Daphnia’s Adaptive Molecular Responses to the Cyanobacterial Neurotoxin Anatoxin-α Are Maternally Transferred
Abstract
1. Introduction
2. Results
2.1. Clones
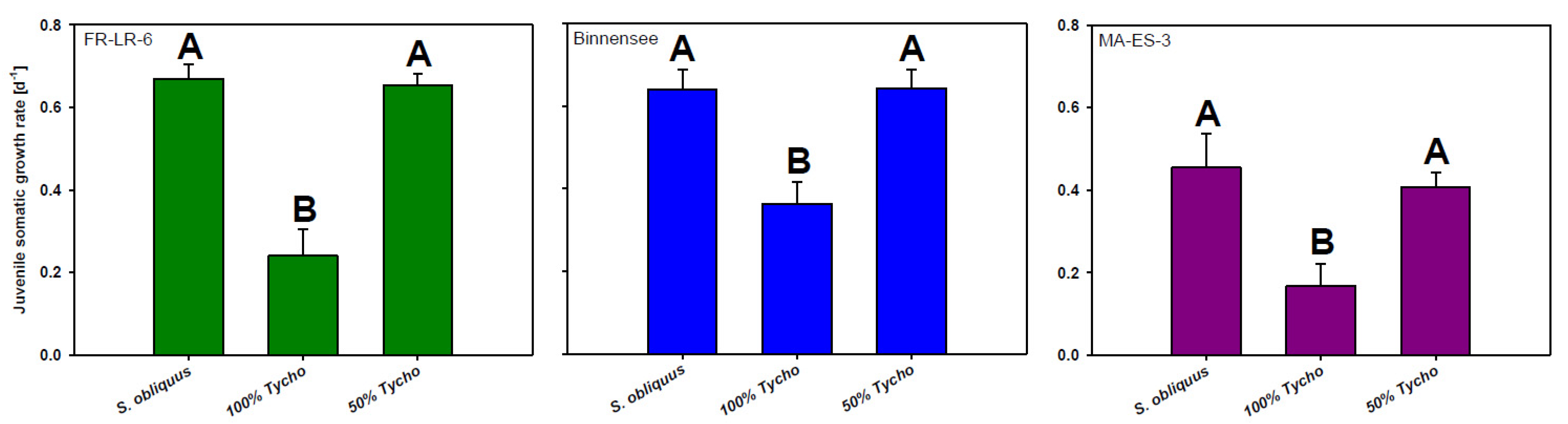
2.1.1. Somatic Growth Rates of the Three D. magna Clones
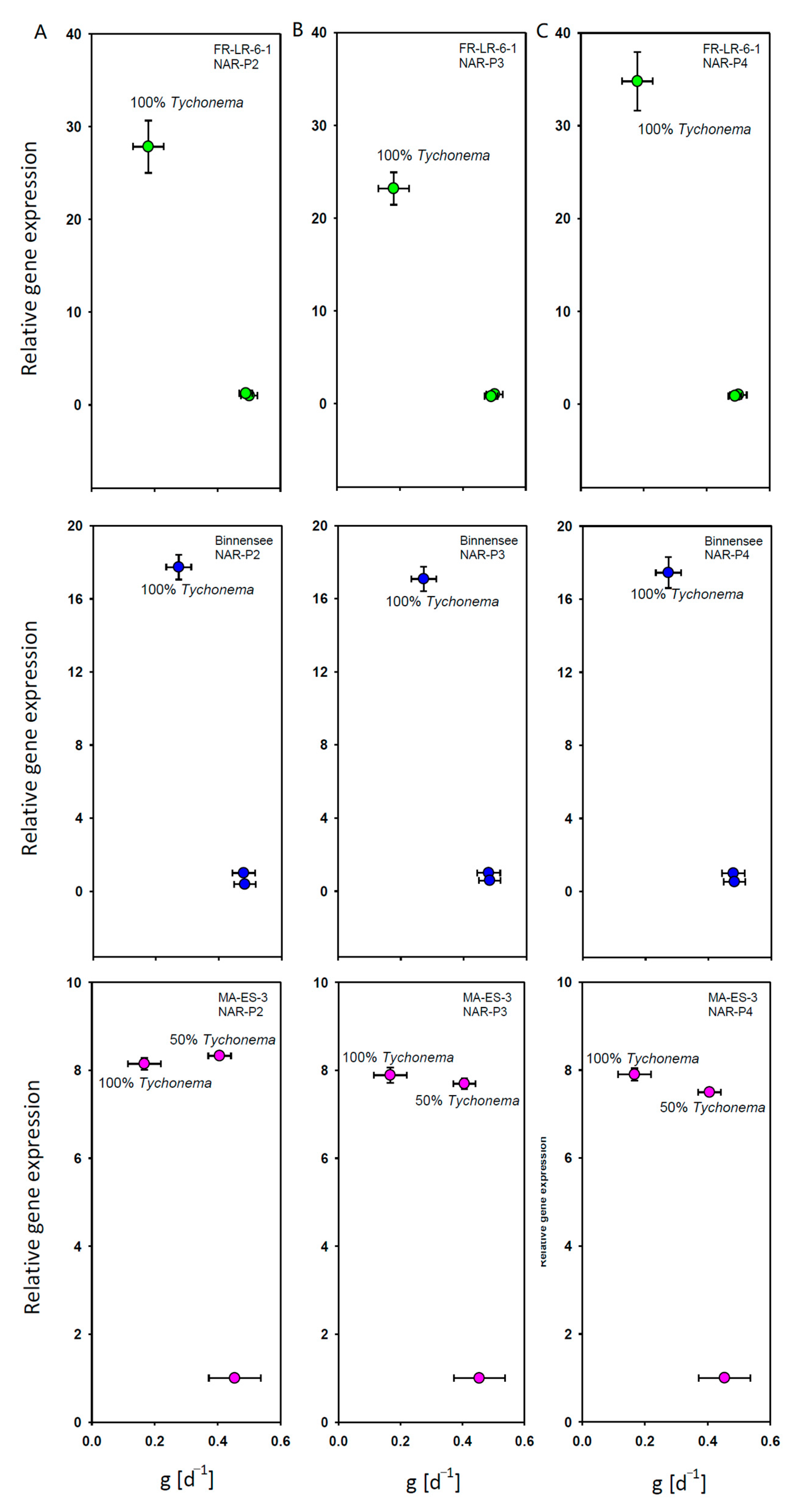
2.1.2. Gene Expression
2.2. Maternal Effects
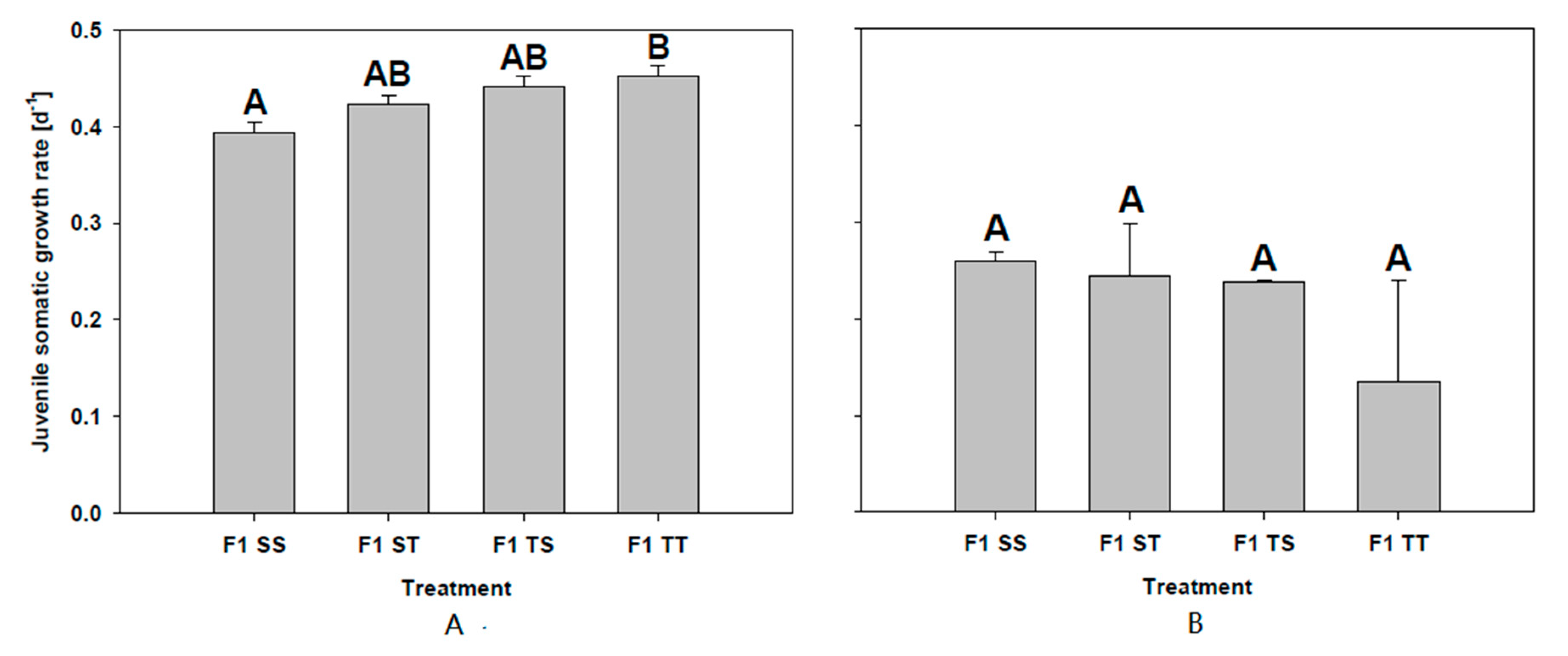
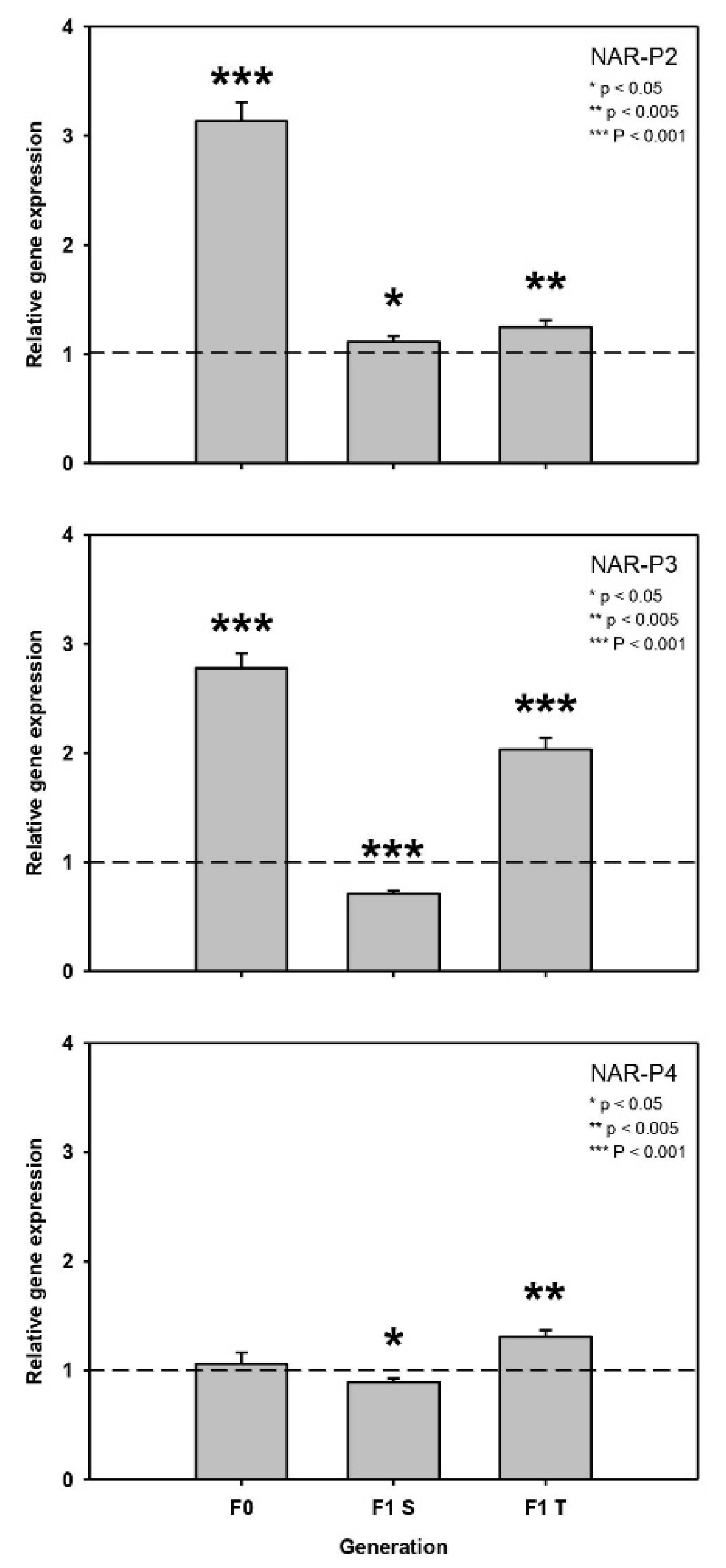
2.2.1. First Experiment: 50% T. bourrellyi
2.2.2. Second Experiment: 100% T. bourrellyi
3. Discussion
4. Materials and Methods
4.1. Cultures
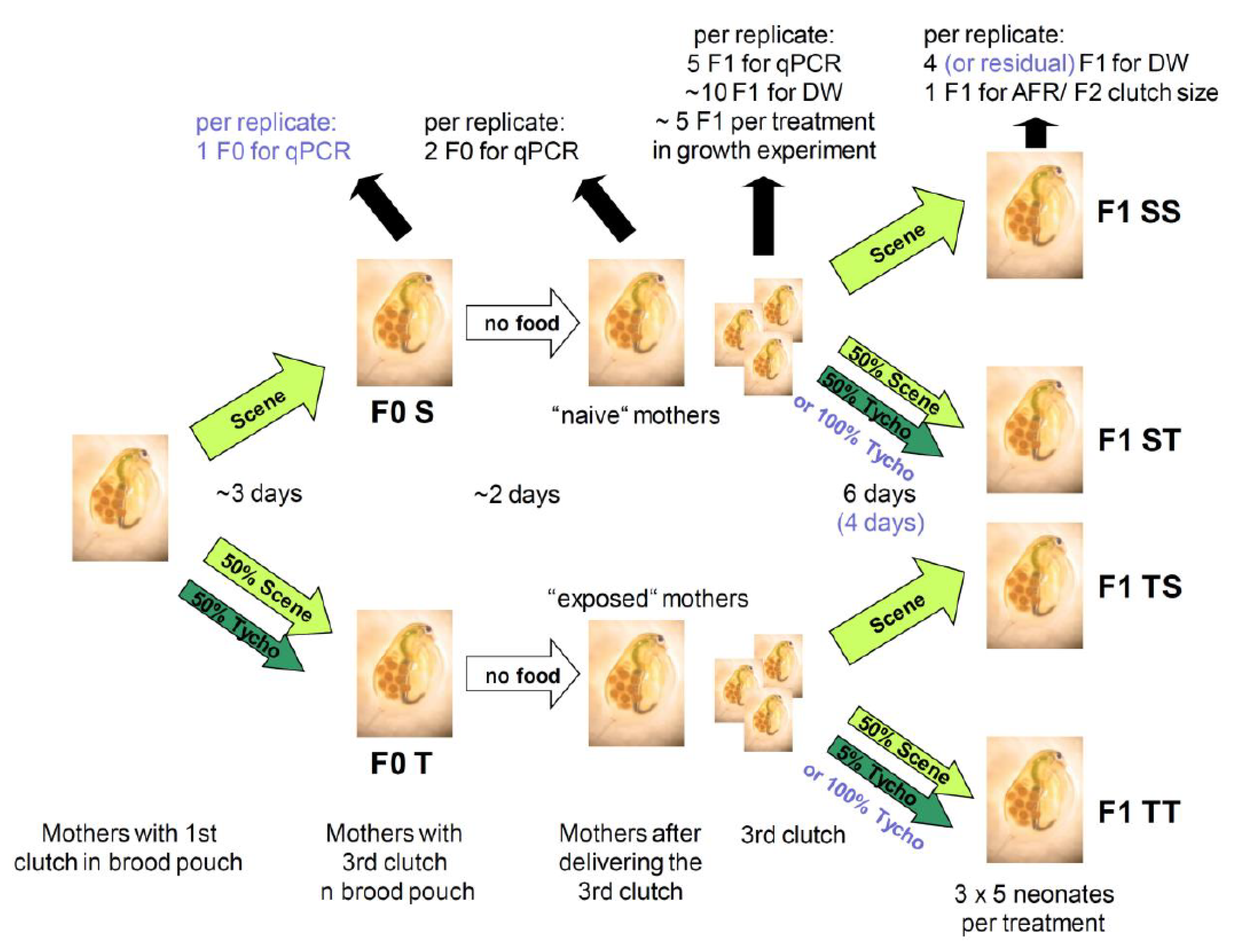
4.2. Experiment Set-Ups
4.2.1. Clone Experiments
4.2.2. Maternal Effect Experiments
4.3. Gene Expression
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paerl:, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 64–72. [Google Scholar] [CrossRef]
- Gademann, K.; Portmann, C. Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- U.S. EPA (United States Environmental Protection Agency). 2015 Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-a. EPA 820R15104, Washington, DC; June 2015; USEPA: Washington, DC, USA, 2015. Available online: http://water.epa.gov/drink/standards/hascience.cfm (accessed on 2 February 2021).
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Sivonen, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae. Mitt. Int. Ver. Limnol. 1964, 15, 796e804. [Google Scholar]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Kalinowska, R.; Skowroński, T. Cyanotoxin diversity and food web bioaccumulation in a reservoir with decreasing phosphorus concentrations and perennial cyanobacterial blooms. Harmful Algae 2013, 28, 118–125. [Google Scholar] [CrossRef]
- Bumke-Vogt, C.; Mailahn, W.; Chorus, I. Anatoxin-a and neurotoxic cyanobacteria in German lakes and reservoirs. Environ. Toxicol. 1999, 14, 117–125. [Google Scholar] [CrossRef]
- Osswald, J.; Carvalho, A.P.; Claro, J.; Vasconcelos, V. Effects of cyanobacterial extracts containing anatoxin-a and of pure anatoxin-a on early developmental stages of carp. Ecotoxicol. Environ. Saf. 2009, 72, 473–478. [Google Scholar] [CrossRef]
- Cerasino, L.; Salmaso, N. Diversity and distribution of cyanobacterial toxins in the Italian subalpine lacustrine district. Oceanol. Hydrobiol. Stud. 2012, 41, 54e63. [Google Scholar] [CrossRef]
- Shams, S.; Capelli, C.; Cerasino, L.; Ballot, A.; Dietrich, D.R.; Sivonen, K.; Salmaso, N. Anatoxin-a producing Tychonema (Cyanobacteria) in European waterbodies. Water Res. 2015, 69, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, K.F.; Huntley, N.; Duffy, M.A.; Hunter, M.D. Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system. Proc. R. Soc. B 2019, 286, 20182231. [Google Scholar] [CrossRef] [PubMed]
- Claska, M.E.; Gilbert, J.J. The effect of temperature on the response of Daphnia to toxic cyanobacteria. Freshw. Ecol. 1998, 39, 221–232. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Lantz, M.; Wiedner, C. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatchenkoi in northeastern Germany. Toxicon 2010, 56, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Rellán, S.; Osswald, J.; Saker, M.; Gago-Martinez, A.; Vasconcelos, V. First detection of anatoxin-a in human and animal dietary supplements containing cyanobacteria. Food Chem. Toxicol. 2009, 47, 2189–2195. [Google Scholar] [CrossRef]
- Thomas, P.; Stephens, M.; Wilkie, G.; Amar, M.; Lunt, G.G.; Whiting, P.; Gallagher, T.; Pereira, E.; Alkondon, M.; Albuquerque, E.X.; et al. (+)-Anatoxin-a is a potent agonist at neuronal nicotinic acetylcholine receptors. J. Neurochem. 1993, 60, 2308–2311. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (cynobacteria) aqueous extracts on the acetylcholineesterade activity of aquatic species. Environ. Toxicol. Chem. 2001, 20, 1228–1235. [Google Scholar] [CrossRef]
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The toxicity of cyanobacterial toxins in the mouse: II anatoxin-a. Hum. Exp. Toxicol. 1999, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxification. Biochim. Biophys. Acta 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, R.; Dao, T.S.; Wiegand, C. Transgenerational effects of microcystin-LR on Daphnia magna. J. Exp. Biol. 2012, 215, 2795–2805. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Sadler, T.; Motameny, S.; Ben-Khalifa, K.; Frommolt, P.; Altmüller, J.; Konrad, K.; Von Elert, E. Deciphering the genetic basis of microcystin tolerance. BMC Genom. 2014, 15, 776. [Google Scholar] [CrossRef]
- Sadler, T.; Von Elert, E. Physiological interaction of Daphnia and Microcystis with regard to cyanobacterial secondary metabolites. Aquat. Toxicol. 2014, 156, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Kurmayer, R.; Martin-Creuzburg, D. Toward disentangling the multiple nutritional constraints imposed by Planktothrix: The significance of harmful secondary metabolites and sterol limitation. Front. Microbiol. 2020, 11, 586120. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Zitt, A.; Kroth, P.; Mueller, S.; Von Elert, E. Gene expression and activity of digestive proteases in Daphnia: Effects of cyanobacterial protease inhibitors. BMC Physiol. 2010, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, A.; Kuster, C.J.; Von Elert, E. Molecular mechanisms of tolerance to cyanobacterial protease inhibitors revealed by clonal differences in Daphnia magna. Mol. Ecol. 2012, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.F.; Baumann, H.; Codd, G.A.; Jüttner, F. Sensitivity and adaptation of aquatic organisms to oscillapeptin J and [D-Asp3,(E)-Dhb7]microcystin-RR. Arch. Fuer Hydrobiol. 2006, 167, 547–559. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Hasselmann, M.; Von Elert, E. Positive selection of digestive proteases in Daphnia: A mechanism for local adaptation to cyanobacterial protease inhibitors. Mol. Ecol. 2020, 29, 912–919. [Google Scholar] [CrossRef]
- Gustafsson, S.; Rengefors, K.; Hansson, L.A. Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 2005, 86, 2561–2567. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Von Elert, E. Cyanobacterial protease inhibitors lead to maternal transfer of increased protease gene expression in Daphnia. Oecologia 2013, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Radersma, R.; Hegg, A.; Noble, D.W.A.; Uller, T. Timing of maternal exposure to toxic cyanobacteria and offspring fitness in Daphnia magna: Implications for the evolution of anticipatory maternal effects. Ecol. Evol. 2018, 8, 12727–12736. [Google Scholar] [CrossRef]
- Bownik, A.; Pawlik-Skowrońska, B. Early indicators of behavioral and physiological disturbances in Daphnia magna (Cladocera) induced by cyanobacterial neurotoxin anatoxin-a. Sci. Total Environ. 2019, 695, 133913. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.Q.D.; Ferrão Filho, A.D.S. Effects of an Anatoxin-a (s)-producing strain of Anabaena spiroides (Cyanobacteria) on the survivorship and somatic growth of two Daphnia similis clones. J. Environ. Prot. 2013, 4, 12–18. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Pflugmacher, S.; James, K.J.; Furey, A. Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat. Toxicol. 2004, 68, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Osswald, J.; Azevedo, J.; Vasconcelos, V.; Guilhermino, L. Experimental determination of the bioconcentration factors for anatoxin-a in juvenile rain-bow trout (Oncorhynchus mykiss). Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 77–86. [Google Scholar]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Freese, H.M.; Martin-Creuzburg, D. Food quality of mixed bacteria–algae diets for Daphnia magna. Hydrobiologia 2013, 715, 63–76. [Google Scholar] [CrossRef]
- Perry, T.; Heckel, D.G.; McKenzie, J.A.; Batterham, P. Mutations in Dα1 or Dβ2 nicotinic acetylcholine receptor subunits can confer resistance to neonicotinoids in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008, 38, 520–528. [Google Scholar] [CrossRef]
- Von Elert, E.; Jüttner, F. Phosphorus limitation not light controls the exudation of allelopathic compounds by Trichormus doliolum. Limnol. Oceanogr. 1997, 42, 1796–1802. [Google Scholar] [CrossRef]
- Lampert, W.; Rothhaupt, K.O. Alternating dynamics of rotifers and Daphnia magna in a shallow lake. Arch. Hydrobiol. 1992, 120, 447–456. [Google Scholar]
- Schwarzenberger, A.; Courts, C.; Von Elert, E. Target gene approaches: Gene expression in Daphnia magna exposed to predator-borne kairomones or to microcystin-producing and microcystin-free Microcystis aeruginosa. BMC Genom. 2009, 10, 527. [Google Scholar] [CrossRef]
- Heckmann, L.H.; Connon, R.; Hutchinson, T.H.; Maund, S.J.; Sibly, R.M.; Callaghan, A. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genom. 2006, 7, 175–182. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Gene Name | Primer Forward (5′–3′) | Primer Reverse (5′–3′) | Length | acc # |
|---|---|---|---|---|---|
| NAR-P2 | Neuronal nicotinic acetyl-choline subunit | GGCGCAGACCTCTCTTCTAC | GGTTGTGATGCCGAGAGTCA | 125 bp | JGI_V11_55424 |
| Nar-P3 | Neuronal nicotinic acetylcholine subunit | ATTTGCTTGGTGTCGTTCGC | AGAATGATCCGGCGCATGAT | 107 bp | JGI_V11_66098 |
| NAR-P4 | Nicotinic acetylcholine receptor subunit beta 1 | CACAACCACACGAAACCCAC | GAAGACCAAGACGCACAGGA | 129 bp | JGI_V11_321681 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarzenberger, A.; Martin-Creuzburg, D. Daphnia’s Adaptive Molecular Responses to the Cyanobacterial Neurotoxin Anatoxin-α Are Maternally Transferred. Toxins 2021, 13, 326. https://doi.org/10.3390/toxins13050326
Schwarzenberger A, Martin-Creuzburg D. Daphnia’s Adaptive Molecular Responses to the Cyanobacterial Neurotoxin Anatoxin-α Are Maternally Transferred. Toxins. 2021; 13(5):326. https://doi.org/10.3390/toxins13050326
Chicago/Turabian StyleSchwarzenberger, Anke, and Dominik Martin-Creuzburg. 2021. "Daphnia’s Adaptive Molecular Responses to the Cyanobacterial Neurotoxin Anatoxin-α Are Maternally Transferred" Toxins 13, no. 5: 326. https://doi.org/10.3390/toxins13050326
APA StyleSchwarzenberger, A., & Martin-Creuzburg, D. (2021). Daphnia’s Adaptive Molecular Responses to the Cyanobacterial Neurotoxin Anatoxin-α Are Maternally Transferred. Toxins, 13(5), 326. https://doi.org/10.3390/toxins13050326




