haje venom. F7 fraction contains a mixture of cytotoxins, neurotoxins and cardiotoxins, showing 63, 36 and 1%, respectively. Eight peptide cytotoxins—cytotoxin 1 (8%), cytotoxin 2 (23%), cytotoxin 4 (8%), cytotoxin 5 (23%), cytotoxin 7 (8%), cytotoxin 10 (15%) and cytotoxin 11 (15%)—were identified in F7 fraction from the Naja haje venom. Moreover, the Naja haje venom possesses neurotoxins such as muscarinic toxins, long neurotoxins, weak neurotoxins and the predominate one—the so-called “short neurotoxins”—and constitute 56%. That being said, it is clear that F7 fraction from the Naje haje venom contains a number of toxins that may contribute to venom toxicity toward cancer cells.
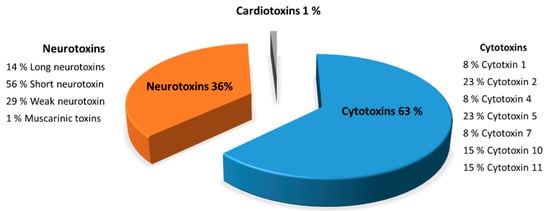
Figure 7.
Characterization of the F7 fraction of Naja haje venom by mass spectrometry (LC/MS-MS).
3. Discussion
Treatment options for HCC are divided into surgical resection of the tumor, and the treatment with the multi-kinase inhibitors such as sorafenib; however, it showed only partial clinical efficacy in patients with liver cancer [24,25]. Moreover, it was established that HCC rapidly becomes sorafenib-resistant [26]. To improve treatment options, the discovery of new drugs for HCC is necessary to improve the clinical treatment of those patients.
Venoms have attracted the attention of researchers engaged in the identification of active components and the development of new drug candidates because of their high sensitivity and specificity for target molecules. They have been used in traditional medicine, mainly in Asia and Africa. Cobra venom has been used to treat joint pain, inflammation and arthritis in traditional Indian medicine [20]. Several studies have shown the remission of tumor cells after treatment with molecules derived from venoms [27]. The potential of anti-cancer therapy based on animal venom proteins and peptides from (scorpions, snakes, bees, spiders and toads) has been studied for decades [28].
This present work focuses on studying the anticancer effect of the Moroccan cobra Naja haje venom and its fractions purified by gel filtration chromatography against hepatocellular carcinoma using the MCTS model. This 3D culture allowed us to mimic the tumor microenvironment by cultivating cancer cell line Huh 7.5 with WI38 human fibroblast cell, LX2 human hepatic stellate cell line, and human endothelial cell (HUVEC). The toxicity of venom and these fractions against normal hepatocytes was tested on the Fa2N-4 cell line.
Our results showed that F7 and F8 fractions of gel filtration chromatography at a dose of 50 μg/mL exhibited an anti-HCC effect. F7 and F8 fractions significantly reduced the size of HCC cell line-derived spheroids by decreasing the RFP signal intensity related to cell proliferation. F8 fraction showed significant cytotoxicity towards normal hepatocytes at 50 μg/mL. On the other hand, F7 fraction showed low cytotoxicity. The results of the dose–response curve showed that F7 fraction has a dose-dependent anti-HCC effect.
A previous study has shown the antitumor activity of Naja haje venom (NHV) and its fractions (NHVI, NHV-Ia, NHV-Ib, NHV-Ic, NHV-II, NHV-III, and NHV-IV) against several human cell lines, namely lymphoblastic leukemia (1301) cell, hepatocellular carcinoma (Hep-G2), colon carcinoma (HCT-116), cervical carcinoma (HeLa), histiocytic lymphoma, and breast adenocarcinoma (MCF-7). This study revealed that NHV was highly cytotoxic to Hep-G2 and 1301 cells with an IC50 values of 6.52 and 4.74 µg/mL, respectively. Additionally, NHV-I fraction, unlike the others, possessed a potential cytotoxic effect against Hep-G2, HeLa, and 1301 cells [29].
Snake venoms in the Elapidae family have been shown to have a significant anti-tumor activity [30]. The crude venom of Naja naja oxiana is a powerful inducer of apoptosis in the cancer cell line, including HCC, with minimal side effects on normal cells. A study on the venom toxins of Naja naja oxiana showed that the treatment of HepG2 cells with these toxins at concentrations of 15 µg/mL induced cell cycle arrest, and both types of cell death—apoptosis and necrosis—were observed [31]. The crude venom of Naja naja oxiana induced an increase in the level of reactive oxygen species (ROS) via the disruption in the mitochondrial of the liver of HCC rats. This process resulted in a drop in matrix metalloproteases (MMP), impaired mitochondrial swelling and release of cytochrome c, which can induce the initiation of the apoptosis signaling pathway by activation of caspase-3 in HCC rats [32]. The Caspian cobra venom Naja naja oxiana has revealed that cytotoxins I and II can easily penetrate into living cancer cells and accumulate in lysosomes, which suggest that lysosomal damage is the cause of cell death induced by these two toxins [33]. BthTX-I is a myotoxin isolated from the venom of the viper Bothrops jararacussu; this myotoxin revealed cytotoxic activity for the human HepG2 cell line by inducing cell death by apoptosis. BthTX-I may be able to promote the delay in the G0/G1 phase of the cell cycle of murine tumor cells [34]. The viper venom Echis Pyramidum has shown a reduction in a dose-dependent cell viability against the HepG2 cell line, by induction of morphological changes and an apoptotic profile compared to an untreated cell control [22]. Venom from Medusa Nemopilema nomurai inhibits the proliferation of HepG2 cells at doses (0.8 to 1.2 µg/mL) by induction of apoptotic cell death, while showing no toxicity to hepatocyte, fibroblasts and keratinocytes at the same concentrations. They did not observe any toxic side effects liver and heart tissue, such as changes in the level of enzymes linked to function or histological abnormalities in vivo [35]. Macrothele raven venom can inhibit cells invasion and metastasis in the subrenal capsule xenograft model of liver cancer in a dose-dependent manner; its antitumor activity seems to be related to the inhibiting of the signaling PI3K-Akt-mTOR and increase its expression [36]. A study on the venom of another spider called Haplopelma hainanum exhibited potent inhibition effects in HepG2 cell proliferation, through reducing the potential of the mitochondrial membrane, caspase-3 and 9 activation, and inducing apoptosis by a pathway dependent on mitochondria [37].
The characterization of gel filtration chromatography F7 fraction of Naja haje venom by Nano-LC-MS/MS showed that this fraction is composed of cytotoxins, neurotoxins and cardiotoxins, showing 63, 36 and 1%, respectively. A presidential study on Moroccan cobra Naja haje venom revealed that this venom is a very complex mixture that has a total of 76 proteins identified from the database that can be assigned into nine protein families, short neurotoxins, long neurotoxins, weak neurotoxins, neurotoxin-like proteins, muscarinic toxins, cardiotoxins, cytotoxins, cobra venom factor (CVF), L-amino-acid oxidases (LAAO), acetylcholinesterase (AChE), snake venom metalloproteinases (SVMP), cysteine rich secretory proteins (CRISP), venom nerve growth factor (vNGF), phospholipases A2 (PLA2), vespryns, and kunitz-type inhibitor [38]. The anticancer effect of F7 fraction is probably due to the bioactive peptides found in the toxic Naja haje venom that may act by inhibiting specific ion channels related to HCC cancer—for example, blocking the voltage-gated shaker potassium channel that was found to mediate tumor cell proliferation by binding to HERG [39], a potassium channel protein that increases in concentration on the cell surface of cancer cells. Moreover, those bioactive venoms may act by activating caspase 9 and 3 and inducing poly (ADP-ribose) polymerase (PARP) cleavage that may deplete the stores of cellular NAD+ and induce a progressive ATP depletion, and thus, apoptotic cell death [40]. Several studies have shown many novel modes of anti-cancer mechanism beyond venom peptides in membrane pore formation. For further study, it is necessary to elucidate the mechanism of anticancer effects depending on the sequence or structure of venom peptides [41].
This article highlights the possible therapeutic option for Naja haje venom and its fractions on HCC. However, there is still a long way to go before these venom fractions can be successfully used as therapeutic agents against HCC as several clinical trials have to be made for the drug product immunotoxicity. Moreover, purification of the active fraction is needed to identify the molecule responsible for anti-HCC activity.
4. Conclusions
This work shows that the snake Naja haje venom, specifically the F7 fraction from the gel filtration chromatograph, showed a dose-dependent effect on the reduction of the size of the HCC-MCTSs, and thus the decrease of RFP intensity of the cells of the cancerous line, indicating the cytotoxic effect of this fraction against HCCs. In this regard, we propose that it is necessary to continue our research and carry out a refraction by filtration gel chromatography of the F7 fraction from cobra Naja haje venom in order to elucidate the effect of each fraction obtained by HPLC and subsequently identify the molecule(s) responsible for cytotoxicity against HCC cells.
5. Materials and Methods
5.1. Cell lines and Culture Conditions
The HCC cell line Huh7.5-red fluorescent protein-(RFP)-NLS-IPS reporter cell line was kindly provided by Dr. Mark Windisch (Institut Pasteur Korea, Gyeonggi-do, Korea). Stromal cells WI38 (human fibroblasts), LX2 (human hepatic stellate cells), and HUVECs (human umbilical vein endothelial cells) were obtained from ATCC (Manassas, VA, USA). Fa2N-4 cells (an immortalized normal hepatocyte cell line) were obtained from Xenotech (Lenexa, KS, USA).
Huh7.5 cell line were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Welgene) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. WI38 cells were cultured in minimum essential media (MEM; Welgene, Daegu, Korea) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1× penicillin-streptomycin (P/S; Gibco). LX2 cells were cultured in DMEM supplemented with 10% heat-inactivated FBS and 1× P/S. HUVECs were cultured in endothelial basal medium (EBM) obtained from Promo Cells. All cell lines were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Fa2N-4 cells were plated to the flask with serum-containing plating medium (XenoTech, Lenexa, KS, USA). After cell attachment (3 to 6 h), plating medium was replaced with MFE serum free supporting (SF) medium (XenoTech), which is a nutrient-rich medium for maintaining Fa2N-4 cells in culture.
5.2. Snake venom
The venom of Naja haje was obtained by manual stimulation of the Cobra venom gland kept in captivity at the Serpentarium of the animal unit at the Pasteur Institute of Morocco. The pooled venom was centrifuged at 15,000× g for 15 min at 4 °C to remove debris, before lyophilization and storage at −20 °C until use [42].
5.3. Fractionation of Venom by Gel Filtration Chromatography
Naja haje venom (161 mg) was fractionated in a glass column (2.6 × 100 cm; Pharmacia, Uppsala, Sweden) with a Sephadex G75 Medium gel (Sigma Aldrich, Lyon, France) and equilibrated with 10% acetic acid. A continuous flow rate of 26 mL/h is provided by a Pump P-1 peristaltic pump (Amersham Biosciences, Piscataway, NJ, USA). The collection was carried out by a Frac-920 automatic fraction collector (Amersham Biosciences, Piscataway, NJ, USA) at the rate of 2.5 mL in each tube [43].
5.4. Cytotoxicity Test of Venoms and Its Fractions to Normal Hepatocytes (Fa5N4)
Fa2N-4 cells were seeded at a density of 3.5 × 103 cells/well in 384-well plates. After 24 h of incubation, crude venom and its fractions were resolubilized in phosphate-buffered saline (PBS; Lonza) at a concentration of 10 and 50 µg/mL, then added and incubated for 48 h. A solution of PBS was used as a negative control and 12.5 µM sorafenib (Santa Cruz Biotechnology) as a positive control. Thus, cells were fixed with 4% PFA for 10 min at room temperature and washed twice with DPBS. Hoechst 33342 was used for nuclear staining. To capture enough cells (>1000) for analysis, five image fields were collected from each well, starting at the center of the well. All of the image analysis was acquired with an Operetta high-content imaging system and Harmony software. Cell counts were calculated and normalized to the control (PBS) [26].
5.5. Anticancer Activity of Venoms and Its Fractions against MCTS (Multicellular Tumor Spheroid)
Single cells were suspended at 6 × 103 cells/well (384-well ultra-low attachment round-bottom ULA microplates (Greiner Bio-One, Monroe, NC, USA) in DMEM media at 37 °C for three days. To produce MCTSs, four kinds of cells (RFP-Huh7.5 cells, LX2 cells, WI38 cells, and HUVECs) suspended in DMEM media were seeded at a density of 6 × 103 cells/well at 37 °C for three days. Crude venom and its fractions were resolubilized in phosphate-buffered saline (PBS; Lonza) at a concentration of 10 and 50 µg/mL, then added and incubated for an additional seven days. A solution of PBS was used as a negative control and 12.5 µM sorafenib (Santa Cruz Biotechnology) as a positive control. All of the image, area and intensity analysis of spheroid was performed using the HCS system and Harmony software. The criteria of spheroid area were calculated by average of positive control and negative control. RFP-intensity criteria were calculated by difference of average and standard deviation of negative control [26].
5.6. Dose Response Curve
Fractions that showed anti-cancerous activity with low cytotoxicity were tested at different concentrations (50, 25, 12.5, 6.3, 3.1, 1.6, 0.8, 0.4, 0.2 and 0.1 μg/mL) in 3D culture in order to establish the dose–response curve of these fractions against spheroids of the hepatocellular carcinoma by the same protocol on MCTSs. Sorafenib was tested as a positive control at different concentrations (starting from 100 µM, 10 points, twofold serial dilution) [26].
5.7. Molecules Identification in the F7 Fraction of the Naja haje Venom Nano-Liquid Chromatography Coupled to Tandem Mass Spectrometry (Nano-LC-MS/MS)
5.7.1. Reduction, Alkylation and Trypsin Digestion
The lyophilized Naja haje fraction was suspended in 10 µL of 4 M urea in 100 mM NH4HCO3 (v:v), then mixed with 10 mM of freshly prepared dithiothreitol (DTT) in 100 mM NH4HCO3 (v:v). The mixture was sonicated and flushed with nitrogen before being heated for two hours at 60 °C. The free sulfhydryl groups of cysteine were alkylated by adding 55 mM of iodoacetamide (IAA) in 100 mM NH4HCO3 (v:v), the samples were incubated at room temperature for 20 min, then 5 µl of 30 nM DTT were added and incubated over one hour to remove the excess of IAA. Subsequently, the reduced and alkylated fraction was digested overnight at 37 °C, with 1 µg of sequencing Grade Modified Trypsin (Promega) in 50 mM NH4HCO3. The enzymatic reaction was quenched by adding 5 µL of formic acid (FA) 5%. Then, the digested fraction was dried and resuspended in 0.1% (v:v) FA and 5% (v:v) acetonitrile (ACN) [44].
5.7.2. Nano-LC-MS/MS Analysis
Proteomic analysis of digested Naja haje fraction was carried out in an Agilent 1200 Series HPLC-Chip/MS system connected to a 6520 Quadrupole-Time of Flight (Q-TOF) mass spectrometer, equipped with a nano-electrospray source (Agilent Technologies, Santa Clara, CA, USA). For the online fractionation, two microliters of tryptic peptides were loaded and enriched on a 160 nL RP-C18 trap column, then separated on an analytical nano-column (150 mm × 75 µm) packed with ZORBAX SB-C18, 5 µm, 300Å (G4240-62010; Agilent Technologies). The separation was maintained over 25 min at 450 nL/min, using a linear gradient from 3 to 80% ACN in 0.1% FA. The eluted peptides were operated in positive mode in the Q-TOF mass spectrometer; the mass range was set from 290 to 1700 m/z and from 59 to 1700 for the MS and MS/MS scans, respectively. The total cycle time was two seconds. In each cycle, five of the most abundant precursor ions were subjected to MS/MS fragmentation; the ions with single charge were excluded, while the collision energy was automatically adjusted according to m/z [44].
5.7.3. Data Processing
MS-MS data files were processed using the Peaks 7.5 software (Bioinformatics Solutions Inc, Waterloo, ON, Canada) against the UniProtKB/Swiss-Prot database downloaded in November 2018 from NCBI. Specific parameters were set as follows: to carry out the research, mass tolerance of parents and fragments set at 50 ppm and 0.3 Da, respectively; enzymatic specificity, trypsin; three as the maximum missed cleavages for trypsin; variable modification, oxidation (M), carbamidomethylation, pyro-glu of Q and E, dehydration and amidation; no fixed modification was taken into account; instrument, ESI-Q-TOF; taxonomy, all; database, UniProtKB/Swiss-Prot. The characterized proteins/peptides have been classified into different families on the basis of their function according to the UniProt database (https://www.uniprot.org, accessed on 23 February 2019) [44].
5.8. Statistical Analysis
The results were expressed as mean ± standard deviation (S.D.) of minimum two independent experiments in duplicate (n = 2).
The data analyses were performed in GraphPad for statistical analysis. To compare the different effects of the fractions, statistical analysis will be assessed with a one-way ANOVA test followed by Dunnett’s multiple comparisons test for parametric data.
Author Contributions
Conceptualization, N.O., H.-R.S., D.S. and N.S-A.; methodology, A.L., S.-Y.L., J.-Y.H., I.G. and K.D.; software, A.L., J.-Y.H., B.D. and F.C.; validation N.O., H.-R.S., D.S., N.S-A. and N.M.; formal analysis, A.L., S.-Y.L. and J.-Y.H.; investigation, A.L., S.-Y.L., J.-Y.H. and Z.A.-K.; resources, N.O., H.-R.S., D.S. and N.S.-A.; data curation, A.L., S.-Y.L., J.-Y.H., I.G. and K.D.; writing—original draft preparation, A.L., S.C., B.D.; writing—review and editing, A.L., S.-Y.L., J.-Y.H., I.G., K.M., R.C. and K.D.; visualization, A.L.; supervision, N.O., H.-R.S., D.S. and N.S.-A.; project administration, N.S.-A.; funding acquisition, N.O., H.-R.S., D.S. and N.S.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research foundation of Korea (NRF), grant funded by the Korea government (MSIP) (2017M3A9G7072864 and NRF-2017M3A9G6068246). This research was funded by Institut Pasteur (Venoms in ACIP project (Anti-Cancer Innovant Polytherapy)—Animal venom biomolecules: Tools for oncogenesis study and targeted therapy against Hepatocellular carcinoma).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank everyone who has shared documents and work in progress, including the Moroccan, Korea, French and Tunisia Pasteur Institutes. Many thanks are also dedicated to both Philippe Chan and David Vaudry researchers in the Normandie Univ, UNIROUEN, Rouen Proteomic platform (PISSARO), Institute for research and innovation in biomedicine (IRIB), Rouen, France.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Alsinet, C.; Chang, C.Y.; Llovet, J.M. Molecular Approaches to Treatment of HCC. Dig. Liver Dis. 2010, 42, S264–S272. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma. 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Roberts, L.R. Hepatocellular Carcinoma: A Global View. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in Targeted Therapies for Hepatocellular Carcinoma in the Genomic Era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef]
- Vyas, V.K.; Brahmbhatt, K.; Bhatt, H.; Parmar, U. Therapeutic Potential of Snake Venom in Cancer Therapy: Current Perspectives. Asian Pac. J. Trop. Biomed. 2013, 3, 156–162. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Snake Venom: A Potent Anticancer Agent—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23244070/ (accessed on 6 May 2021).
- Lord, R.; Suddle, A.; Ross, P.J. Emerging Strategies in the Treatment of Advanced Hepatocellular Carcinoma: The Role of Targeted Therapies. Int. J. Clin. Pract. 2011, 65, 182–188. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef]
- Wörns, M.-A.; Galle, P.R. Hepatocellular Carcinoma in 2017: Two Large Steps Forward, One Small Step Back. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 74–76. [Google Scholar] [CrossRef]
- Guo, G.; Yao, G.; Zhan, G.; Hu, Y.; Yue, M.; Cheng, L.; Liu, Y.; Ye, Q.; Qing, G.; Zhang, Y.; et al. N-Methylhemeanthidine Chloride, a Novel Amaryllidaceae Alkaloid, Inhibits Pancreatic Cancer Cell Proliferation via down-Regulating AKT Activation. Toxicol. Appl. Pharmacol. 2014, 280, 475–483. [Google Scholar] [CrossRef]
- Wang, L.; Martins-Green, M. Pomegranate and Its Components as Alternative Treatment for Prostate Cancer. Int. J. Mol. Sci. 2014, 15, 14949–14966. [Google Scholar] [CrossRef]
- Harvey, A.L. Toxins and Drug Discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Grishin, E.V.; Vassilevski, A.A. Diversity of Potassium Channel Ligands: Focus on Scorpion Toxins. Biochemistry 2015, 80, 1764–1799. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Possani, L.D. Overview of Scorpion Toxins Specific for Na+ Channels and Related Peptides: Biodiversity, Structure-Function Relationships and Evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Haldar, S.; Giri, B.; Mishra, R.; Saha, A.; Dasgupta, S.; Gomes, A. Experimental Osteoporosis Induced in Female Albino Rats and Its Antagonism by Indian Black Scorpion (Heterometrus Bengalensis C.L.Koch) Venom. Toxicon 2008, 53, 60–68. [Google Scholar] [CrossRef]
- Costa Torres, A.F.; Dantas, R.T.; Toyama, M.H.; Diz Filho, E.; Zara, F.J.; Rodrigues de Queiroz, M.G.; Pinto Nogueira, N.A.; Rosa de Oliveira, M.; de Oliveira Toyama, D.; Monteiro, H.S.A.; et al. Antibacterial and Antiparasitic Effects of Bothrops Marajoensis Venom and Its Fractions: Phospholipase A2 and L-Amino Acid Oxidase. Toxicon 2010, 55, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Doumanov, J.; Mladenova, K.; Topouzova-Hristova, T.; Stoitsova, S.; Petrova, S. Effects of Vipoxin and Its Components on HepG2 Cells. Toxicon 2015, 94, 36–44. [Google Scholar] [CrossRef]
- Gomes, A.; Bhattacharya, S.; Chakraborty, M.; Bhattacharjee, P.; Mishra, R.; Gomes, A. Anti-Arthritic Activity of Indian Monocellate Cobra (Naja Kaouthia) Venom on Adjuvant Induced Arthritis. Toxicon 2010, 55, 670–673. [Google Scholar] [CrossRef]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef]
- Mahmoud, G.H.; Saber, S.A.; El-Fiky, A.A.E.-F.; Mohamed, A.F. In Vitro Evaluation of Anticancer Potential of Echispyramidum Venom (Viperidae) and Related Genetic and Apoptotic Profile Alterations. Egypt. J. Hosp. Med. 2019, 76, 3891–3900. [Google Scholar] [CrossRef]
- Mahadevappa, R.; Ma, R.; Kwok, H.F. Venom Peptides: Improving Specificity in Cancer Therapy. Trends Cancer 2017, 3, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sood, G.K. Hepatocellular Carcinoma Review: Current Treatment, and Evidence-Based Medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Bruix, J.; da Fonseca, L.G.; Reig, M. Insights into the Success and Failure of Systemic Therapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 617–630. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-Y.; Kim, S.; Choi, I.; Kim, S.-H.; Shum, D.; Heo, J.; Kim, A.-R.; Kim, K.M.; Seo, H.R. Inhibitors of Na + /K + ATPase Exhibit Antitumor Effects on Multicellular Tumor Spheroids of Hepatocellular Carcinoma. Sci. Rep. 2020, 10, 5318. [Google Scholar] [CrossRef] [PubMed]
- Heinen, T.E.; da Veiga, A.B.G. Arthropod Venoms and Cancer. Toxicon 2011, 57, 497–511. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, C.; Chen, Z.; Wang, W.; Huang, M. Expression, Purification and Characterization of Recombinant Jerdonitin, a P-II Class Snake Venom Metalloproteinase Comprising Metalloproteinase and Disintegrin Domains. Toxicon 2010, 55, 375–380. [Google Scholar] [CrossRef] [PubMed]
- El Hakim, A.E.; Gamal-Eldeen, A.M.; Shahein, Y.E.; Mansour, N.M.; Wahby, A.F.; Abouelella, A.M.K. Purification and Characterization of a Cytotoxic Neurotoxin-like Protein from Naja Haje Haje Venom That Induces Mitochondrial Apoptosis Pathway. Arch. Toxicol. 2011, 85, 941–952. [Google Scholar] [CrossRef]
- Fakhri, A.; Omranipour, R.; Fakhri, S.; Mirshamsi, M.; Zangeneh, F.; Vatanpour, H.; Pourahmad, J. Naja Naja Oxiana Venom Fraction Selectively Induces ROS-Mediated Apoptosis in Human Colorectal Tumor Cells by Directly Targeting Mitochondria. Asian Pac. J. Cancer Prev. 2017, 18, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, K.; Vatanpour, H.; Zare, A.; Shirazi, F.H.; Nakhjavani, M. Anticancer Activity a of Caspian Cobra (Naja Naja Oxiana) Snake Venom in Human Cancer Cell Lines Via Induction of Apoptosis. Iran. J. Pharm. Res. 2016, 15, 101–112. [Google Scholar]
- Seydi, E.; Babaei, S.; Fakhri, A.; Pourahmad, J. Selective Toxicity of Caspian Cobra (Naja Oxiana) Venom on Liver Cancer Cell Mitochondria. Asian Pac. J. Trop. Biomed. 2017, 7, 460–465. [Google Scholar] [CrossRef]
- Av, F.; Gv, S.; Mv, A.; Di, R.; Yn, U.; As, A. Cancer Cell Injury by Cytotoxins from Cobra Venom Is Mediated through Lysosomal Damage. Biochem. J. 2005, 390, 11–18. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Dong, P.; Zhao, D.; Wang, C.; Tao, Z.; Sun, M.-Z. Akbu-LAAO Exhibits Potent Anti-Tumor Activity to HepG2 Cells Partially through Produced H2O2 via TGF-β Signal Pathway. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer Effect of Nemopilema Nomurai Jellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evid. Based Complement. Altern. Med. 2017, 2017, e2752716. [Google Scholar] [CrossRef] [PubMed]
- Hou Effects of Macrothele Raven Venom on Intrarenal Invasion and Metastasis of H22 Liver Cancer Cells in Mice. Available online: https://www.cancerjournal.net/article.asp?issn=0973-1482;year=2017;volume=13;issue=4;spage=725;epage=729;aulast=Hou (accessed on 6 May 2021).
- Lian, W.; Lian, H.; Li, Q.; Hu, A.; Liu, S. The Venom of Spider Haplopelma Hainanum Suppresses Proliferation and Induces Apoptosis in Hepatic Cancer Cells by Caspase Activation in Vitro. J. Ethnopharmacol. 2018, 225, 169–177. [Google Scholar] [CrossRef]
- Malih, I.; Ahmad Rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic Analysis of Moroccan Cobra Naja Haje Legionis Venom Using Tandem Mass Spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-Gated Potassium Channels as Therapeutic Drug Targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Hammouda, M.B.; Montenegro, M.F.; Sánchez-del-Campo, L.; Zakraoui, O.; Aloui, Z.; Riahi-Chebbi, I.; Karoui, H.; Rodríguez-López, J.N.; Essafi-Benkhadir, K. Lebein, a Snake Venom Disintegrin, Induces Apoptosis in Human Melanoma Cells. Toxins 2016, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-Based Peptide Therapy: Insights into Anti-Cancer Mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef]
- Oukkache, N.; Chgoury, F.; Lalaoui, M.; Cano, A.A.; Ghalim, N. Comparison between Two Methods of Scorpion Venom Milking in Morocco. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 5. [Google Scholar] [CrossRef]
- Sarray, S.; Berthet, V.; Calvete, J.J.; Secchi, J.; Marvaldi, J.; Ayeb, M.E.; Marrakchi, N.; Luis, J. Lebectin, a Novel C-Type Lectin from Macrovipera Lebetina Venom, Inhibits Integrin-Mediated Adhesion, Migration and Invasion of Human Tumour Cells. Lab. Investig. 2004, 84, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; Malosse, C.; Lafnoune, A.; Darkaoui, B.; Chakir, S.; Sabatier, J.-M.; Chamot-Rooke, J.; Cadi, R.; Oukkache, N. Mass Spectrometry-Based Top-down and Bottom-up Approaches for Proteomic Analysis of the Moroccan Buthus Occitanus Scorpion Venom. FEBS Open Bio 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).