Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review
Abstract
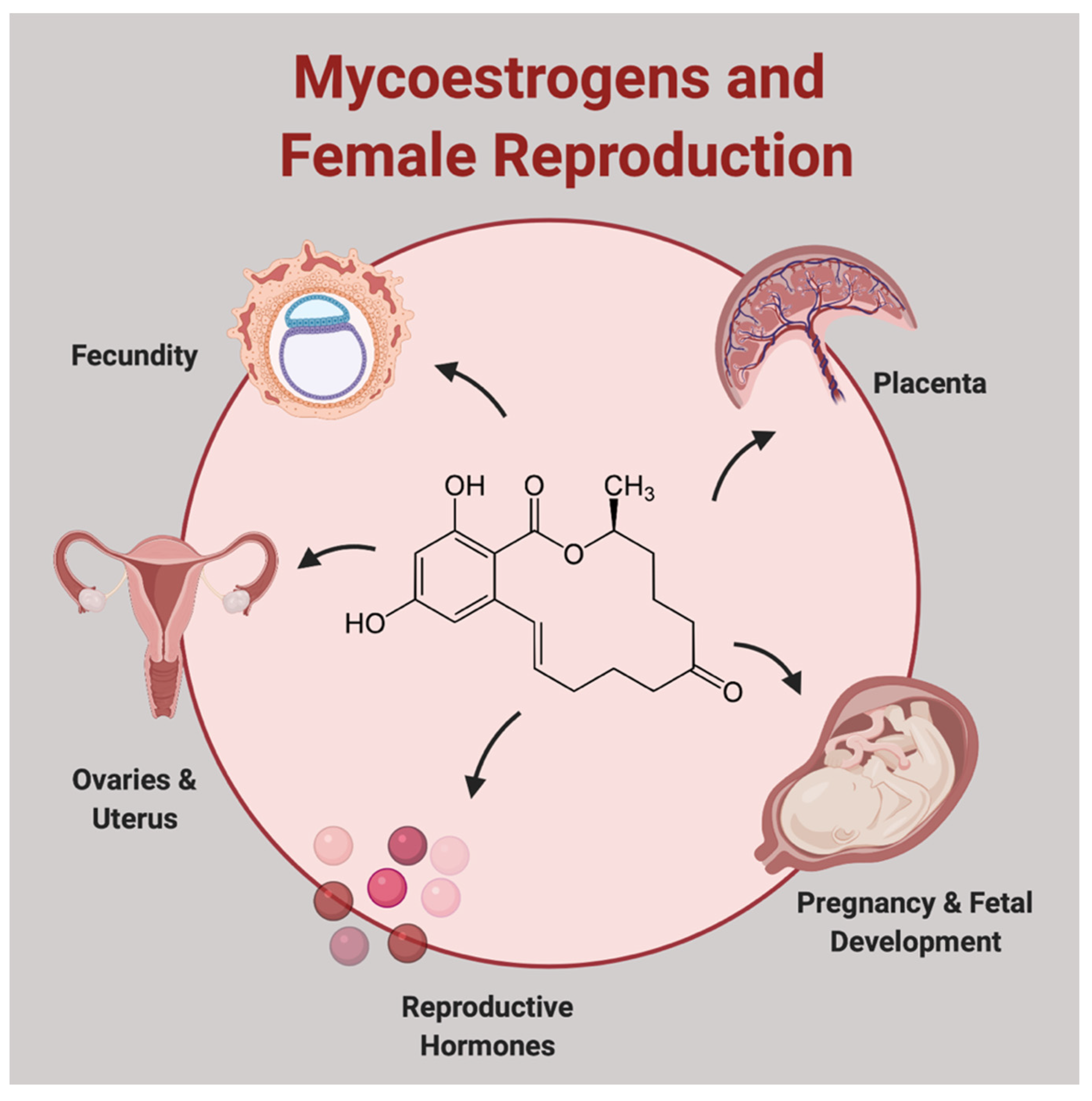
1. Introduction
2. Results
2.1. ZEN and Reproductive Hormone Regulation in the Non-Pregnant State
2.2. ZEN and the Ovaries
2.3. ZEN and the Uterus
2.4. ZEN and the Placenta
2.5. ZEN and Fertilization, Pregnancy, and Fetal Development
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Mycotoxin Fact Sheet. 9 May 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 23 May 2021).
- Khaneghah, A.M.; Fakhri, Y.; Raeisi, S.; Armoon, B.; Sant’Ana, A.S. Prevalence and concentration of ochratoxin A, zearalenone, deoxynivalenol and total aflatoxin in cereal-based products: A systematic review and meta-analysis. Food Chem. Toxicol. 2018, 118, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Etienne, M.; Jemmali, M. Effects of feeding corn infested by Fusarium to sows. Comptes Rendus des Seances de l’Academie des Sci. Ser. D Sci. Nat. 1979, 288, 779–782. [Google Scholar]
- Belchev, L. Pathomorphological changes in the estrogenic syndrome of swine. Vet.-Med. Nauk. 1979, 16, 33–40. [Google Scholar]
- Mshelia, L.P.; Selamat, J.; Samsudin, N.I.P.; Rafii, M.Y.; Mutalib, N.-A.A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Berrada, H.; Miere, D.; Loghin, F.; Mañes, J. Study on Trichothecene and Zearalenone Presence in Romanian Wheat Relative to Weather Conditions. Toxins 2019, 11, 163. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Lešić, T.; Zadravec, M.; Vahčić, N.; Staver, M.M.; Markov, K. Deoxynivalenol and zearalenone in unprocessed cereals and soybean from different cultivation regions in Croatia. Food Addit. Contam. Part B 2017, 10, 268–274. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Nikov, G.N.; Hopkins, N.E.; Boue, S.; Alworth, W.L. Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environ. Health Perspect. 2000, 108, 867–872. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Hao, R.; Grimaldi, M.; Thrikawala, S.; Boulahtouf, A.; Aït-Aïssa, S.; Brion, F.; Gustafsson, J.Å.; Balaguer, P.; Bondesson, M. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 380, 114709. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.-Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mitak, M.; Gojmerac, T.; Cvrtila, D.; Cvetnić, Ž. Effect of atrazine and zearalenone on the numer of receptor binding sites for 3H-estradiol in the rat uterus cytosol. Vet. Med. 2012, 47, 12–16. [Google Scholar] [CrossRef]
- Oliver, W.; Miles, J.; Diaz, D.; Dibner, J.; Rottinghaus, G.; Harrell, R. Zearalenone enhances reproductive tract development, but does not alter skeletal muscle signaling in prepubertal gilts. Anim. Feed. Sci. Technol. 2012, 174, 79–85. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Padilla-Banks, E.; Clark, G.; Newbold, R.R. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J. Chromatogr. B 2002, 777, 179–189. [Google Scholar] [CrossRef]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Food Consumption Data as a Tool to Estimate Exposure to Mycoestrogens. Toxins 2020, 12, 118. [Google Scholar] [CrossRef]
- Dellafiora, L.; Warth, B.; Schmidt, V.; Del Favero, G.; Mikula, H.; Fröhlich, J.; Marko, D. An integrated in silico/in vitro approach to assess the xenoestrogenic potential of Alternaria mycotoxins and metabolites. Food Chem. 2018, 248, 253–261. [Google Scholar] [CrossRef]
- Christensen, A.; Bentley, G.E.; Cabrera, R.; Ortega, H.H.; Perfito, N.; Wu, T.J.; Micevych, P. Hormonal Regulation of Female Reproduction. Horm. Metab. Res. 2012, 44, 587–591. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Yin, S.; Shan, A.; Gao, R.; Qu, Z.; Liu, M.; Nie, S. Toxic Effects of Maternal Zearalenone Exposure on Uterine Capacity and Fetal Development in Gestation Rats. Reprod. Sci. 2014, 21, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Yang, C.W.; Huang, L.B.; Niu, Q.S.; Jiang, S.Z.; Chi, F. Zearalenone Altered the Serum Hormones, Morphologic and Apoptotic Measurements of Genital Organs in Post-weaning Gilts. Asian-Australas. J. Anim. Sci. 2015, 28, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Sun, X.-F.; Feng, Y.-Z.; Cheng, S.-F.; Li, B.; Li, Y.-P.; Shen, W.; Li, L. The impact of Zearalenone on the meiotic progression and primordial follicle assembly during early oogenesis. Toxicol. Appl. Pharmacol. 2017, 329, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Parandin, R.; Behnam-Rassouli, M.; Mahdavi-Shahri, N. Effects of Neonatal Exposure to Zearalenone on Puberty Timing, Hypothalamic Nuclei of AVPV and ARC, and Reproductive Functions in Female Mice. Reprod. Sci. 2017, 24, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, N.; Momtaz, S.; Baeeri, M.; Navaei-Nigjeh, M.; Hosseini, R.; Abdollahi, M. Molecular and biochemical evidence on the role of zearalenone in rat polycystic ovary. Toxicon 2018, 154, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, K.; Wei, H.; Shen, L. Isolation and characterization of the Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice. Toxicon 2018, 155, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Shrivastava, V.K.; Saleh, R.; Henkel, R.; Agarwal, A. Protective effects of saffron against zearalenone-induced alterations in reproductive hormones in female mice (Mus musculus). Clin. Exp. Reprod. Med. 2018, 45, 163–169. [Google Scholar] [CrossRef]
- Li, R.; Andersen, C.L.; Hu, L.; Wang, Z.; Li, Y.; Nagy, T.; Ye, X. Dietary exposure to mycotoxin zearalenone (ZEA) during post-implantation adversely affects placental development in mice. Reprod. Toxicol. 2019, 85, 42–50. [Google Scholar] [CrossRef]
- Biehl, M.; Prelusky, D.; Koritz, G.; Hartin, K.; Buck, W.; Trenholm, H. Biliary Excretion and Enterohepatic Cycling of Zearalenone in Immature Pigs. Toxicol. Appl. Pharmacol. 1993, 121, 152–159. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Behrens, G.H.; Ingebrigtsen, K.; Langseth, W.; Berndt, S.; Haugen, T.B.; Grotmol, T. Placental transfer of the estrogenic mycotoxin zearalenone in rats. Reprod. Toxicol. 2001, 15, 545–550. [Google Scholar] [CrossRef]
- Koraichi, F.; Videmann, B.; Mazallon, M.; Benahmed, M.; Prouillac, C.; Lecoeur, S. Zearalenone exposure modulates the expression of ABC transporters and nuclear receptors in pregnant rats and fetal liver. Toxicol. Lett. 2012, 211, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Preindl, K.; Manser, P.; Wick, P.; Marko, D.; Buerki-Thurnherr, T. Transfer and Metabolism of the Xenoestrogen Zearalenone in Human Perfused Placenta. Environ. Health Perspect. 2019, 127, 107004. [Google Scholar] [CrossRef]
- Lange, I.G.; Daxenberger, A.; Meyer, H.H.D.; Meyts, E.R.-D.; Skakkebæk, N.E.; Veeramachaneni, D.N.R. Quantitative assessment of foetal exposure to trenbolone acetate, zeranol and melengestrol acetate, following maternal dosing in rabbits. Xenobiotica 2002, 32, 641–651. [Google Scholar] [CrossRef]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef]
- Migdalof, B.H.; Dugger, H.A.; Heider, J.G.; Coombs, R.A.; Terry, M.K. Biotransformation of zeranol: Disposition and metabolism in the female rat, rabbit, dog, monkey and man. Xenobiotica 1983, 13, 209–221. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Schauerhamer, B.; Christensen, C.M.; Niku-Paavola, M.L.; Nummi, M. Incidence of zearalenol (Fusarium mycotoxin) in animal feed. Appl. Environ. Microbiol. 1979, 38, 749–750. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- FDA. New Animal Drugs; Updating Tolerances for Residues of New Animal Drugs in Food; Food and Drug Administration: Silver Spring, MD, USA, 2019; p. 33001. [Google Scholar]
- Cai, Y.; McLaughlin, M.; Zhang, K. Advancing the FDA/Office of Regulatory Affairs Mycotoxin Program: New Analytical Method Approaches to Addressing Needs and Challenges. J. AOAC Int. 2020, 103, 705–709. [Google Scholar] [CrossRef]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R.; Teeguarden, J.G. Urine and serum biomonitoring of exposure to environmental estrogens II: Soy isoflavones and zearalenone in pregnant women. Food Chem. Toxicol. 2016, 95, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bandera, E.V.; Chandran, U.; Buckley, B.; Lin, Y.; Isukapalli, S.; Marshall, I.; King, M.; Zarbl, H. Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci. Total Environ. 2011, 409, 5221–5227. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Alvito, P.; Assunção, R.; Oliveira, C.A. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019, 128, 21–34. [Google Scholar] [CrossRef]
- Lorenz, N.; Dänicke, S.; Edler, L.; Gottschalk, C.; Lassek, E.; Marko, D.; Rychlik, M.; Mally, A. A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res. 2019, 35, 27–46. [Google Scholar] [CrossRef]
- Rivera-Núñez, Z.; Barrett, E.S.; Szamreta, E.A.; Shapses, S.A.; Qin, B.; Lin, Y.; Zarbl, H.; Buckley, B.; Bandera, E.V. Urinary mycoestrogens and age and height at menarche in New Jersey girls. Environ. Health 2019, 18, 24. [Google Scholar] [CrossRef]
- Massart, F.; Meucci, V.; Saggese, G.; Soldani, G. High Growth Rate of Girls with Precocious Puberty Exposed to Estrogenic Mycotoxins. J. Pediatr. 2008, 152, 690–695.e1. [Google Scholar] [CrossRef] [PubMed]
- Asci, A.; Durmaz, E.; Erkekoglu, P.; Pasli, D.; Bircan, I.; Kocer-Gumusel, B. Urinary zearalenone levels in girls with premature thelarche and idiopathic central precocious puberty. Minerva Pediatr. 2014, 66, 571–578. [Google Scholar] [PubMed]
- De Rodriguez, C.A.; Bongiovanni, A.M.; de Borrego, L.C. An epidemic of precocious development in Puerto Rican children. J. Pediatrics 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, L.; Chen, Y.; Sun, T.; Wang, N.; Chen, X.; Yang, Z.; Ge, J.; Jiang, S. Comparative study of stress response, growth and development of uteri in post-weaning gilts challenged with zearalenone and estradiol benzoate. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, U.; Rudolf, F.O.; Pandey, K.; Kadokawa, H. The non-steroidal mycoestrogen zeranol suppresses luteinizing hormone secretion from the anterior pituitary of cattle via the estradiol receptor GPR30 in a rapid, non-genomic manner. Anim. Reprod. Sci. 2015, 156, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, U.; Kadokawa, H. The nonsteroidal mycoestrogen zearalenone and its five metabolites suppress LH secretion from the bovine anterior pituitary cells via the estradiol receptor GPR30 in vitro. Theriogenology 2015, 84, 1342–1349. [Google Scholar] [CrossRef]
- He, J.; Wei, C.; Li, Y.; Liu, Y.; Wang, Y.; Pan, J.; Liu, J.; Wu, Y.; Cui, S. Zearalenone and alpha-zearalenol inhibit the synthesis and secretion of pig follicle stimulating hormone via the non-classical estrogen membrane receptor GPR30. Mol. Cell. Endocrinol. 2018, 461, 43–54. [Google Scholar] [CrossRef]
- Tan, S.-J.; Ge, W.; Wang, J.-J.; Liu, W.-X.; Zhao, Y.; Shen, W.; Li, L. Zearalenone-induced aberration in the composition of the gut microbiome and function impacts the ovary reserve. Chemosphere 2020, 244, 125493. [Google Scholar] [CrossRef]
- Altavilla, D.; Saitta, A.M.; Galeano, M.; Squadrito, G.; Marino, D.; Minutoli, L.; Calapai, G.; Deodato, B.; D’Anna, R.; Corrado, F.; et al. The Phytoestrogen α-Zearalenol Reverses Endothelial Dysfunction Induced by Oophorectomy in Rats. Lab. Investig. 2001, 81, 125–132. [Google Scholar] [CrossRef]
- Denli, M.; Blandon, J.C.; Salado, S.; Guynot, M.E.; Pérez, J.F. Effect of dietary zearalenone on the performance, reproduction tract and serum biochemistry in young rats. J. Appl. Anim. Res. 2016, 45, 619–622. [Google Scholar] [CrossRef]
- Zielonka, Ł.; Gajęcka, M.; Lisieska-Żołnierczyk, S.; Dąbrowski, M.; Gajęcki, M.T. The Effect of Different Doses of Zearalenone in Feed on the Bioavailability of Zearalenone and Alpha-Zearalenol, and the Concentrations of Estradiol and Testosterone in the Peripheral Blood of Pre-Pubertal Gilts. Toxins 2020, 12, 144. [Google Scholar] [CrossRef]
- Rykaczewska, A.; Gajęcka, M.; Onyszek, E.; Cieplińska, K.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Babuchowski, A.; Gajęcki, M.T.; Zielonka, Ł. Imbalance in the Blood Concentrations of Selected Steroids in Pre-pubertal Gilts Depending on the Time of Exposure to Low Doses of Zearalenone. Toxins 2019, 11, 561. [Google Scholar] [CrossRef]
- Wu, F.; Cui, J.; Yang, X.; Liu, S.; Han, S.; Chen, B. Effects of zearalenone on genital organ development, serum immunoglobulin, antioxidant capacity, sex hormones and liver function of prepubertal gilts. Toxicon 2021, 189, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Wang, C.C.; Leung, L.K. Effect of zeranol on expression of apoptotic and cell cycle proteins in murine placentae. Toxicology 2013, 314, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Alam, H.Z.; Rorie, J.; Ruggles, D.I. Effects of zearalenone on in utero development in rats. Food Chem. Toxicol. 2006, 44, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, L.; Zhang, N.; Li, C.; Zhang, J.; Xiao, Z.; Qi, D. Gestational Zearalenone Exposure Causes Reproductive and Developmental Toxicity in Pregnant Rats and Female Offspring. Toxins 2017, 9, 21. [Google Scholar] [CrossRef]
- Pan, P.; Ying, Y.; Ma, F.; Zou, C.; Yu, Y.; Li, Y.; Li, Z.; Fang, Y.; Huang, T.; Ge, R.-S.; et al. Zearalenone disrupts the placental function of rats: A possible mechanism causing intrauterine growth restriction. Food Chem. Toxicol. 2020, 145, 111698. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Sun, X.-F.; Feng, Y.-Z.; Li, B.; Li, Y.-P.; Yang, F.; Nyachoti, C.M.; Shen, W.; Sun, S.-D.; Li, L. Zearalenone exposure impairs ovarian primordial follicle formation via down-regulation of Lhx8 expression in vitro. Toxicol. Appl. Pharmacol. 2017, 317, 33–40. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Xiong, B.; Zheng, W.-J.; Duan, X.; Cui, X.-S.; Kim, N.-H.; Wang, Q.; Xu, Y.-X.; Sun, S.-C. Oocyte quality in mice is affected by a mycotoxin-contaminated diet. Environ. Mol. Mutagen. 2014, 55, 354–362. [Google Scholar] [CrossRef]
- Chen, F.; Wen, X.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol. Lett. 2019, 301, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Yang, X.; Huang, K.; Luo, Y.; Zhu, L.; Li, Y.; Xu, W. Zinc inhibits the reproductive toxicity of Zearalenone in immortalized murine ovarian granular KK-1 cells. Sci. Rep. 2015, 5, 14277. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, L.; Chen, K.; Li, C.; Wang, Y.; Wang, G. Melatonin alleviates β-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells. Environ. Toxicol. Pharmacol. 2019, 68, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, M.-Y.; Li, N.; Wang, J.-J.; Ge, W.; Tan, S.-J.; Shen, W.; Li, L. Zearalenone exposure triggered porcine granulosa cells apoptosis via microRNAs-mediated focal adhesion pathway. Toxicol. Lett. 2020, 330, 80–89. [Google Scholar] [CrossRef]
- Yi, Y.; Wan, S.; Hou, Y.; Cheng, J.; Guo, J.; Wang, S.; Khan, A.; Sun, N.; Li, H. Chlorogenic acid rescues zearalenone induced injury to mouse ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2020, 194, 110401. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and Their Human Metabolites Show Distinct Agonistic and Antagonistic Properties on Estrogen Receptor (ER) and ER in Human Cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Minervini, F.; Dell’Aquila, M.; Maritato, F.; Minoia, P.; Visconti, A. Toxic effects of the mycotoxin zearalenone and its derivatives on in vitro maturation of bovine oocytes and 17β-estradiol levels in mural granulosa cell cultures. Toxicol. Vitr. 2001, 15, 489–495. [Google Scholar] [CrossRef]
- Sambuu, R.; Takagi, M.; Namula, Z.; Otoi, T.; Shiga, S.; DOS Santos, R.R.; Fink-Gremmels, J. Effects of Exposure to Zearalenone on Porcine Oocytes and Sperm During Maturation and Fertilization In Vitro. J. Reprod. Dev. 2011, 57, 547–550. [Google Scholar] [CrossRef]
- Alm, H.; Greising, T.; Brüssow, K.-P.; Torner, H.; Tiemann, U. The influence of the mycotoxins deoxynivalenol and zearalenol on in vitro maturation of pig oocytes and in vitro culture of pig zygotes. Toxicol. Vitr. 2002, 16, 643–648. [Google Scholar] [CrossRef]
- Han, J.; Wang, T.; Fu, L.; Shi, L.-Y.; Zhu, C.-C.; Liu, J.; Zhang, Y.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Altered oxidative stress, apoptosis/autophagy, and epigenetic modifications in Zearalenone-treated porcine oocytes. Toxicol. Res. 2015, 4, 1184–1194. [Google Scholar] [CrossRef]
- Sambuu, R.; Takagi, M.; Shiga, S.; Uno, S.; Kokushi, E.; Namula, Z.; Otoi, T.; Miyamoto, A.; Deguchi, E.; Fink-Gremmels, J. Detection of Zearalenone and Its Metabolites in Naturally Contaminated Porcine Follicular Fluid by Using Liquid Chromatography-Tandem Mass Spectrometry. J. Reprod. Dev. 2011, 57, 303–306. [Google Scholar] [CrossRef]
- Silva, T.E.S.; De Brito, D.C.C.; De Sá, N.A.R.; Da Silva, R.F.; Ferreira, A.C.A.; Da Silva, J.Y.G.; Guedes, M.I.F.; Rodrigues, A.P.R.; Dos Santos, R.R.; De Figueiredo, J.R. Equol: A Microbiota Metabolite Able to Alleviate the Negative Effects of Zearalenone during In Vitro Culture of Ovine Preantral Follicles. Toxins 2019, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, F.; Caloni, F.; Schutz, L.F.; Totty, M.L.; Spicer, L.J. Individual and combined effects of deoxynivalenol and α-zearalenol on cell proliferation and steroidogenesis of granulosa cells in cattle. Environ. Toxicol. Pharmacol. 2015, 40, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Giannoccaro, A.; Fornelli, F.; Dell’Aquila, M.E.; Minoia, P.; Visconti, A. Influence of mycotoxin zearalenone and its derivatives (alpha and beta zearalenol) on apoptosis and proliferation of cultured granulosa cells from equine ovaries. Reprod. Biol. Endocrinol. 2006, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Yoshizawa, K.; Danbara, N.; Tsujita-Kyutoku, M.; Yuri, T.; Uehara, N.; Tsubura, A. Prepubertal Zearalenone Exposure Suppresses N-Methyl-N-nitrosourea-Induced Mammary Tumorigenesis but Causes Severe Endocrine Disruption in Female Sprague-Dawley Rats. Nutr. Cancer 2003, 47, 164–170. [Google Scholar] [CrossRef]
- Denli, M.; Blandon, J.C.; Guynot, M.E.; Salado, S.; Pérez, J.F. Efficacy of activated diatomaceous clay in reducing the toxicity of zearalenone in rats and piglets. J. Anim. Sci. 2015, 93, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.C.; Montiani-Ferreira, F.; Locatelli-Dittrich, R.; Santin, E.; Alberton, G.C. Effects of zearalenone in prepubertal gilts. Pesquisa Veterinaria Brasileira 2011, 31, 656–662. [Google Scholar] [CrossRef][Green Version]
- Nikaido, Y.; Danbara, N.; Tsujita-Kyutoku, M.; Yuri, T.; Uehara, N.; Tsubura, A. Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo 2005, 19, 487–494. [Google Scholar]
- Samik, A.; Safitri, E. Mycotoxin binders potential on histological of ovary mice exposed by zearalenone. Vet. World 2017, 10, 353–357. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Y.-M.; Zhang, L.; Zhao, Z.-M.; Zhao, J.; Peng, S.-Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol. Cell. Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef]
- Yuri, T.; Nikaido, Y.; Shimano, N.; Uehara, N.; Shikata, N.; Tsubura, A. Effects of prepubertal zeranol exposure on estrogen target organs and N-methyl-N-nitrosourea-induced mammary tumorigenesis in female Sprague-Dawley rats. In Vivo 2005, 18, 755–761. [Google Scholar]
- Kriszt, R.; Winkler, Z.; Polyák, Á.; Kuti, D.; Molnár, C.; Hrabovszky, E.; Kalló, I.; Szőke, Z.; Ferenczi, S.; Kovács, K.J. Xenoestrogens Ethinyl Estradiol and Zearalenone Cause Precocious Puberty in Female Rats via Central Kisspeptin Signaling. Endocrinology 2015, 156, 3996–4007. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, E.J.; Santos, R.R.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Transgenerational toxicity of Zearalenone in pigs. Reprod. Toxicol. 2012, 34, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Jiang, S.; Yuan, X.; Yang, W.; Yang, Z.; Huang, L. Effects of zearalenone-diet on expression of ghrelin and PCNA genes in ovaries of post-weaning piglets. Anim. Reprod. Sci. 2016, 168, 126–137. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhou, M.; Huang, L.B.; Yang, W.R.; Yang, Z.B.; Jiang, S.Z.; Ge, J.S. Zearalenone-Promoted Follicle Growth through Modulation of Wnt-1/beta-Catenin Signaling Pathway and Expression of Estrogen Receptor Genes in Ovaries of Postweaning Piglets. J. Agric. Food Chem. 2018, 66, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Rybarczyk, L.; Zwierzchowski, W.; Jakimiuk, E.; Obremski, K.; Gajęcki, M. The effect of experimental, long-term exposure to low-dose zearalenone mycotoxicosis on the histological condition of ovaries in sexually immature gilts. Theriogenology 2011, 75, 1085–1094. [Google Scholar] [CrossRef]
- Zwierzchowski, W.; Przybyłowicz, M.; Obremski, K.; Zielonka, L.; Skorska-Wyszyńska, E.; Gajecka, M.; Polak, M.; Jakimiuk, E.; Jana, B.; Rybarczyk, L.; et al. Level of zearalenone in blood serum and lesions in ovarian follicles of sexually immature gilts in the course of zearalenone micotoxicosis. Pol. J. Vet. Sci. 2005, 8, 209–218. [Google Scholar] [PubMed]
- Otrocka-Domagala, I.; Rotkiewiczl, T.; Mikolajczyk, A.; Gajecka, M.; Polak, M. Influence of zearalenone on reproductive system cell proliferation in gilts. Pol. J. Vet. Sci. 2003, 6, 239–245. [Google Scholar]
- Tiemann, U.; Tomek, W.; Schneider, F.; Vanselow, J. Effects of the mycotoxins α- and β-zearalenol on regulation of progesterone synthesis in cultured granulosa cells from porcine ovaries. Reprod. Toxicol. 2003, 17, 673–681. [Google Scholar] [CrossRef]
- Hou, Y.J.; Zhu, C.C.; Xu, Y.X.; Cui, X.S.; Kim, N.H.; Sun, S.C. Zearalenone exposure affects mouse oocyte meiotic maturation and granulosa cell proliferation. Environ. Toxicol. 2015, 30, 1226–1233. [Google Scholar] [CrossRef]
- Zhu, C.C.; Hou, Y.J.; Han, J.; Cui, X.S.; Kim, N.H.; Sun, S.C. Zearalenone exposure affects epigenetic modifications of mouse eggs. Mutagenesis 2014, 29, 489–495. [Google Scholar] [CrossRef]
- Zhang, F.L.; Li, N.; Wang, H.; Ma, J.M.; Shen, W.; Li, L. Zearalenone Exposure Induces the Apoptosis of Porcine Granulosa Cells and Changes Long Noncoding RNA Expression To Promote Antiapoptosis by Activating the JAK2-STAT3 Pathway. J. Agric. Food Chem. 2019, 67, 12117–12128. [Google Scholar] [CrossRef]
- Devine, T.; Rosenkrans, C.; Philipp, D.; Davis, A.; Lester, T.; Rorie, R.; Looper, M. Growth, reproductive development, and estrous behavior of beef heifers treated with growth promotants. Prof. Anim. Sci. 2015, 31, 114–119. [Google Scholar] [CrossRef]
- Jakimiuk, E.; Rybarczyk, L.; Zwierzchowski, W.; Obremski, K.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Effect of experimental long-term exposure to low-dose zearalenone mycotoxicosis on selected morphometric parameters of the reproductive tract in sexually-immature gilts. Bull. Vet. Inst. Pulawy 2010, 54, 25–28. [Google Scholar]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed. Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Lemke, S.L.; Mayura, K.; Reeves, W.R.; Wang, N.; Fickey, C.; Phillips, T.D. Investigation of organophilic montmorillonite clay inclusion in zearalenone-contaminated diets using the mouse uterine weight bioassay. J. Toxicol. Environ. Health A 2001, 62, 243–258. [Google Scholar] [CrossRef]
- Afriyie-Gyawu, E.; Wiles, M.C.; Huebner, H.J.; Richardson, M.B.; Fickey, C.; Phillips, T.D. Prevention of Zearalenone-Induced Hyperestrogenism in Prepubertal Mice. J. Toxicol. Environ. Health Part A 2005, 68, 353–368. [Google Scholar] [CrossRef]
- Heneweer, M.; Houtman, R.; Poortman, J.; Groot, M.; Maliepaard, C.; Peijnenburg, A. Estrogenic Effects in the Immature Rat Uterus after Dietary Exposure to Ethinylestradiol and Zearalenone Using a Systems Biology Approach. Toxicol. Sci. 2007, 99, 303–314. [Google Scholar] [CrossRef]
- Turcotte, J.C.; Hunt, P.J.; Blaustein, J.D. Estrogenic effects of zearalenone on the expression of progestin receptors and sexual behavior in female rats. Horm. Behav. 2005, 47, 178–184. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, L.J.; Yang, W.R.; Huang, L.B.; Zhou, X.M.; Jiang, S.Z.; Yang, Z.B. Effects of zearalenone on the localization and expression of the growth hormone receptor gene in the uteri of post-weaning piglets. Asian-Australas J. Anim. Sci. 2018, 31, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Stopa, E.; Gajęcka, M.; Babińska, I.; Zielonka, Ł.; Gajęcki, M. The effect of experimental exposure to low doses of zearalenone on uterine histology and morphometry in prepubertal bitches. Theriogenology 2014, 82, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, L.; Shao, M.; Wang, Y.; Yang, W.; Huang, L.; Zhou, X.; Jiang, S.; Yang, Z. Effects of Zearalenone Exposure on the TGF-β1/Smad3 Signaling Pathway and the Expression of Proliferation or Apoptosis Related Genes of Post-Weaning Gilts. Toxins 2018, 10, 49. [Google Scholar] [CrossRef]
- Nikaido, Y.; Yoshizawa, K.; Danbara, N.; Tsujita-Kyutoku, M.; Yuri, T.; Uehara, N.; Tsubura, A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 2004, 18, 803–811. [Google Scholar] [CrossRef]
- Zhao, F.; Li, R.; Xiao, S.; Diao, H.; Viveiros, M.M.; Song, X.; Ye, X. Postweaning Exposure to Dietary Zearalenone, a Mycotoxin, Promotes Premature Onset of Puberty and Disrupts Early Pregnancy Events in Female Mice. Toxicol. Sci. 2013, 132, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hu, J.; Xiao, C.; Dai, Y.; Ding, X.; Xu, Y. Exploration of ZEA cytotoxicity to mouse endometrial stromal cells and RNA-seq analysis. J. Biochem. Mol. Toxicol. 2016, 31, e21874. [Google Scholar] [CrossRef]
- Wollenhaupt, K.; Jonas, L.; Tiemann, U.; Tomek, W. Influence of the mycotoxins α- and β-zearalenol (ZOL) on regulators of cap-dependent translation control in pig endometrial cells. Reprod. Toxicol. 2004, 19, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, M.; Dai, Y.; Ding, X.; Xiao, C.; Ji, H.; Xu, Y. Exploration of Bcl-2 family and caspases-dependent apoptotic signaling pathway in Zearalenone-treated mouse endometrial stromal cells. Biochem. Biophys. Res. Commun. 2016, 476, 553–559. [Google Scholar] [CrossRef]
- Giammarino, A.; Manera, M.; Robbe, D.; Perugini, M.; Minervini, F.; Amorena, M. Influence of mycotoxins on spontaneous contraction in myometrial strips of prepubertal lamb. Res. Vet. Sci. 2008, 84, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Walf, V.M.; Christians, A.; Pfaffl, M.; Meyer, H.H. Modification of mRNA expression after treatment with anabolic agents and the usefulness for gene expression-biomarkers. Anal. Chim. Acta 2007, 586, 73–81. [Google Scholar] [CrossRef]
- Song, T.; Yang, W.; Huang, L.; Yang, Z.; Jiang, S. Zearalenone exposure affects the Wnt/β-catenin signaling pathway and related genes of porcine endometrial epithelial cells in vitro. Anim. Biosci. 2021, 34, 993–1005. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Ni, C.; Dai, Y.; Guo, Y. Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol. Med. Rep. 2018, 17, 7797–7806. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.; Vetrano, A.M.; Laskin, J.D.; Aleksunes, L.M. Localization of the placental BCRP/ ABCG2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity. Placenta 2017, 55, 29–36. [Google Scholar] [CrossRef]
- Szilagyi, J.T.; Gorczyca, L.; Brinker, A.; Buckley, B.; Laskin, J.D.; Aleksunes, L.M. Placental BCRP/ABCG2Transporter Prevents Fetal Exposure to the Estrogenic Mycotoxin Zearalenone. Toxicol. Sci. 2018, 168, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Prouillac, C.; Videmann, B.; Mazallon, M.; Lecoeur, S. Induction of cells differentiation and ABC transporters expression by a myco-estrogen, zearalenone, in human choriocarcinoma cell line (BeWo). Toxicology 2009, 263, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Prouillac, C.; Koraichi, F.; Videmann, B.; Mazallon, M.; Rodriguez, F.; Baltas, M.; Lecoeur, S. In vitro toxicological effects of estrogenic mycotoxins on human placental cells: Structure activity relationships. Toxicol. Appl. Pharmacol. 2012, 259, 366–375. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, W.; Leung, L.K. Zeranol upregulates corticotropin releasing hormone expression in the placental cell line JEG-3. Toxicol. Lett. 2013, 219, 218–222. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Leung, L.K. Zeranol induces COX-2 expression through TRPC-3 activation in the placental cells JEG-3. Toxicol. Vitr. 2016, 35, 17–23. [Google Scholar] [CrossRef]
- Kunishige, K.; Kawate, N.; Inaba, T.; Tamada, H. Exposure to Zearalenone During Early Pregnancy Causes Estrogenic Multitoxic Effects in Mice. Reprod. Sci. 2016, 24, 421–427. [Google Scholar] [CrossRef]
- Wu, L.; Duan, Q.; Gao, D.; Wang, Y.; Xue, S.; Li, W.; Lei, M. Zearalenone Blocks Autophagy Flow and Induces Cell Apoptosis During Embryo Implantation in Gilts. Toxicol. Sci. 2020, 175, 126–139. [Google Scholar] [CrossRef]
- Althali, N.J.; Hassan, A.M.; Abdel-Wahhab, M.A. Effect of grape seed extract on maternal toxicity and in utero development in mice treated with zearalenone. Environ. Sci. Pollut. Res. 2019, 26, 5990–5999. [Google Scholar] [CrossRef]
- Trout, W.E.; Herr, C.T.; Richert, B.T.; Singleton, W.L.; Haglof, S.A.; Diekman, M.A. Effects of Zeranol® upon luteal maintenance and fetal development in peripubertal gilts. Anim. Reprod. Sci. 2007, 99, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Schoevers, E.J.; Daemen, I.J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Exposure of Oocytes to the Fusarium Toxins Zearalenone and Deoxynivalenol Causes Aneuploidy and Abnormal Embryo Development in Pigs. Biol. Reprod. 2007, 77, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rodriguez, O.C.; Memili, E. Mycotoxin Alpha-Zearalenol Impairs the Quality of Preimplantation Porcine Embryos. J. Reprod. Dev. 2012, 58, 338–343. [Google Scholar] [CrossRef]
- Nazar, M.; Lee, S.H.; Lee, C.I.; Khan, M.S.; Ijaz, M.; Anjum, A.A.; Setyawan, E.M.N.; Jang, G. Epigenetic disruption analysis of zearalenol on different stages of in-vitro culture of parthenogenetically activated bovine embryos. Int. J. Appl. Res. Vet. Med. 2017, 15, 135–141. [Google Scholar]
- Yao, X.; Jiang, H.; Gao, Q.; Li, Y.-H.; Xu, Y.N.; Kim, N.-H. Melatonin alleviates defects induced by zearalenone during porcine embryo development. Theriogenology 2020, 151, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, K.-H.; Sun, M.-H.; Lan, M.; Wan, X.; Zhang, Y.; Sun, S.-C. Protective Effects of Melatonin Against Zearalenone Toxicity on Porcine Embryos in vitro. Front. Pharmacol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhi, Y.; Xu, H.; Fang, H.; Jia, X. Zearalenone causes embryotoxicity and induces oxidative stress and apoptosis in differentiated human embryonic stem cells. Toxicol. Vitr. 2019, 54, 243–250. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, T.Y.; Chan, F.L.; Chen, S.; Leung, L.K. Assessing the effect of food mycotoxins on aromatase by using a cell-based system. Toxicol. In Vitro 2014, 28, 640–646. [Google Scholar] [CrossRef]
- Chang, K.; Kurtz, H.J.; Mirocha, C.J. Effects of the mycotoxin zearalenone on swine reproduction. Am. J. Vet. Res. 1979, 40, 1260–1267. [Google Scholar]
- Ruzsas, C.; Biro-Gosztonyi, M.; Wöller, L.; Mess, B. Effect of the fungal toxin (zearalenone) on the reproductive system and fertility of male and female rats. Acta Biol. Acad. Sci. Hung. 1979, 30, 335–345. [Google Scholar]
- Delfosse, V.; Grimaldi, M.; Cavailles, V.; Balaguer, P.; Bourguet, W. Structural and Functional Profiling of Environmental Ligands for Estrogen Receptors. Environ. Health Perspect. 2014, 122, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Juul, A.; Leffers, H.; Skakkebæk, N.E.; Andersson, A.-M. The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens. Hum. Reprod. Updat. 2006, 12, 341–349. [Google Scholar] [CrossRef]
- Stokes, W.S. Selecting appropriate animal models and experimental designs for endocrine disruptor research and testing studies. ILAR J. 2004, 45, 387–393. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]


| Study Type | Population | Exposure | Comparators | Outcomes |
|---|---|---|---|---|
| In vivo studies | Any female mammalian animal model, age, or life stage at exposure or outcome assessment. | Exposure to mycoestrogens at all ranges of doses, duration, and routes of exposure. Excluded: exposure to mixtures of chemicals and unmeasured doses of mycoestrogens. | Experimental animals receiving vehicle-only treatment. | Reproductive hormone levels, ovary and uterine weight, morphological and pathological changes in ovary or uterus, oocyte maturation rate, duration of estrus cycle, placental changes, implantation rate, pregnancy rate, gestational weight gain, resorbed/dead fetuses, live birth rate, fetal growth. |
| In vitro and ex vivo studies | Cells lines derived from ovaries, uterus, and anterior pituitary; and zygotes, blastocysts, embryos. | Exposure to mycoestrogens including all ranges of doses and durations. Excluded: exposure to mixtures of chemicals including mycoestrogens. | Cells receiving vehicle-only treatment. | Cell viability, reactive oxygen species, apoptosis, cell proliferation, fertilization rate, blastocyst formation and development, embryotoxicity, corticotropic-releasing hormone levels. |
| Outcome | LH * | FSH * | PRO * | P4 * | E2 * | T * | Experimental Model (Strain) | Dose (Compound, Route) | Age (Duration) | [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|
| Circulating Hormones During the Non-Pregnant State | ↓ | ↓ | ↓ | Mouse (CD-1) | 20 to 40 μg/kg (ZEN, IG) | 4 wks (14 days) | [60] | |||
| ↓ | ↑ | Mouse (BALB/C) | 0.2 to 2 mg/kg (ZEN, SQ) | PND 1–5 | [25] | |||||
| ↑ | ↓ | Mouse (BALB/C) | 10 mg/kg (ZEN, IG) | 3 wks (14 days) | [27] | |||||
| ↓ | ↓ | ↓ | ↓ | Mouse (Parkes) | 2.5 mg/kg (ZEN, IP) | 8 wks (up to 90 days) | [28] | |||
| ↑ | ↓ | ↑ | ↓ | ↑ | Rat (Wistar Albino) | 0.1 and 1 mg/kg (ZEN, IG) | 9 wks (3 mos) | [26] | ||
| NC | Rat (Sprague-Dawley, ovariectomized) | 1 mg/kg (α-ZOL, IM) | 4 wks | [61] | ||||||
| NC | Rat (Sprague-Dawley) | 0.5 to 3.6 mg/kg (ZEN, diet) | 9 wks (28 days) | [62] | ||||||
| NC | ↓ | Pig (NR, ovariectomized) | 7.5 mg/kg (ZEN, IP) | NR, 24 h | [59] | |||||
| ↑ | ↑↓ | Pig (NR) | 20 and 40 μg/kg (ZEN, capsule PO) | Pre-pubertal (48 days) | [63] | |||||
| ↑↓ | ↑↓ | ↑↓ | Pigs (NR) | 5 to 15 μg/kg (ZEN, capsule PO) | Pre-pubertal (42 days) | [64] | ||||
| ↓ | ↓ | Pig (Landrace × Yorkshire) | 200 to 1600 μg/kg (ZEN, diet) | Pre-pubertal (14 days) | [65] | |||||
| ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | Pig (Landrace × Yorkshire × Duroc) | 1.1 to 3.2 mg/kg (ZEN, diet) | 2 weeks (18 days) | [23] | |
| ↓ | NC | NC | ↑ | Pigs (Duroc × Landrace × Large White) | 1 mg/kg (ZEN, diet) | 4 weeks (35 days) | [56] | |||
| Circulating Hormones During Pregnancy | ↑↓ | NC | ↓ | Mouse (ICR) | 1 to 100 mg/kg (ZER, IG) | 8 wks (GD 13.5–16.5) | [66] | |||
| ↓↑ | ↓↑ | ↓↑ | ↓↑ | ↓ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, IG) | NR (GD 6–19) | [67] | ||
| ↑ | ↑ | ↑ | ↓ | ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | NR (GD 0–7) | [22] | ||
| ↑ | ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, diet) | NR (GD 1–21) | [68] | |||||
| ↓ | ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, IG) | 60 days (GD 14–21) | [69] |
| In Vivo Studies | |||||
|---|---|---|---|---|---|
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Experimental Model (Strain) | Dose (Compound, Route) | Age (Duration) | [Ref] |
| Ovary Weight | ↑ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet ) | NR, GD 0–7 | [22] |
| NC | Rat (Sprague-Dawley) | 0.2 and 10 mg/kg (ZEN, SC) | PND 15–19 | [87] | |
| ↑ | Rat (Sprague-Dawley) | 6 mg/kg (ZEN, diet) | 3 wks (28 days) | [88] | |
| NC | Rat (Sprague-Dawley) | 0.5 to 3.6 mg/kg (ZEN, diet) | 3 wks (28 days) | [62] | |
| ↑ | Pig (Danbred) | 0.75 mg/kg (ZEN, diet) | 4 wks (21 days) | [89] | |
| ↑ | Pig (Duroc × Landrace × Yorkshire) | 1.1 to 3.2 mg/kg (ZEN, diet) | 2 wks (18 days) | [23] | |
| ↑ | Pig (Large White × Landrace × Pietrain) | 6 mg/kg (ZEN, diet) | 2 mos (26 days) | [88] | |
| Pathological or Morphological Changes in Ovary | ↑ | Mouse (CD-1) | 20 to 40 μg/kg (ZEN, SQ) | GD 12.5–18.5 | [24] |
| ↑ | Mouse (CD-1) | 10 mg/kg (ZEN and ZER, SQ) | 2 weeks (up to 24 wks) | [90] | |
| ↑ | Mouse (BALB/C) | 0.2 to 2 mg/kg (ZEN, SQ) | PND 1–5 | [25] | |
| ↑ | Mouse (BALB/C) | 10 mg/kg (ZEN, IG) | 3 wks (14 days) | [27] | |
| ↑↓ | Mouse (NR) | 0.1 mg/day (ZEN, IG) | 8 wks (10 days) | [91] | |
| ↑ | Mouse (Parkes) | 2.5 mg/kg (ZEN, IP) | 8 wks (up to 90 days) | [28] | |
| ↑ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet ) (ZEN, diet) | GD 0–7 | [22] | |
| ↑↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, diet) | GD 0–20 | [68] | |
| ↑ | Rat (Sprague-Dawley) | 0.2, 1, 5 mg/kg (ZEN, IG) | PND 15–19 | [92] | |
| ↑ | Rat (Sprague-Dawley) | 0.1 to 10 mg/kg (ZER, SQ) | PND 15–19 | [93] | |
| NC | Rat (Wistar Albino) | 0.1 to 1 mg/kg (ZEN, IG) | 9 wks (3 mos) | [26] | |
| ↑ | Rat (Wistar) | 10 mg/kg (ZEN, IG) | PND 18 (10 days) | [94] | |
| ↑↓ | Pig (York × Finnish × Landrace) | 200 to 1000 μg/kg (ZEN, diet) | GD 0–112 | [95] | |
| ↑ | Pig (Duroc × Landrace × Yorkshire) | 1.1 to 3.2 mg/kg (ZEN, diet) | 2 wks (18 days) | [23] | |
| ↑ | Pig (Duroc × Landrace × Yorkshire) | 1.04 mg/kg (ZEN, diet) | 4 wks (35 days) | [96] | |
| ↑ | Pig (Duroc × Landrace × Large White) | 0.5 to 1.5 mg/kg (ZEN, diet) | 4 wks (35 days) | [97] | |
| ↑ | Pig (NR) | 20 to 40 μg/kg (ZEN, capsule PO) | 2 mos (48 days) | [98] | |
| ↑ | Pig (Large White × Landrace) | 200 μg/kg (ZEN, capsule PO) | 3 mos (8 days) | [99] | |
| ↑ | Pig (Large White × Landrace) | 200 to 400 μg/kg (ZEN, capsule PO) | 4 mos | [100] | |
| In Vitro Studies | |||||
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Cell Type, Model (Strain) | Concentration (Compound) | Exposure Time | [Ref] |
| Cell Viability | ↓ | Granular KK-1 Cells, Mouse (NR) | 20 μM (ZEN) | 24 h | [73] |
| ↓ | Granulosa Cells, Mouse (Kunming) | 15 to 150 μM (ZEN) | 24 h | [72] | |
| ↓ | Granulosa Cells, Mouse (Kunming) | 30 μM (ZEN) | 24 h | [77] | |
| NC, NC | Granulosa Cells, Pig (NR) | 5 to 30 μM (α-ZOL, β-ZOL) | 48 h | [101] | |
| Reactive Oxygen Species | ↑ | Granular KK-1 cells, (Mouse) | 20 μM (ZEN) | 24 h | [73] |
| ↑ | Oocytes, Pig (NR) | 5 to 30 μM (ZEN) | 44 h | [82] | |
| ↑ | Ovaries, Sheep (NR) | 1 μmol/L (ZEN) | 3 days | [84] | |
| Oocyte Maturation Rate | ↓ | Granulosa Cell, Mouse (ICR) | 10 to 50 μM (ZEN) | 24 h | [102] |
| ↓, ↓ | Oocytes, Pig (Landrace) | 3.5 to 90 μM (α-ZOL, β-ZOL) | 48 h | [81] | |
| ↓ | Oocytes, Pig (NR) | 1 to 1000 μg/L (ZEN) | 71 h | [80] | |
| ↓, ↓, ↓ | Oocytes, Cow (NR) | 0.3 to 30 μg/ml (ZEN, α-ZOL, ZAN) | 24 h | [79] | |
| Epigenetic Modifications | ↑ | Oocytes, Mouse (ICR) | 10 to 50 μM (ZEN) | 12 h | [103] |
| ↑ | Oocytes, Pig (NR) | 5 to 30 μM (ZEN) | 44 h | [82] | |
| Apoptosis or Markers of Apoptosis | ↑ | Granular KK-1 Cells, Mouse (NR) | 20 μM (ZEN) | 24 h | [73] |
| ↑ | Ovaries, Mouse (CD-1) | 10 to 30 μM (ZEN) | 72 h | [70] | |
| ↑ | Granulosa Cells, Mouse (Kunming White) | 15 to 150 μM (ZEN) | 24 h | [72] | |
| ↑ | Granulosa Cells, Pig (NR) | 60 to 120 μM (ZEN) | 24 h | [74] | |
| ↑ | Oocytes, Pig (NR) | 5 to 30 μM (ZEN) | 44 h | [82] | |
| ↑ | Granulosa Cells, Pig (NR) | 5 to 30 μM (ZEN) | 48 h | [104] | |
| ↑ | Granulosa Cells, Pig (NR) | 10 to 30 uM (ZEN) | 48 h | [76] | |
| ↓ | Granulosa Cells, Cow (NR) | 5 to 200 uM (β-ZOL) | 24 h | [75] | |
| ↑, ↑, ↑ | Granulosa Cells, Horse (NR) | 1 × 10−7 to 0.1 μM (ZEN, α-ZOL, β-ZOL) | 72 h | [86] | |
| Cell Proliferation | ↓ | Granulosa Cell, Mouse (ICR) | 10 to 50 μM (ZEN) | 24 h | [102] |
| ↓ | Granulosa Cells, Pig (NR) | 60 to 120 μM (ZEN) | 24 h | [74] | |
| ↑ | Granulosa Cells, Cow (NR) | 0.09 to 3.1 μM (α-ZOL) | 48 h | [85] | |
| ↓ | Granulosa Cells, Cow (NR) | 5 to 200 μM (β-ZOL) | 24 h | [75] | |
| ↑, NC, NC | Granulosa Cells, Horse (NR) | 1 × 10−7 to 0.1 μM (ZEN, α-ZOL, β-ZOL) | 72 h | [86] | |
| In Vivo Studies | |||||
|---|---|---|---|---|---|
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Experimental Model (Strain) | Dose (Compound, Route) | Age (Duration) | [Ref] |
| Uterine Weight | ↑ | Mouse (B6C3F1) | 25 or 35 mg/kg (ZEN, diet) | 2.5 wks (6 days) | [109] |
| ↑ | Mouse (CD-1) | 10−2 to 106 μg/kg (ZEN, α-ZOL, SQ) | 2.5 wks (3 days) | [18] | |
| ↑ | Mouse (B6C3F1) | 35 mg/kg (ZEN, diet) | 2.5 wks (7 days) | [110] | |
| ↑ | Mouse (ICR, ovariectomized) | 0.5 to 1000 ng/kg (ZEN, a-ZOL, SQ) | 6 wks (3 days) | [15] | |
| ↑ | Mouse (BALB/C) | 10 mg/kg (ZEN, IG) | 3 wks (14 days) | [27] | |
| ↑ | Rat (Sprague-Dawley) | 0.03 to 10 μg/kg (ZEN, PO) | PND 21–24 | [111] | |
| ↓ | Rat (Sprague Dawley, ovariectomized) | 1 mg/kg (a-ZOL, IM) | 4 wks (28 days) | [61] | |
| ↑ | Rat (Sprague Dawley, ovariectomized) | 0.2 to 2 mg/kg (ZEN, SQ) | NR (3 days) | [112] | |
| ↑ | Rat (Sprague-Dawley) | 0.03 to 10 μg/kg (ZEN, PO) | PND 21–24 | [111] | |
| ↑ | Rat (Sprague-Dawley) | 0.5 to 3.6 mg/kg (ZEN, diet) | 3 wks (28 days) | [62] | |
| ↑ | Rat (Sprague-Dawley) | 6 mg/kg (ZEN, diet) | 3 wks (28 days) | [88] | |
| ↑ | Pig (Danbred) | 0.75 mg/kg (ZEN, diet) | 4 wks (21 days) | [89] | |
| ↑ | Pig (Duroc × Landrace × Large White × Pietrain) | 1.5 mg/kg (ZEN, diet) | 4 wks (35 days) | [17] | |
| ↑ | Pig (Duroc × Landrace × Large White) | 0.5 to 1.5 mg/kg (ZEN, diet) | 5 wks (35 days) | [113] | |
| ↑ | Pig (Large White × Landrace × Pietrain) | 0.8 mg/kg (ZEN, diet) | 7 wks (26 days) | [88] | |
| Pathological or Morphological Changes in Uterus | ↑ | Mouse (CD-1) | 10−2 to 106 μg/kg (ZEN, α-ZOL, SQ) | 2.5 wks (3 days) | [18] |
| ↑ | Mouse (BALB/C) | 10 mg/kg (ZEN, IG) | 3 wks (14 days) | [27] | |
| ↑ | Mouse (Parkes) | 2.5 mg/kg (ZEN, IP) | 8 wks (up to 90 days) | [28] | |
| ↑ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, diet) | GD 0–20 | [68] | |
| NC | Rat (Sprague-Dawley) | 0.1 to 10 mg/kg (ZER, SQ) | PND 15–19 | [93] | |
| ↑ | Rat (Sprague-Dawley) | 0.2 to 5 mg/kg (ZEN, IG) | PND 15–19 | [92] | |
| ↑ | Rat (Sprague-Dawley) | 0.03 to 10 μg/kg (ZEN, PO) | PND 21–24 | [111] | |
| ↑ | Rat (Sprague-Dawley) | 0.2 to 5 mg/kg (ZEN, IG) | PND 15–19 | [92] | |
| ↑ | Dog (NR) | 50 to 75 μg/kg (ZEN, capsule PO) | 10 wks (42 days) | [114] | |
| ↑ | Pig(Danbred) | 0.75 mg/kg (ZEN, diet) | 4 wks (21 days) | [89] | |
| ↑ | Pig (Duroc × Landrace × Large White) | 1 mg/kg (ZEN, diet) | 4 wks (35 days) | [56] | |
| ↑ | Pig (Duroc × Landrace × Large White) | 0.5 to 1.5 mg/kg (ZEN, diet) | 5 wks (35 days) | [113] | |
| ↑ | Pig (Duroc × Landrace × Large White) | 0.5 to 1.5 mg/kg (ZEN, diet) | 5 wks (35 days) | [115] | |
| Estradiol-Binding Sites in Uterine Cytosol | ↓ | Rat (Sprague-Dawley) | 2.5 mg (ZEN, IG) | 3 mos (5 days) | [16] |
| Irregular Estrus Cycling | ↑ | Mouse (CD-1) | 0.5 or 10 mg/kg (ZEN, SQ) | GD 15–19 | [116] |
| ↑ | Mouse (C57BL/6J) | 0.002 to 40 ppm (ZEN, PO) | GD 0.5–4.5 | [117] | |
| ↑ | Mouse (CD-1) | 10 mg/kg (ZEN and ZER, SQ) | 2 wks (up to 24 wks | [90] | |
| ↑ | Mouse (BALB/C) | 0.2 to 2 mg/kg (ZEN, SQ) | PND 1–5 | [25] | |
| ↑ | Rat (Sprague-Dawley) | 0.1 to 10 mg/kg (ZER, SQ) | PND 15–19 | [93] | |
| ↑ | Rat (Sprague-Dawley) | 0.2 and 10 mg/kg (ZEN, SQ) | PND 15–19 | [87] | |
| ↑ | Rat (Sprague-Dawley) | 0.2, 1, 5 mg/kg (ZEN, IG) | PND 15–19 | [92] | |
| NC | Rat (Sprague-Dawley) | 0.5 to 3.6 mg/kg (ZEN, diet) | 3 wks (28 days) | [62] | |
| In Vitro Studies | |||||
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Cell Type, Model (Strain) | Concentration (Compound) | Exposure Time | [Ref] |
| Cell Viability | ↓ | Endometrial Stromal Cells, Mouse (NR) | 25 to 125 μM (ZEN) | 6 to 48 h | [118] |
| NC, NC | Granulosa Cells, Pig (NR) | 7.5 to 30 μM (α-ZOL, β-ZOL) | 24 or 48 h | [101] | |
| NC, ↓ | Endometrial Cells, Pig (Landrace) | 7.5 to 30 μM (α-ZOL, β-ZOL) | 24 h | [119] | |
| Apoptosis or Markers of Apoptosis | ↑ | Endometrial Stromal cells, Mouse (NR) | 25 to 125 μM (ZEN) | 24 h | [120] |
| ↑ | Endometrial Cells, Mouse (Strain NR) | 25 to 125 μM (ZEN) | 6 to 48 h | [118] | |
| Cell Proliferation | ↓ | Granulosa Cells, Pig (NR) | 7.5 to 30 μM (α-ZOL, β-ZOL) | 24 or 48 h | [101] |
| NC, ↓ | Endometrial Cells, Pig (Landrace) | 7.5 to 30 μM (α-ZOL, β-ZOL) | 24 h | [119] | |
| Myometrial Contractility | ↑↓ | Uterine smooth muscle, ex vivo | 10−11 to 10−6 M (ZEN, α-ZOL, β-ZOL) | 3 min | [121] |
| In Vivo Studies | |||||
|---|---|---|---|---|---|
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Experimental Model (Strain) | Dose (Compound, Route) | Age (Duration) | [Ref] |
| Placental Weight | ↓ | Mouse (C57/BL6/129) | 40 ppm (ZEN, diet) | 8 wks (GD 5.5–13.5) | [29] |
| NC | Mouse (Slc:ICR) | 2 to 8 mg/kg (ZEN, SQ) | 8 wks (GD 1–5) | [131] | |
| ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, IG) | 60 days (GD 7–14) | [69] | |
| Placental Thickness | ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, IG) | 60 days (GD 7–14) | [69] |
| Placental Hemorrhage | ↑ | Mouse (C57/BL6/129) | 40 ppm (ZEN, diet) | 2 mos GD 5.5–13.5 | [29] |
| Implantation Sites | ↓ | Mouse (C57BL/6J) | 0.002 to 40 ppm (ZEN, diet) | 3 wks (up to 5 wks) | [117] |
| ↓ | Mouse (Slc:ICR) | 2 to 8 mg/kg (ZEN, SQ) | 8 wks (GD 1–5) | [131] | |
| ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| Embryo Size | ↓ | Pig (Landrace × Large White) | 1 to 10 mg/kg (ZEN, diet) | NR (GD 7–14) | [132] |
| Pregnancy Rate | ↓ | Mouse (C57BL/6J) | 0.002 to 40 ppm (ZEN, diet) | 3 wks (up to 5 wks) | [117] |
| ↓ | Mouse (C57BL/6J) | 0.8 to 40 ppm (ZEN, PO) | GD1—up to 10 wks | [117] | |
| ↓ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, IG) | NR (GD 6–19) | [67] | |
| NC | Cow (Charolais × Balancer) | 36 mg (ZER, implant) | 8 mos (195 days) | [105] | |
| Gestational Weight Gain | ↓ | Mouse (ICR) | 1 to 100 mg/kg (ZER, IG) | 8 wks (GD 13.5–16.5) | [66] |
| ↓ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| ↓ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, PO) | NR (GD 6–19) | [67] | |
| ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, PO) | NR, GD 0–20 | [68] | |
| Resorbed Fetuses and Fetal Deaths | ↑ | Mouse (ICR) | 1 to 100 mg/kg (ZER, IG) | 8 wks (GD 13.5–16.5) | [66] |
| ↑ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| ↑ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, PO) | NR (GD 6–19) | [67] | |
| ↑ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| Number of Live Births | ↓ | Mouse (Slc:ICR) | 2 to 8 mg/kg (ZEN, SQ) | 8 wks (GD 1–5) | [131] |
| ↓ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| ↓ | Mouse (ICR) | 1 to 100 mg/kg (ZER, IG) | 8 wks (GD 13.5–16.5) | [66] | |
| ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, diet) | NR, GD 0–20 | [68] | |
| ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| ↓ | Pig (crossbred) | 36 mg (ZER, implant) | 5.5 mos old (58 days) | [134] | |
| Fetal Weight | ↓ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] |
| ↓ | Mouse (Slc:ICR) | 2 to 8 mg/kg (ZEN, SQ) | 8 wks (GD 1–5) | [131] | |
| ↓ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| ↓ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, IG) | NR (GD 6–19) | [67] | |
| ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| ↓ | Pig (crossbred) | 36 mg (ZER, implant) | 5.5 mos old (58 days) | [134] | |
| ↓ | Rat (Sprague-Dawley) | 5 to 20 mg/kg (ZEN, IG) | 60 days (GD 7–14) | [69] | |
| Fetal Length | ↓ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, IG) | NR (GD 6–19) | [67] |
| ↓ | Rat (Sprague-Dawley) | 0.3 to 146 mg/kg (ZEN, diet) | 9 wks (GD 0–7) | [22] | |
| ↓ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| ↓ | Pig (crossbred) | 36 mg (ZER, implant) | 5.5 mos old (58 days) | [134] | |
| Fetal Skeletal Abnormalities | ↑ | Rat (Sprague-Dawley) | 1 to 8 mg/kg (ZEN, IG) | NR (GD 6–19) | [67] |
| ↑ | Mouse (Albino) | 25 mg/kg (ZEN, IG) | 10 wks (GD 6–13) | [133] | |
| In Vitro Studies | |||||
| Outcome | Impact * (↑, ↓, ↑↓, NC) | Cell Type, Model | Concentration (Compound) μM (α-ZOL, β-ZOL) | Exposure Time | [Ref] |
| Fertilization Rate | ↑↓ | Porcine oocytes | 1 to 1000 μg/L (ZEN) | During fertilization | [80] |
| Blastocyst Formation and Development | ↓ | Porcine zygotes | 3.75 to 30 μM (α-ZOL) | 5 days | [81] |
| ↓ | COCs | 0.312–31.2 μmol/L (ZEN, α-ZOL β-ZOL) | 44 h | [135] | |
| ↓ | Fertilized porcine embryos | 3 to 60 μM (α-ZOL) | 24–48 h post-insemination | [136] | |
| ↓ | Bovine oocytes | 3 to 30 μM (α-ZOL β-ZOL) | During in vitro maturation | [137] | |
| ↓ | Porcine blastocysts | 5 to 50 μM (ZEN) | 24 h | [138] | |
| ↓ | Porcine embryos | 10 μM (ZEN) | 144 h | [139] | |
| Embryotoxicity | ↑ | mESC | 2–20 μg/mL (ZEN) | 24 h | [140] |
| ↑ | hESC | 2–20 μg/mL (ZEN) | 24 h | [140] | |
| Corticotropic-Releasing Hormone | ↑ | JEG-3 cells | 0.01 to 100 nM (ZER) | 24 h | [129] |
| Human Chorionic Gonadotropin | ↑ | BeWo cells | 0.1 to 200 μM (ZEN) | 24 to 72 h | [127] |
| ↑, NC, NC | BeWo cells | 0.1 to 100 μM (ZEN, α-ZOL, β-ZOL) | 48 h | [128] | |
| Aromatase | ↓ | JEG-3 cells | 0.001 to 100 μM (ZEN, ZER) | 24 h | [141] |
| Transient Receptor Potential Channels | ↑↓ | JEG-3 cells | 0.01 to 100 nM (ZER) | [130] | 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. https://doi.org/10.3390/toxins13060373
Kinkade CW, Rivera-Núñez Z, Gorcyzca L, Aleksunes LM, Barrett ES. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins. 2021; 13(6):373. https://doi.org/10.3390/toxins13060373
Chicago/Turabian StyleKinkade, Carolyn W., Zorimar Rivera-Núñez, Ludwik Gorcyzca, Lauren M. Aleksunes, and Emily S. Barrett. 2021. "Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review" Toxins 13, no. 6: 373. https://doi.org/10.3390/toxins13060373
APA StyleKinkade, C. W., Rivera-Núñez, Z., Gorcyzca, L., Aleksunes, L. M., & Barrett, E. S. (2021). Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins, 13(6), 373. https://doi.org/10.3390/toxins13060373





